Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems
Abstract
:1. Introduction
2. Materials and Methods
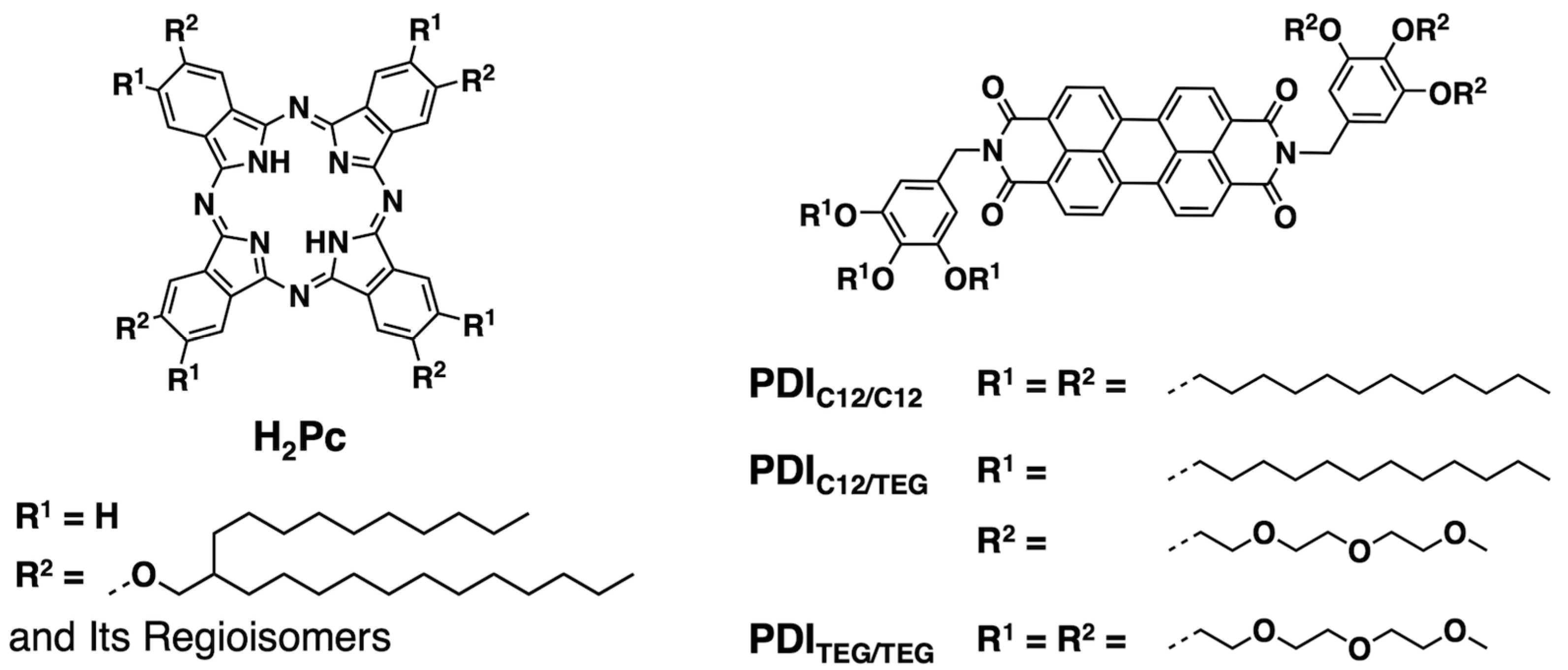
2.1. Synthesis and Characterization of H2Pc and PDIs
2.2. Characterization of LC Mesophases
2.3. Evaluation of Intracolumnar Molecular Order
3. Results and Discussion
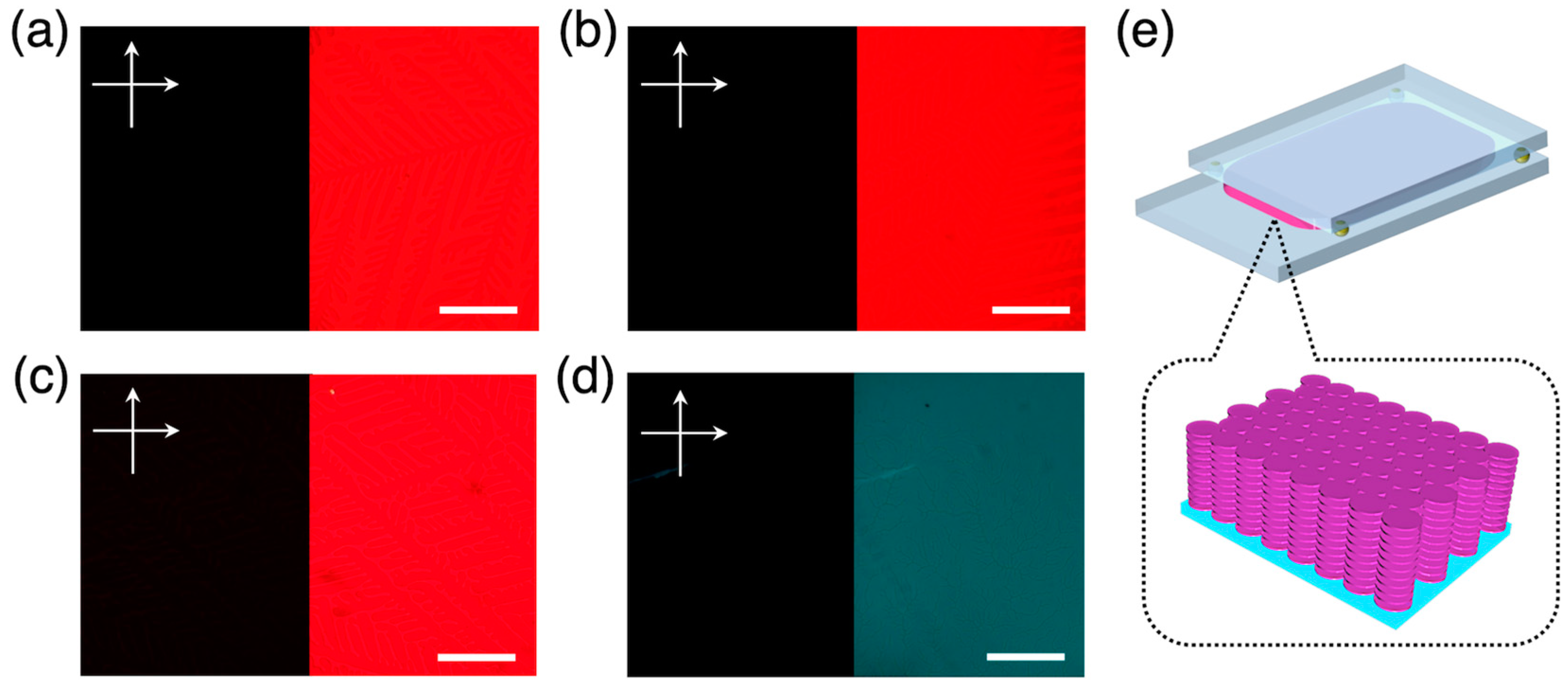
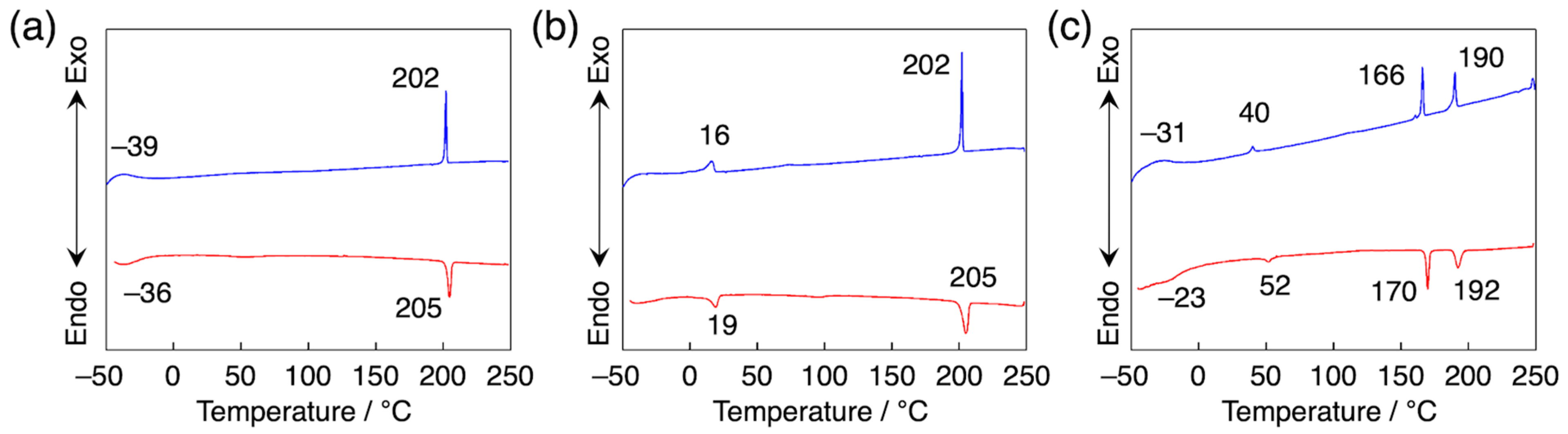
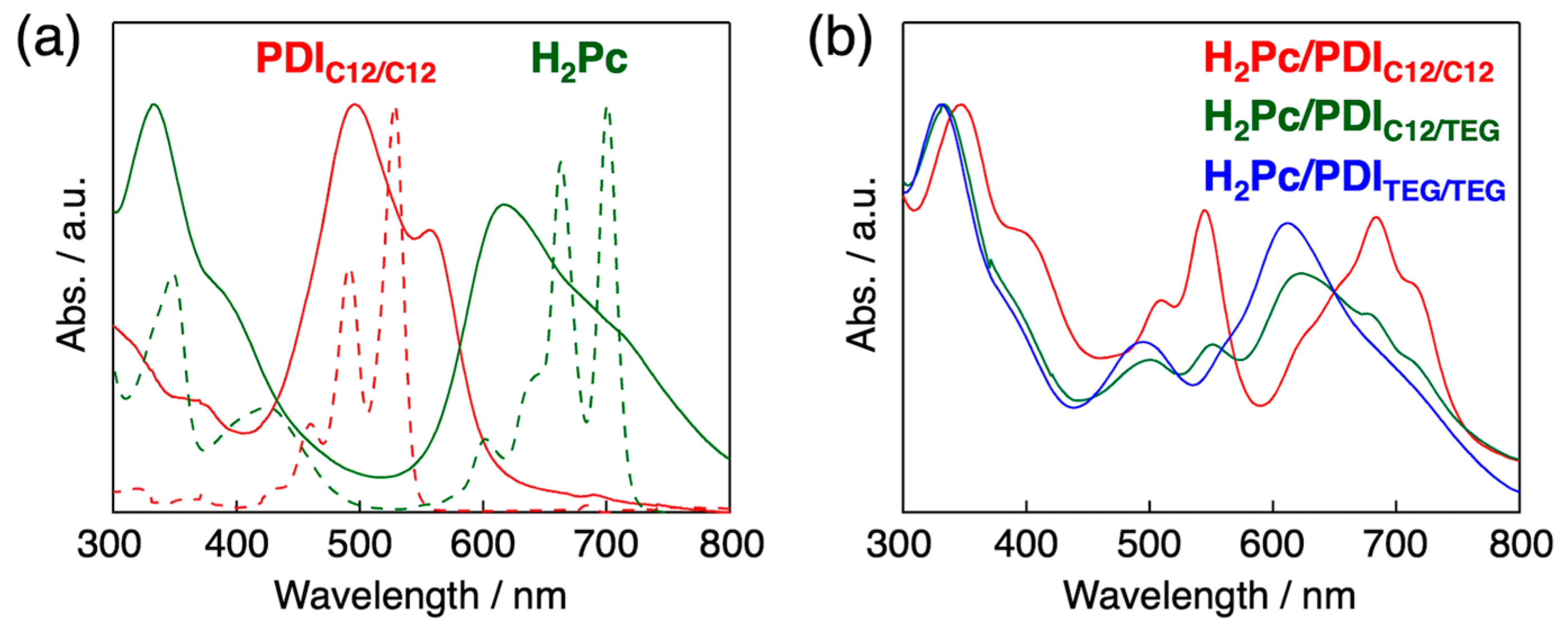
3.1. Homeotropic Alignment Capability of H2Pc and PDIs
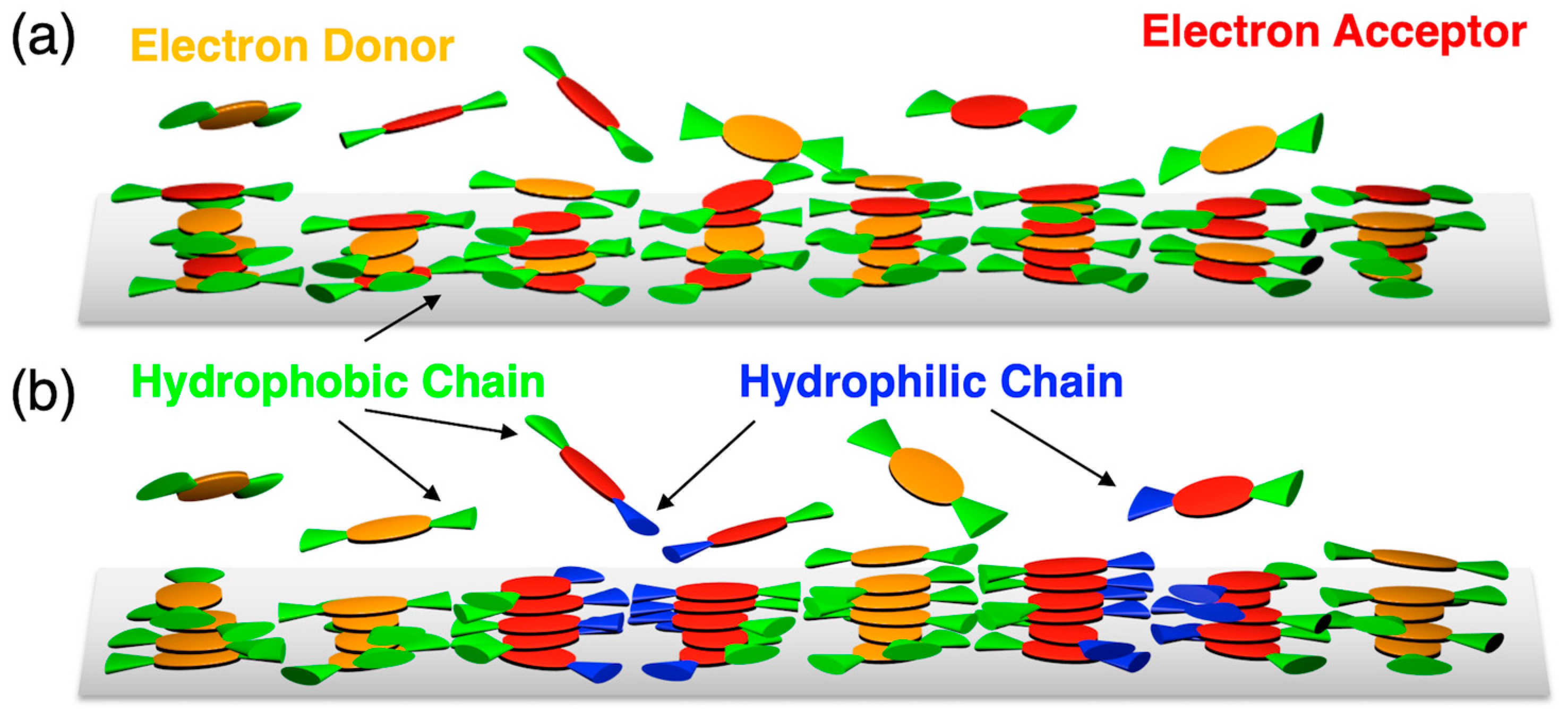
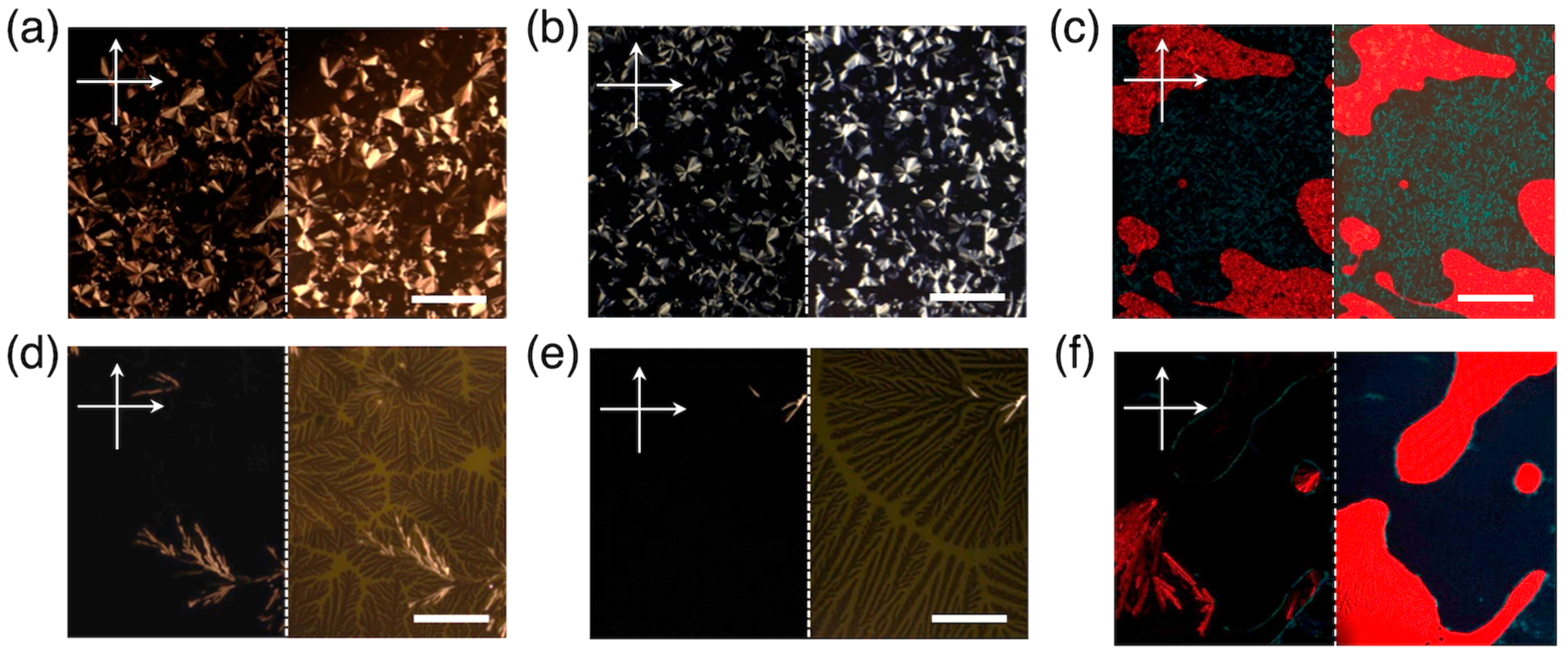
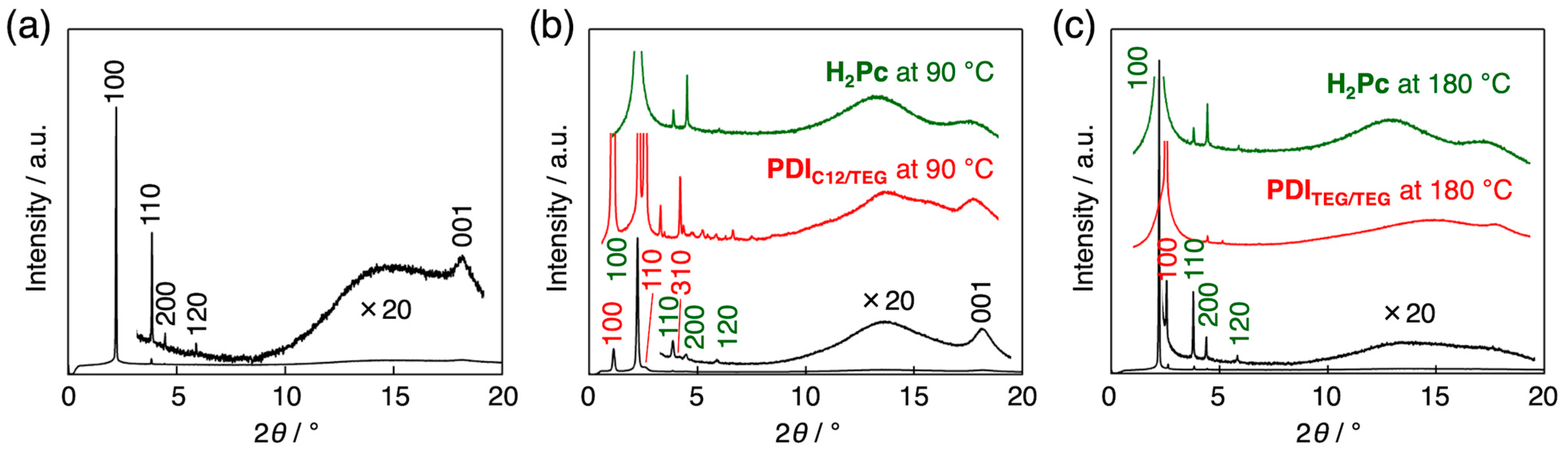
3.2. Orientation, Phase Transition Behavior, and Phase Structure of H2Pc/PDI Mixtures
3.3. Intracolumnar Molecular Order in H2Pc/PDI Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robeson, L.M. Polymer Blends: A Comprehensive Review; Hanser Publishers: Munich, Germany, 2007. [Google Scholar]
- Paul, D.R. Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Barlow, J.W.; Paul, D.R. Polymer blends and alloys—A review of selected considerations. Polym. Sci. Eng. 1981, 21, 985–996. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.; Kim, G.-U.; Lee, W.; Kim, B.J. Recent Advances, Design Guidelines, and Prospects of All-Polymer Solar Cells. Chem. Rev. 2019, 119, 8028–8086. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, Z.; Yang, K.; Niu, L.; Ma, X.; Zhou, Z.; Zhang, X.; Zhang, F. Recent progress in all-small-molecule organic photovoltaics. J. Mater. Chem. A 2022, 10, 6291–6329. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Meng, L.; Han, C.; Wan, X.; Chen, Y. Recent Progress in All-Small-Molecule Organic Solar Cells. Small 2023, 19, e2205594. [Google Scholar] [CrossRef]
- Wu, X.; Tam, T.L.D.; Chen, S.; Salim, T.; Zhao, X.; Zhou, Z.; Lin, M.; Xu, J.; Loo, Y.-L.; Leong, W.L. All-Polymer Bulk-Heterojunction Organic Electrochemical Transistors with Balanced Ionic and Electronic Transport. Adv. Mater. 2022, 34, e2206118. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Huang, P.-Y.; Ramesh, M.; Chang, H.-C.; Chen, L.-M.; Yeh, C.-M.; Fung, C.-L.; Wu, M.-C.; Liu, C.-C.; Kim, C.; et al. Solution-Processed Small-Molecule Bulk Heterojunction Ambipolar Transistors. Adv. Funct. Mater. 2014, 24, 2057–2063. [Google Scholar] [CrossRef]
- Sugiyasu, K.; Kawano, S.; Fujita, N.; Shinkai, S. Self-Sorting Organogels with p-n Heterojunction Points. Chem. Mater. 2008, 20, 2863–2865. [Google Scholar] [CrossRef]
- Molla, M.R.; Das, A.; Ghosh, S. Chiral induction by helical neigbour: Spectroscopic visualization of macroscopic-interaction among self-sorted donor and acceptor π-stacks. Chem. Commun. 2011, 47, 8934–8936. [Google Scholar] [CrossRef] [PubMed]
- Prasanthkumar, S.; Ghosh, S.; Nair, V.C.; Saeki, A.; Seki, S.; Ajayaghosh, A. Organic Donor−Acceptor Assemblies form Coaxial p–n Heterojunctions with High Photoconductivity. Angew. Chem. Int. Ed. 2015, 54, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Lee, J.R.; Wallace, M.; Jäckel, F.; Cowan, A.J.; Adams, D.J. Self-sorted photoconductive xerogels. Chem. Sci. 2016, 7, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Seki, A.; Yoshio, M.; Mori, Y.; Funahashi, M. Ferroelectric Liquid-Crystalline Binary Mixtures Based on Achiral and Chiral Trifluoromethylphenylterthiophenes. ACS Appl. Mater. Interfaces 2020, 12, 53029–53038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nakano, K.; Nakamura, M.; Araoka, F.; Tajima, K.; Miyajima, D. Noncentrosymmetric Columnar Liquid Crystals with the Bulk Photovoltaic Effect for Organic Photodetectors. J. Am. Chem. Soc. 2020, 142, 3326–3330. [Google Scholar] [CrossRef] [PubMed]
- Franca, L.G.; Dos Santos, P.L.; Pander, P.; Cabral, M.G.B.; Cristiano, R.; Cazati, T.; Monkman, A.P.; Bock, H.; Eccher, J. Delayed Fluorescence by Triplet-Triplet Annihilation from Columnar Liquid Crystal Films. ACS Appl. Electron. Mater. 2022, 4, 3486–3494. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Chen, X.; Fan, Y.; Huang, J.; Yu, H.; Yang, S.; Chen, E.-Q. Precisely Controllable Artificial Muscle with Continuous Morphing based on “Breathing” of Supramolecular Columns. Adv. Mater. 2023, 35, e2211648. [Google Scholar] [CrossRef]
- Nunes da Silva, F.; Marchi Luciano, H.; Stadtlober, C.H.; Farias, G.; Durola, F.; Eccher, J.; Bechtold, I.H.; Bock, H.; Gallardo, H.; Vieira, A.A. Columnar Liquid Crystalline Glasses by Combining Configurational Flexibility with Moderate Deviation from Planarity: Extended Triaryltriazines. Chem. Eur. J. 2023, 29, e202203604. [Google Scholar] [CrossRef]
- Takahashi, H.; Kohri, M.; Kishikawa, K. Axially Polar-Ferroelectric Columnar Liquid Crystalline System That Maintains Polarization upon Switching to the Crystalline Phase: Implications for Maintaining Long-Term Polarization Information. ACS Appl. Nano Mater. 2023, 6, 10531–10538. [Google Scholar] [CrossRef]
- Delage-Laurin, L.; Swager, T.M. Liquid Crystalline Magneto-Optically Active Peralkylated Azacoronene. JACS Au 2023, 3, 1965–1974. [Google Scholar] [CrossRef]
- Zucchi, G.; Donnio, B.; Geerts, Y.H. Remarkable miscibility between disk- and lathlike mesogens. Chem. Mater. 2005, 17, 4273–4277. [Google Scholar] [CrossRef]
- Zucchi, G.; Viville, P.; Donnio, B.; Vlad, A.; Melinte, S.; Mondeshki, M.; Graf, R.; Spiess, H.W.; Geerts, Y.H.; Lazzaroni, R. Miscibility between Differently Shaped Mesogens: Structural and Morphological Study of a Phthalocyanine-Perylene Binary System. J. Phys. Chem. B 2009, 113, 5448–5457. [Google Scholar] [CrossRef]
- Sakurai, T.; Yoneda, S.; Sakaguchi, S.; Kato, K.; Takata, M.; Seki, S. Donor/Acceptor Segregated π-Stacking Arrays by Use of Shish-Kebab-Type Polymeric Backbones: Highly Conductive Discotic Blends of Phthalocyaninatopolysiloxanes and Perylenediimides. Macromolecules 2017, 50, 9265–9275. [Google Scholar] [CrossRef]
- Thiebaut, O.; Bock, H.; Grelet, E. Face-on Oriented Bilayer of Two Discotic Columnar Liquid Crystals for Organic Donor-Acceptor Heterojunction. J. Am. Chem. Soc. 2010, 132, 6886–6887. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Shi, K.; Sato, H.; Tashiro, K.; Osuka, A.; Saeki, A.; Seki, S.; Tagawa, S.; Sasaki, S.; Masunaga, H.; et al. Prominent Electron Transport Property Observed for Triply Fused Metalloporphyrin Dimer: Directed Columnar Liquid Crystalline Assembly by Amphiphilic Molecular Design. J. Am. Chem. Soc. 2008, 130, 13812–13813. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Jahr, M.; Gutmann, J. Star-shaped oligobenzoates with a naphthalenechromophore as potential semiconducting liquid crystal materials? J. Mater. Chem. 2008, 14, 2995–3003. [Google Scholar] [CrossRef]
- Sakurai, T.; Tsutsui, Y.; Choi, W.; Seki, S. Intrinsic Charge Carrier Mobilities at Insulator–Semiconductor Interfaces Probed by Microwave-based Techniques: Studies with Liquid Crystalline Organic Semiconductors. Chem. Lett. 2015, 44, 1401–1403. [Google Scholar] [CrossRef]
- Sakurai, T.; Tsutsui, Y.; Kato, K.; Takata, M.; Seki, S. Preferential formation of columnar mesophases via peripheral modification of discotic π-systems with immiscible side chain pairs. J. Mater. Chem. C 2016, 4, 1490–1496. [Google Scholar] [CrossRef]
- Funahashi, M. Anisotropic electrical conductivity of n-doped thin films of polymerizable liquid-crystalline perylene bisimide bearing a triethylene oxide chain and cyclotetrasiloxane rings. Mater. Chem. Front. 2017, 1, 1137–1146. [Google Scholar] [CrossRef]
- Tant, J.; Geerts, Y.H.; Lehmann, M.; De Cupere, V.; Zucchi, G.; Laursen, B.W.; Bjornholm, T.; Lemaur, V.; Marcq, V.; Burquel, A.; et al. Liquid Crystalline Metal-Free Phthalocyanines Designed for Charge and Exciton Transport. J. Phy. Chem. B 2005, 109, 20315–20323. [Google Scholar] [CrossRef]
- Kato, K.; Tanaka, Y.; Yamauchi, M.; Ohara, K.; Hatsui, T. A Statistical approach to correct X-ray response non-uniformity in microstrip detectors for high-accuracy and high-resolution total-scattering measurements. J. Synchrotron Radiat. 2019, 26, 762–773. [Google Scholar] [CrossRef]
- Hatsusaka, K.; Ohta, K.; Yamamoto, I.; Shirai, H. Discotic liquid crystals of transition metal complexes, Part 30: Spontaneous uniform homeotropic alignment of octakis(dialkoxyphenoxy)phthalocyaninatocopper(II) complexes. J. Mater. Chem. 2001, 11, 423–433. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Zhang, Y.; Zhao, H.; Zhang, C.; Kong, X.; Mu, L.; Liang, C. The driving force for homeotropic alignment of a triphenylene derivative in a hexagonal columnar mesophase on single substrates. Thin Solid Films 2010, 518, 1973–1979. [Google Scholar] [CrossRef]
- Osawa, T.; Kajitani, T.; Hashizume, D.; Ohsumi, H.; Sasaki, S.; Takata, M.; Koizumi, Y.; Saeki, A.; Seki, S.; Fukushima, T.; et al. Wide-Range 2D Lattice Correlation Unveiled for Columnarly Assembled Triphenylene Hexacarboxylic Esters. Angew. Chem. Int. Ed. 2012, 51, 7990–7993. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Muranaka, A.; Kato, K.; Saeki, A.; Tanaka, T.; Uchiyama, M.; Osuka, A.; Aida, T.; Sakurai, T. A structural parameter to link molecular geometry to macroscopic orientation in discotic liquid crystals: Study of metalloporphyrin tapes. Chem. Commun. 2021, 57, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Schweicher, G.; Gbabode, G.; Quist, F.; Debever, O.; Dumont, N.; Sergeyev, S.; Geerts, Y.H. Homeotropic and Planar Alignment of Discotic Liquid Crystals: The Role of the Columnar Mesophase. Chem. Mater. 2009, 21, 5867–5874. [Google Scholar] [CrossRef]
- Self, J.L.; Zervoudakis, A.J.; Peng, X.; Lenart, W.R.; Macosko, C.W.; Ellison, C.J. Linear, Graft, and Beyond: Multiblock Copolymers as Next-Generation Compatibilizers. JACS Au 2022, 2, 310–321. [Google Scholar] [CrossRef]
- Grimann, M.; Ueberschaer, R.; Tatarov, E.; Fuhrmann-Lieker, T. Phase Separation and Nanostructure Formation in Binary and Ternary Blends of Spiro-Linked Molecular Glasses. J. Phys. Chem. B 2020, 124, 5507–5516. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, T.; Kato, K.; Shimizu, M. Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems. Crystals 2023, 13, 1473. https://doi.org/10.3390/cryst13101473
Sakurai T, Kato K, Shimizu M. Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems. Crystals. 2023; 13(10):1473. https://doi.org/10.3390/cryst13101473
Chicago/Turabian StyleSakurai, Tsuneaki, Kenichi Kato, and Masaki Shimizu. 2023. "Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems" Crystals 13, no. 10: 1473. https://doi.org/10.3390/cryst13101473
APA StyleSakurai, T., Kato, K., & Shimizu, M. (2023). Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems. Crystals, 13(10), 1473. https://doi.org/10.3390/cryst13101473






