Polypyrrole Film Decorated Manganese Oxide Electrode Materials for High-Efficient Aqueous Zinc Ion Battery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.2. Morphology and Structural Characterization
2.3. Electrochemical Characterization
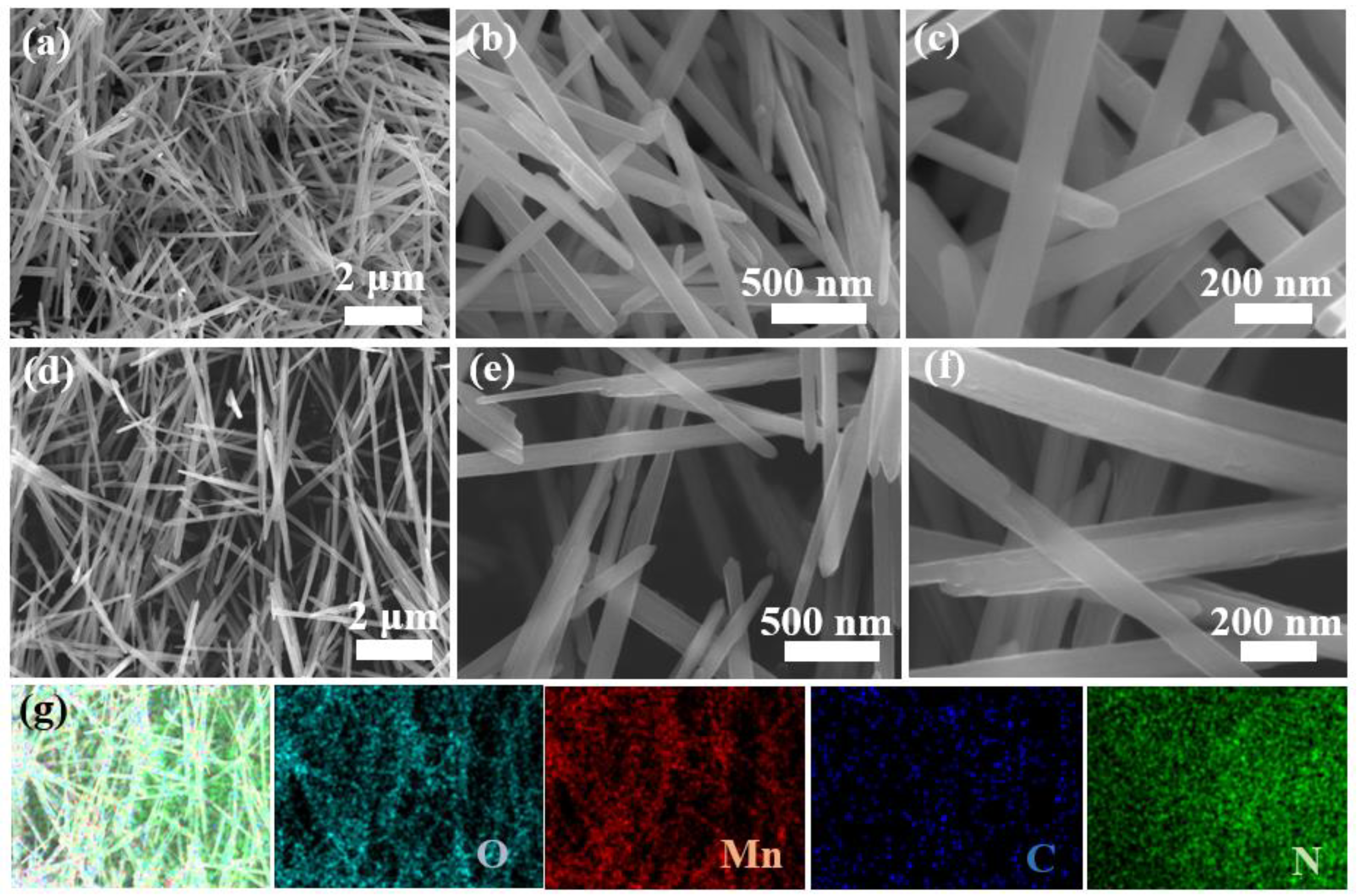
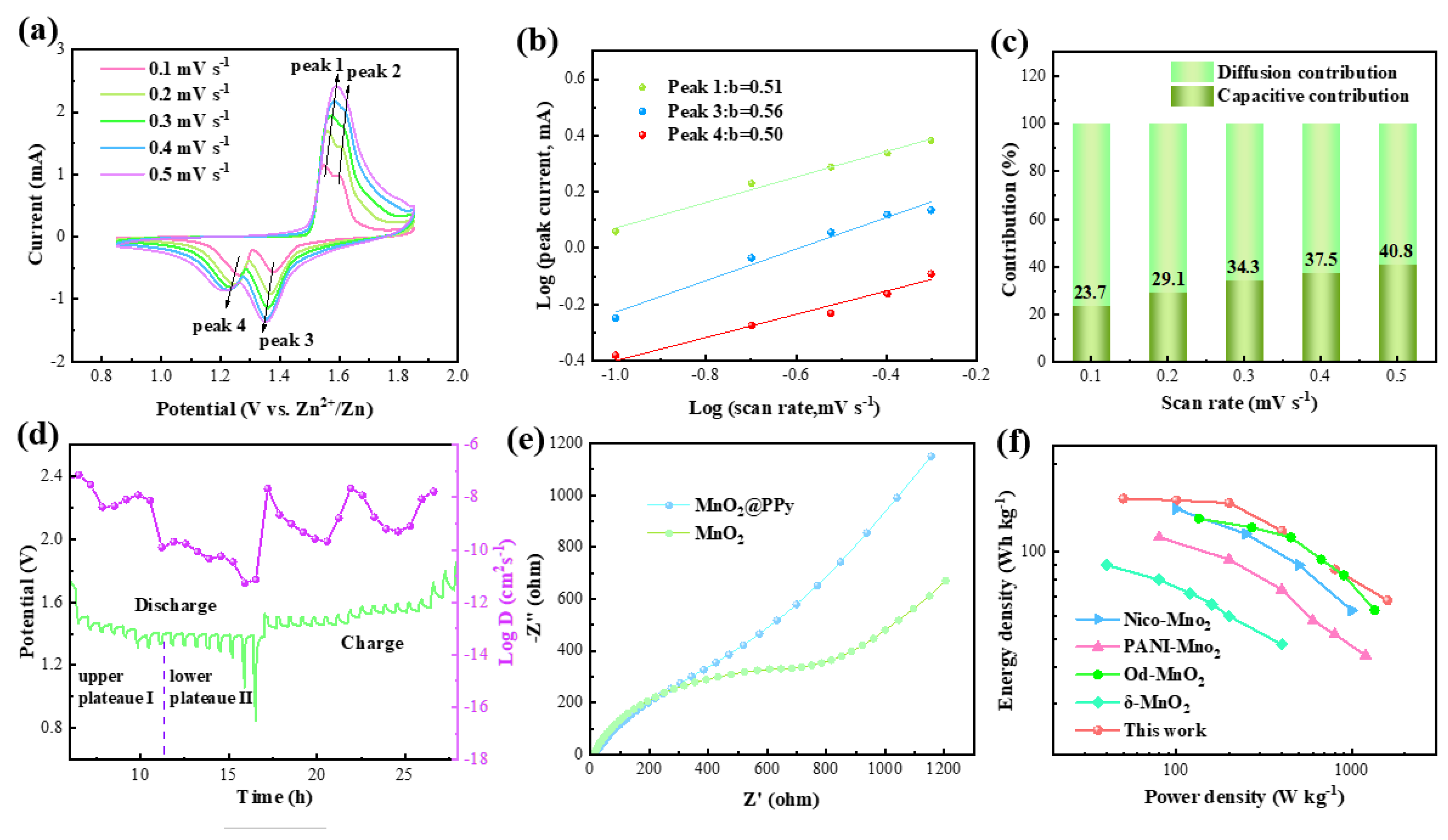
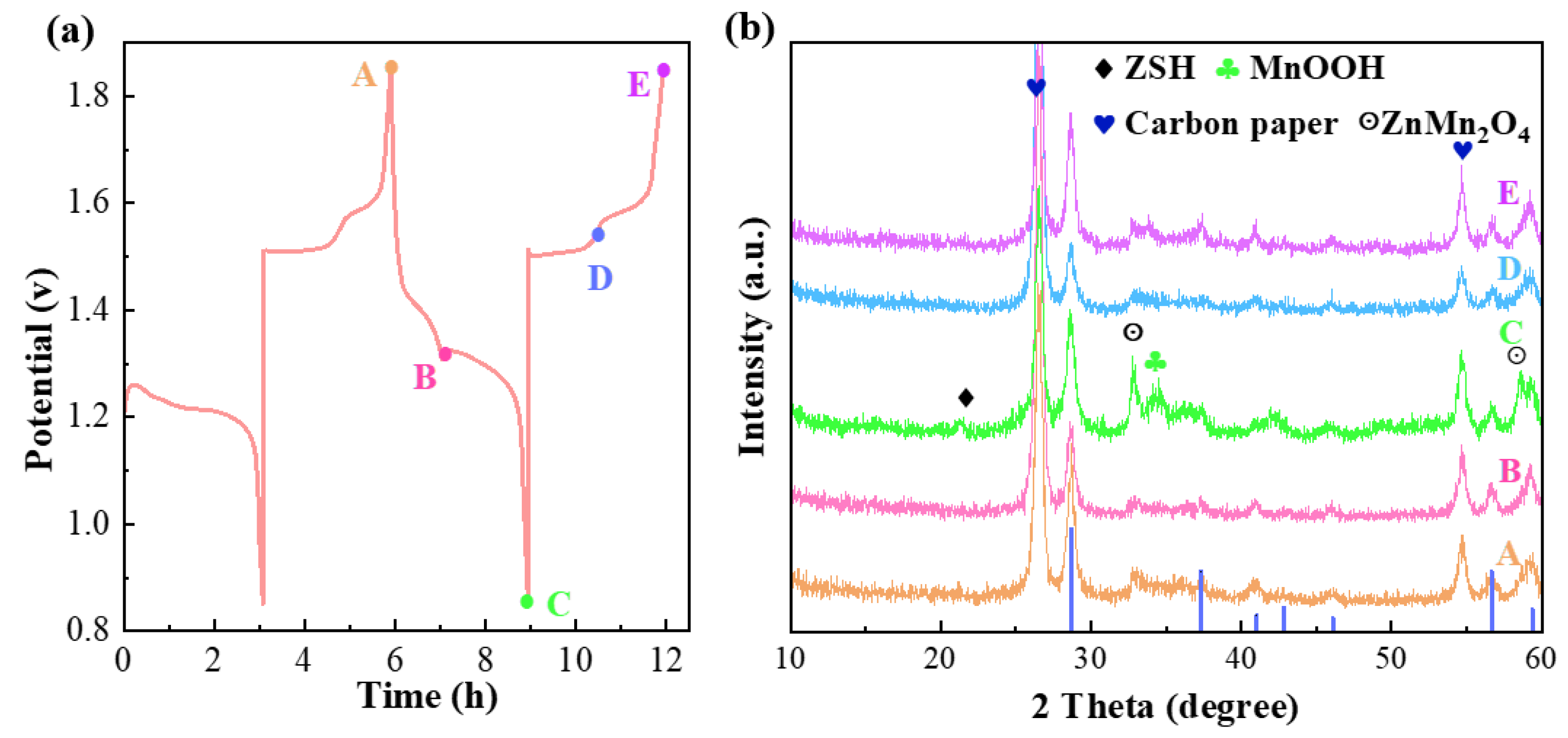
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pang, Z.; Kong, L.; Zhang, H.; Deng, B.; Song, D.; Shi, X.; Ma, Y.; Zhang, L. The optimization of a carbon paper/MnO2 composite current collector for manufacturing a high-performance Li–S battery cathode. Crystals 2022, 12, 1596. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y. General carbon modification avenue to construct highly stable V2O5 electrodes for aqueous zinc-ion batteries. ACS Sustain. Chem. Eng. 2023, 11, 13298–13305. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, K.; Kang, E.; Quan, T.; Sun, T.; Luo, J.; Zhao, S. Simulation investigation on thermal characteristics of thermal battery activation process based on COMSOL. Crystals 2023, 13, 641. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Defect engineering of vanadium-based electrode materials for zinc ion battery. Chin. Chem. Lett. 2023, 34, 107839. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Shen, J.; Liu, F.; Chen, G.; Tao, R.; Ma, S.; Peng, Y.; Lu, Y. Anion-sorbent composite separators for high-rate lithium-ion batteries. Adv. Mater. 2019, 31, 1808338. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. High performance aqueous zinc battery enabled by potassium ion stabilization. J. Colloid Interface Sci. 2022, 628, 33–40. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Zhang, Q.; Xie, C.; Ji, H.; Tang, Y.; Li, Y.; Wang, H. A piece of common cellulose paper but with outstanding functions for advanced aqueous zinc-ion batteries. Mater. Today Energy 2022, 28, 101076. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, R.; Chen, W. Zinc battery goes to anode-free. Nano Res. Energy 2023, 2, e9120053. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Pre-intercalation strategies to layered vanadium-based electrode materials for aqueous zinc batteries. Batt. Supercap. 2023, 6, e202200461. [Google Scholar] [CrossRef]
- Kulkarni, P.; Jung, H. In-situ construction of barium-induced cathode electrolyte interphase to enable mechanostable high-performance zinc-ion batteries. Mater. Today Energy 2023, 32, 101254. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Z.; Lek, D.; Wang, Q. Towards high power density aqueous redox flow batteries. Nano Res. Energy 2023, 2, e9120045. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yamauchi, Y.; Alothman, Z.A.; Kaneti, Y.V.; Wu, X. Enhanced zinc ion storage capability of V2O5 electrode materials with hollow interior cavities. Batter. Supercaps 2021, 4, 1867–1873. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InforMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Z.; Zhang, X.; Bi, S.; Zhou, Z.; Tian, J.; Zhang, Q.; Niu, Z. Non-metal ion Co-insertion chemistry in aqueous Zn/MnO2 batteries. Angew. Chem. Int. Ed. 2021, 60, 7056–7060. [Google Scholar] [CrossRef]
- Huang, Y.; He, W.; Zhang, P.; Lu, X. Nitrogen-doped MnO2 nanorods as cathodes for high-energy Zn-MnO2 batteries. Funct. Mater. Lett. 2018, 11, 1840006. [Google Scholar] [CrossRef]
- Han, M.; Huang, J.; Liang, S.; Shan, L.; Xie, X.; Yi, Z.; Wang, Y.; Guo, S.; Zhou, J. Oxygen defects in β-MnO2 enabling high-performance rechargeable aqueous zinc/manganese dioxide battery. iScience 2020, 23, 100797. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, M.; Qin, R.; Fang, J.; Ren, H.; Yi, H.; Liu, L.; Zhao, W.; Li, Y.; Yao, L.; et al. Oxygen-deficient β-MnO2@graphene oxide cathode for high-rate and long-life aqueous zinc ion batteries. Nano-Micro Lett. 2021, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, Y.; Chen, W.; Yan, Y.; Xue, L.; Zhang, W. CoP3@PPy microcubes as anode for lithium-ion batteries with improved cycling and rate performance. Chem. Eng. J. 2018, 347, 455–461. [Google Scholar] [CrossRef]
- Lia, Z.; Huang, Y.; Zhang, J.; Jin, S.; Zhang, S.; Zhou, H. One-step synthesis of MnOx/PPy nanocomposite as a highperformance cathode for rechargeable zinc-ion battery and insight into its energy storage mechanism. Nanoscale 2020, 12, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, G.; Wu, J.; Liu, L.; Lin, J.; Wei, Y. Asymmetric supercapacitor based on graphene oxide/polypyrrole composite and activated carbon electrodes. Electrochim. Acta 2014, 137, 26–33. [Google Scholar] [CrossRef]
- Li, J.; Cui, L.; Zhang, X. Preparation and electrochemistry of one-dimensional nanostructured MnO2/PPy composite for electrochemical capacitor. Appl. Surf. Sci. 2010, 256, 4339–4343. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Fenta, F.W.; Olbasa, B.W.; Tsai, M.C.; Temesgen, N.T.; Huang, W.H.; Tekaligne, T.M.; Nikodimos, Y.; Wu, S.H.; Su, W.N.; Dai, H.; et al. Structural engineering of α-MnO2 cathode by Ag+ incorporation for high capacity aqueous zinc-ion batteries. J. Power Sources 2022, 548, 232010. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Qin, X.; Wang, Y.; You, Z.; Liu, H.; Shao, M. Superfine MnO2 nanowires with rich defects toward boosted zinc ion storage performance. ACS Appl. Mater. Interfaces 2020, 12, 34949–34958. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wu, C.; Hou, J.; Zhang, Z.; Yan, J.; Wang, G.; Li, Z.; Che, H.; Xing, Z.; Zhang, X. Construction of hollow mesoporous PPy microsphere nanostructures coated with MnO2 nanosheet as high-performance electrodes for supercapacitors. J. Electroanal. Chem. 2023, 928, 117074. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, J.; Yang, X.; Zhang, Y.; Shang, Y.; Hu, X. Decoration NiCo2S4 nanoflakes onto PPy nanotubes as core-shell heterostructure material for high-performance asymmetric supercapacitor. Chem. Eng. J. 2018, 333, 111–121. [Google Scholar] [CrossRef]
- Qi, Y.; Liao, M.; Xie, Y.; Chen, J.; Dong, X.; Wang, Y.; Huang, J.; Xia, Y. Long-life vanadium oxide cathode for zinc battery enabled by polypyrrole intercalation and concentrated electrolyte. Chem. Eng. J. 2023, 470, 143971. [Google Scholar] [CrossRef]
- Hao, J.; Mou, J.; Zhang, J.; Dong, L.; Liu, W.; Xu, C.; Kang, F. Electrochemically induced spinel-layered phase transition of Mn3O4 in high performance neutral aqueous rechargeable zinc battery. Electrochim. Acta 2018, 259, 170–178. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Q.; Liu, H.; Yang, J.; Chen, X.; Yang, L.; Cui, Y.; Huang, W.; Zhao, W.; Song, A.; et al. Tuning phase evolution of β-MnO2 during microwave hydrothermal synthesis for high-performance aqueous Zn ion battery. Nano Energy 2019, 64, 103942. [Google Scholar] [CrossRef]
- Liu, G.; Huang, H.; Bi, R.; Xiao, X.; Ma, T.; Zhang, L. Layered birnessite cathode with a displacement/intercalation mechanism for high-performance aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 20806. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, K.; Chen, S.; Liu, Y.; Tao, Y.; Zhang, X.; Ding, Y.; Dai, S. Proton inserted manganese dioxides as a reversible cathode for aqueous Zn-ion batteries. ACS Appl. Energy Mater. 2020, 3, 319. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, C.; Wu, C.; Dong, L.; Li, J.; Kang, F. Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life. Electrochim. Acta 2017, 229, 422. [Google Scholar] [CrossRef]
- Gao, P.; Ru, Q.; Yan, H.; Cheng, S.; Liu, Y.; Hou, X.; Wei, L.; Ling, F. A durable Na0.56V2O5 nanobelt cathode material assisted by hybrid cationic electrolyte for high-performance aqueous zinc-ion batteries. ChemElectroChem 2020, 7, 283–288. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Wu, L.; Dou, H.; Zhang, X. Microstructural engineering of hydrated vanadium pentoxide for boosted zinc ion thermoelectrochemical cells. J. Mater. Chem. A 2022, 10, 21446–21455. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.; Xia, Y.; Lin, X.; Liu, W.; Ren, M.; Kong, F.; Wang, S.; Lin, C. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef]
- Li, Z.; Chen, D.; An, Y.; Chen, C.; Wu, L.; Chen, Z.; Sun, Y.; Zhang, X. Flexible and anti-freezing quasi-solid-state zinc ion hybrid supercapacitors based on pencil shavings derived porous carbon. Energy Storage Mater. 2020, 28, 307–314. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, R.; Xia, L.; Liu, H.; Gao, Y.; Gan, Z.; Feng, X.; Wang, M.; Meng, G.; Su, Y.; et al. From atomic modification to structure engineering: Layered NiCo–MnO2 with ultrafast kinetics and optimized stress distribution for aqueous zinc ion storage. J. Mater. Chem. A 2023, 11, 11436–11444. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Q.; Cao, Y.; Gao, Y.; Li, A.; Liu, X.; Zhang, X.; Tian, Z.; Liu, R. α-MnO2/super-P with conductive carbon network for rechargeable aqueous zinc ion batteries. Mater. Lett. 2021, 302, 130419. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Zhao, W.; Qin, R.; Ding, S.; Chen, X.; Song, Y.; Yang, L.; Lin, H.; Li, S.; et al. Boosting the energy density of aqueous batteries via facile grotthuss proton transport. Angew. Chem. Int. Ed. 2020, 60, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, M.; Brant, W.R.; Tai, C.W.; Karlsson, G.; Noréus, D. Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater. 2018, 15, 351–360. [Google Scholar] [CrossRef]

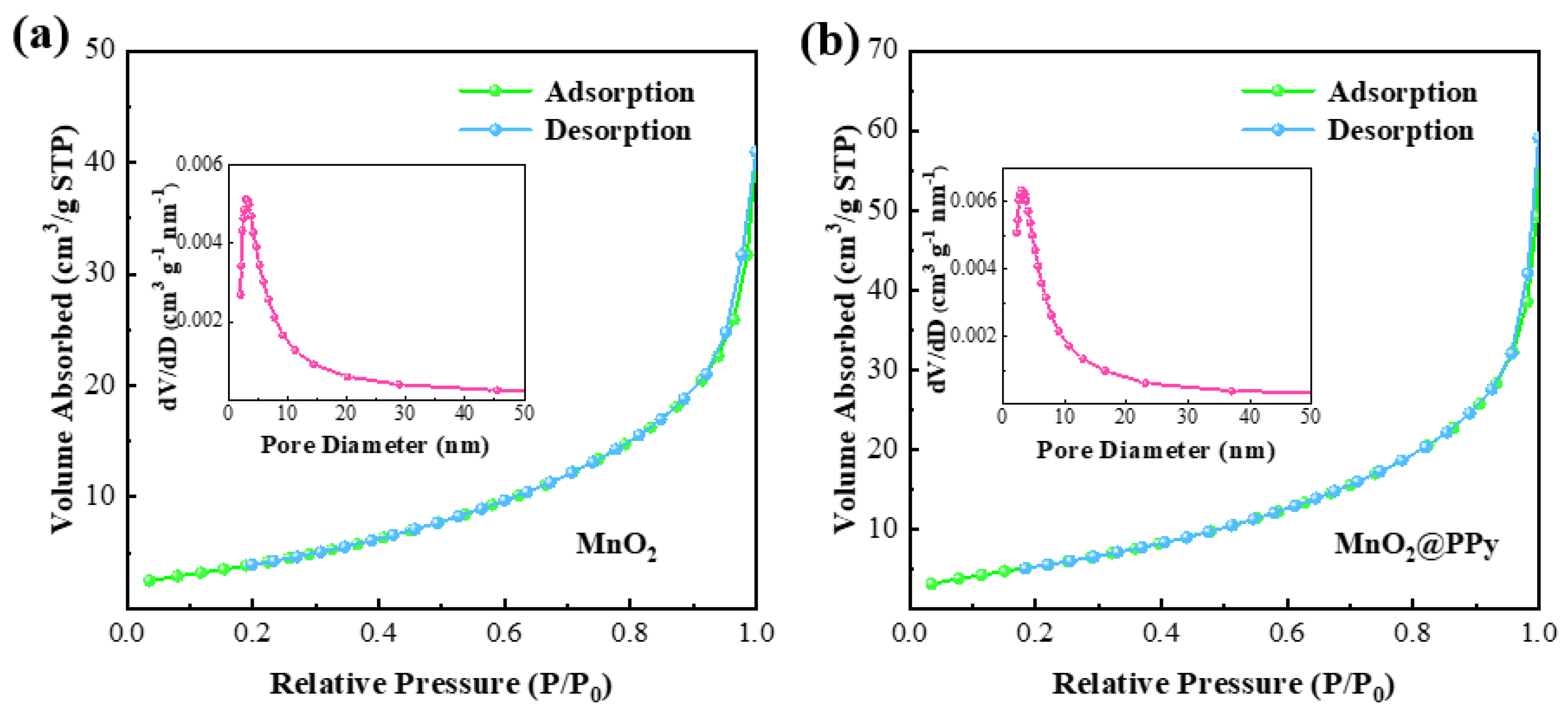

| Samples | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| MnO2 | 18.1 | 0.053 | 11.595 |
| MnO2@PPy | 23.7 | 0.069 | 11.699 |
| Materials | Discharge Capacity (Current Density) | Cyclic Stability (Cycles, Current Density) | Electrolyte | Ref |
|---|---|---|---|---|
| β-MnO2 | 288 mAh g−1 (0.1 C) | 84.3% (1000, 4 C) | 3 M ZnSO4 + 0.2 M MnSO4 | [28] |
| Od-δ-MnO2 | 345 mAh g−1 (0.2 A g−1) | 80% (2000, 5 A g−1) | 1 M ZnSO4 + 0.2 M MnSO4 | [18] |
| (Na, H2O) δ-MnO2 | 389 mAh g−1 (0.2 A g−1) | 70% (700, 0.5 A g−1) | 2 M ZnSO4 + 0.2 M MnSO4 | [29] |
| HxMn2O4 | 281 mAh g−1 (0.1 A g−1) | 93% (1000, 1 A g−1) | 2 M ZnSO4 + 0.1 M MnSO4 | [30] |
| Mn2O3 | 148 mAh g−1 (0.1 A g−1) | 91% (1000, 2 A g−1) | 2 M ZnSO4 + 0.1 M MnSO4 | [31] |
| β-MnO2@PPy | 386 mAh g−1 (0.1 A g−1) | 96% (1000, 1 A g−1) | 2 M ZnSO4 + 0.2 M MnSO4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, Y.; Wu, X. Polypyrrole Film Decorated Manganese Oxide Electrode Materials for High-Efficient Aqueous Zinc Ion Battery. Crystals 2023, 13, 1445. https://doi.org/10.3390/cryst13101445
Liu Y, Zhang Y, Wu X. Polypyrrole Film Decorated Manganese Oxide Electrode Materials for High-Efficient Aqueous Zinc Ion Battery. Crystals. 2023; 13(10):1445. https://doi.org/10.3390/cryst13101445
Chicago/Turabian StyleLiu, Yi, Yuyin Zhang, and Xiang Wu. 2023. "Polypyrrole Film Decorated Manganese Oxide Electrode Materials for High-Efficient Aqueous Zinc Ion Battery" Crystals 13, no. 10: 1445. https://doi.org/10.3390/cryst13101445
APA StyleLiu, Y., Zhang, Y., & Wu, X. (2023). Polypyrrole Film Decorated Manganese Oxide Electrode Materials for High-Efficient Aqueous Zinc Ion Battery. Crystals, 13(10), 1445. https://doi.org/10.3390/cryst13101445







