Abstract
Magnesium (Mg)-based implants were extensively developed and tested to improve the shortages of traditional hard metal implants. Unlike the nail, screw, and plate, pure Mg wire is rarely applied in the musculoskeletal system because of its poor mechanical properties. Therefore, we developed the magnesium–zinc–gadolinium (ZG21) alloy wire, which presented good mechanical properties. Before the in vivo study, the in vitro tests were carried out in this study. The ZG21 wire was scanned by SEM/EDS. The changing rate of weight and pH values were recorded during degradation. The corrosion interface was scanned by SEM/EDS. The cytotoxicity of metal extracts, Mg, Zn, and Gd ions was tested. The osteogenic and angiogenic potential was also evaluated. The ZG21 wire degraded at a stable speed in 14 days. The extracts were diluted ten times, and the correspondent ion concentration presented low cytotoxicity for cell lines of pre-osteoblasts, fibroblasts, and endothelial vessel cells. Pre-osteoblast cell lines cultured with 10% extracts presented significantly higher osteogenic potential. Endothelial vessel cell lines cultured with 2.5, 5, and 10 mM Mg2+ presented significantly higher angiogenic potential. The ZG21 wire could maintain an intact structure when making a surgical knot. Its degradation process and products presented low toxicity and potential for osteogenesis and angiogenesis. The ZG21 wire could be identified as a safe and bioactive material for further in vivo musculoskeletal regeneration.
1. Introduction
Conventional orthopedic implants used to be manufactured with stainless steel, titanium, and cobalt–chromium-based alloys [1]. Although they presented competent biocompatibility and extraordinary wear resistance, the limitations involving shielding effects, implant loosening, infection, and secondary surgery may negatively affect the healing outcomes directly or indirectly [2,3]. To improve these limitations, bioactive materials were applied [4]. Generally, bioactive material can be defined as the machined or fabricated materials needed to release biological factors for enhancing the healing effect [5,6,7,8,9]. As a biodegradable and bioactive material, magnesium-based implants have been investigated in musculoskeletal repair [10,11].
As the first successful orthopedic translation, the MAGNEZIX screw was applied during osteotomy to treat hallux valgus [12]. At the same time, more magnesium-based implants were processed at the preclinical stage. Wang et al. and Cheng et al. developed the magnesium interference screw to enhance bone–tendon junction healing after anterior cruciate ligament reconstruction in rabbits [13,14]. Li et al. applied magnesium rods to accelerate the process of distraction osteogenesis in rats [15]. Zheng et al. developed a titanium–magnesium intramedullary nail in rats to treat the delay union in fracture healing brought by bisphosphonate treatment [16].
In addition to traditional implants such as screws, nails, and plates, the metal wire is widely used in fracture fixation [17], spine fusion [18], and soft tissue repair or reconstruction [19]. However, a limited number of studies have applied magnesium-based wire in the musculoskeletal system because magnesium wire is too brittle to maintain durable mechanical strength during or after implantation [20]. Miao et al. developed the magnesium–zinc–gadolinium (ZG21) alloy to improve the mechanical strength of the wire [21,22]. Therefore, the addition of the Gd means the ZG21 wire could fulfill the requirements for suturing, bunching, and making knots during surgery (Video 1).
Except for the biological effects reported by animal studies, in vitro tests are also involved in evaluating the biological safety [23], osteogenic [24], and angiogenic [15] potential of applied materials. To design an in vivo study efficiently, completing an in vitro test as a pilot study to provide the bioactive potential and safety data is meaningful [25,26]. In this study, in vitro tests were performed, including micromorphology, degradation, cytotoxicity, angiogenesis, and osteogenesis, as a pilot study for the in vivo evaluation. We assumed the ZG21 wire is sufficiently biocompatible and bioactive as a material for musculoskeletal repair.
2. Results
2.1. The Results of SEM/EDS
The diameter of the wire was 15 μm. The S/D value was missing since the precision of the micrometer screw was 1 μm (Figure 1A). The surface of the wire was amplified 200 times and 3000 times (Figure 1B). The EDS map scanning also observed the distribution of Mg, Zn, and Gd (Figure 1C).
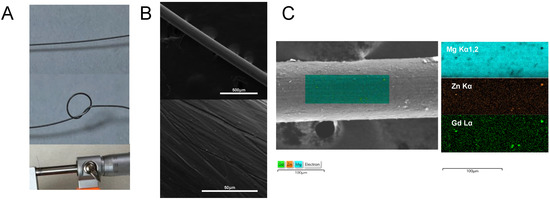
Figure 1.
SEM/EDS of ZG21 alloy wire. (A) The gross look of the wire and the diameter measurement of the wire; (B) SEM image amplified by 200 times and 3000 times; (C) element distribution detected by the EDS map scanning.
2.2. Degradation
For the wire immersed in Hank’s solution, the weight of ZG21 slightly decreased after 72 h. After 144 h, about 60% of the original weight was maintained. After 336 h, only around 25% of the original weight was left (Figure 2B). The initial pH value was 7.4. During the first six hours, the pH value was elevated to 10.2 ± 0.1. The pH value then increased slowly to 10.5 ± 0.1 at 48 h post-degradation and remained stable until the last testing time point (Figure 2C). For the wire immersed in MEM α with 10% FBS, after three days, the pH values of 100%, 50%, and 10% metal extracts were 8.7 ± 0.23, 7.79 ± 0.16, and 7.6 ± 0.05, respectively. The average concentration of Mg, Zn, and Gd was 25.1 ± 2.1 mM, 0.08 ± 0.02 mM, and 0.0007 ± 0.0002 mM, respectively. The major elements of the corrosion interface detected by SEM/EDS were magnesium, oxygen, and phosphate (Figure 2D), which makes it highly possible that it was Mg3(PO4)2 according to the chemical reaction formula (Figure 2A).
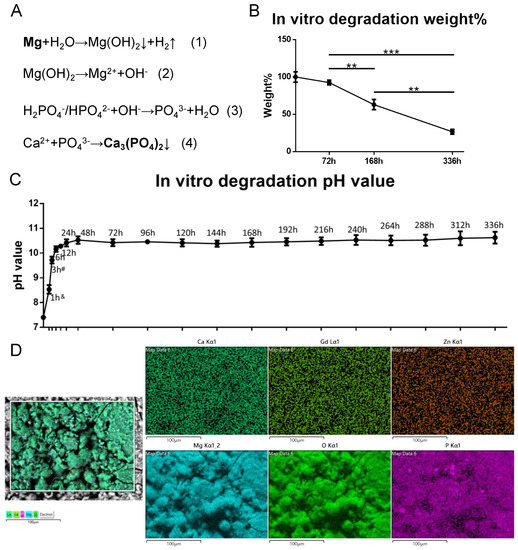
Figure 2.
The in vitro degradation. (A) Chemical reaction during degradation process; (B) weight changing curve during 336 h immersion, n = 3, one-way AONVA, Tukey test, ** p < 0.01, *** p < 0.001; (C). pH value changing curve during 336 h immersion, &: significantly different in each group, #: significantly different for 1 h, 6 h to 120 h, n = 3, one-way AONVA, Tukey test, p < 0.05; (D). SEM/EDS detection on the corrosion interface (72 h immersion).
2.3. Cytotoxicity
As shown in Figure 3A–C, for the MC3T3−E1, L929, and MS−1 cell lines, the 10-times-diluted alloy extracts presented close to 100% cell viability on days 1, 3, and 5. The 2-times-diluted alloy extracts showed around a 90% survival rate on day 1, which dropped around 75% on days 3 and 5 (p < 0.001). The 100% alloy extracts also showed good biocompatibility on day 1, while the viability collapsed under 50% on days 3 and 5 (p < 0.001). Similar to the 10-times-diluted alloy extracts, 1 mM and 2.5 mM Mg2+ presented good biocompatibility for all time points for all three cell lines. The viability of the 5 mM, 10 mM, and 15 mM Mg2+ group was acceptable on day 1 and dropped under 75% on days 3 and 5 for the MC3T3-E1 and L929 cell lines, while the cell viability was still higher than 75% for the MS−1cell line. For all of the cell lines, the cell survival rate was low for all time points with Mg2+ concentrations of 20 mM and 25 mM, which was close to the result of the 100% alloy extracts (p < 0.001). All the set Zn2+ concentrations from 0.001 mM to 0.1 mM presented low cytotoxicity for the MC3T3-E1 cells. The 0.01 mM and 0.1 mM Zn2+ showed a higher cell viability than the control (p < 0.05). All the Zn2+ concentrations presented high cell proliferation with cell viability higher than the control for all L929 and MS−1 cells at all the time points (p < 0.001). As for Gd3+, the tested concentrations did not significantly suppress the proliferation of MC3T3-E1 and L929 cells. For the MS−1 cell line, the 10−5 mM and 10−4 mM Gd3+ kept the cell viability higher than 75% at all times, while the cell viability dropped under 75% with 10−3 mM Gd ions on days 3 and 5 (p < 0.001).
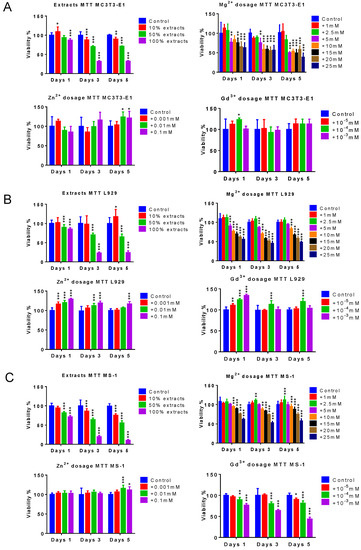
Figure 3.
Cytotoxicity of alloy extracts Mg ions, zinc ions, and gadolinium ions. (A) The cell viability of the pre-osteoblast cell line, MC3T3E−1; (B) the cell viability of the fibroblast cell line L929; (C) the cell viability of the endothelial cell line, MS−1, n = 8, t-test between control and each group, * p < 0.05, ** p < 0.01, *** p < 0.001.
2.4. Osteogenesis
The osteogenic potential of the 10% extracts and 2.5 mM Mg2+ were evaluated based on cytotoxicity results. As shown in Table 1 and Figure 4, on day 14 after induction, the MC3T3−E1 cell line cultured by 10% immersion extracts presented a significantly larger calcium node area than the control group (n = 4, p < 0.001). Interestingly, the group cultured with 2.5 mM Mg2+ and 10mM Mg2+ presented a significantly smaller calcium node area than the control group (n = 4, p < 0.05, and p < 0.01).

Table 1.
Alizarin Red staining positive area.
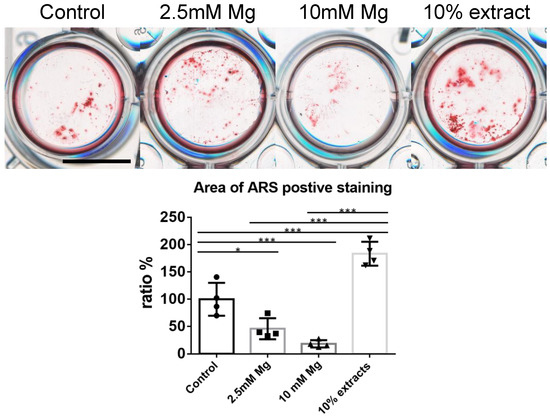
Figure 4.
In vitro osteogenesis induction cultured with control medium, 2.5 mM Mg2+, 10 mM Mg2+, 10% of alloy extracts, scale bar: 10 mm, n = 4, one-way ANOVA, Tukey test, * p < 0.05, *** p < 0.001.
2.5. Angiogenesis
According to cytotoxicity results, the angiogenic potential of 2.5 mM, 5 mM, and 10 mM Mg2+ was evaluated. As for the tube branching length, the control group had significantly lower values than the groups cultured with 2.5 mM, 5 mM, and 10 mM Mg2+ (n = 4, p < 0.05, p < 0.01, p < 0.001, respectively) (Figure 5 and Table 2). As for the total junction number, the control group also had significantly lower values than groups cultured with 2.5 mM, 5 mM, and 10 mM Mg2+ (n = 4, p < 0.01, p < 0.001, p < 0.001, respectively). In addition, the group cultured with 2.5 mM Mg2+ presented a significantly lower total junction number than the group cultured with 10 mM Mg2+ (n = 4, p < 0.05) (Figure 5 and Table 2).
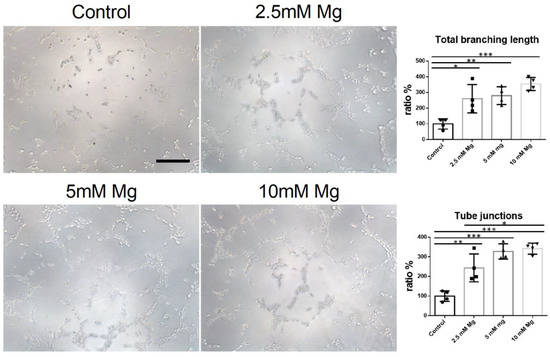
Figure 5.
In vitro angiogenesis induction cultured with control medium, 2.5 mM Mg2+, 5 mM Mg2+, 10 mM Mg2+, scale bar: 10 um, n = 4, one-way AONVA, Tukey test, * p < 0.05, ** p < 0.01, *** p < 0.001.

Table 2.
Tube formation.
3. Discussion
The high-purity Mg presented a similar mechanical strength to screws [27], while as for surgical wire, the unbalanced compression loading and tensile loading made the wire break easily when twisting it into knots [28]. Using the cold drawing technique, Miao et al. investigated different element proportions and sorted out the optimal alloy, Mg97Zn2Gd0.84. This alloy could narrow the ultimate loading of the tensile/compression ratio from 3:1 to 2:1 [21,22]. As the transition element, Zn is mostly used in three-dimensional Mg alloys. According to the published study, the weight of Zn should be kept under 2% or it could accelerate Mg alloy degradation [29,30]. The Mg-Gd alloy presented higher compression and tensile loading, but the high proportion of Gd might be potentially harmful after degradation [31]. Although some rare earth Mg alloy wires such as Mg-Zn-Y-Nd had been developed [32], incorporating Gd into the Mg-Zn alloy was a relatively novel attempt that could significantly decrease the Gd proportion and establish excellent mechanical prosperities.
In the medical translation process, in vitro studies are meaningful in sorting dangerous and bioactive dosages to guide further animal studies. In this study, the cell lines of pre-osteoblast, fibroblast, and vascular endothelial cells were tested since these cells play important roles in tissue regeneration. According to the standard of ISO10993, a cell viability of over 75% indicates a low cytotoxic level [33,34]. The extracts without dilution presented relatively low cell viability for all tested cell lines, especially on days 3 and 5. This result could be induced by a high concentration of Mg ions, hyperosmolality, and an alkaline environment. However, unlike in vivo implantation, the cell culture medium could not be refreshed dynamically. Fischer et al. recommended diluting extracts ten times to suppress the osmotic pressure [35]. Wang et al. further standardized the testing protocol, which has been cited by the FDA [23]. After dilution, the metal extracts showed very low cytotoxicity for pre-osteoblasts, fibroblasts, and endothelial cells. As for the cytotoxicity of Mg2+, a high dosage of Mg2+ could interfere with the transmembrane process of other ions, especially Ca2+. Furthermore, over 20 mM Mg2+ elevates the crystal osmotic pressure. Li et al. reported the endothelial cell line could tolerate a higher concentration of Mg2+, which has been confirmed by our result [9]. It may be because of the high transmembrane activity of endothelial cells. According to published studies [36,37], the concentration of Zn and Gd in this study was safe, which is also confirmed by our results. As a rare earth element, Gd might potentially affect local bone metabolism [38]. As the material that comprises the contrast medium in MRI, the dosage of Gd is usually much higher than it was in this study.
After immersing it in the culture medium or implanting it into the body, Mg degradation was triggered. Firstly, Mg reacted with H2O to produce Mg(OH)2 and hydrogen (Figure 2A(1)). Then, Mg(OH)2 was hydrolyzed into Mg2+ and OH− (Figure 2A(2)). The OH− could bond H2PO4−/HPO42− to PO43− (Figure 2A(3)). Eventually, Mg2+ and PO43− combined to form Mg3(PO4)2 (Figure 2A(4)). This process could explain the Mg, P, and O elements on the corrosion interface detected by SEM/EDS (Figure 2D). The implanted Mg could stimulate bone formation through several pathways. Cheng et al. and Wang et al. reported that the Mg interference screw could elevate TGF-beta family expression [14,39]. Zhang et al. investigated the neuro-skeletal pathway in Mg degradation and found that releasing Mg2+ could stimulate the CGRP secretion from the dorsal root ganglion to promote sub-periosteum ossification [40]. As for the in vitro osteogenic effects of Mg2+, the published studies showed some contradicting results. Huang et al. reported that Mg2+ could elevate Wnt/beta-catenin expression to promote bone node formation [41]. Whereas Montes et al. reported that Mg2+ suppressed the same pathway [42]. Zheng et al. also found that Mg2+ delayed osteogenesis with the standard induction assay [24]. Our study found that 2.5 mM and 5 mM Mg2+ presented a lower osteogenic potential than the control group. Interestingly, the cells cultured with 10% extracts produced the most bone nodes among all the groups, which could match the result of SEM/EDS after degradation. Angiogenesis plays a crucial role in bone formation. Cheng et al. reported that Mg degradation promoted local VEGF, which could promote endochondral ossification [14]. Wang et al. reported that Mg degradation could promote local PDGF−BB, which was crucial in bridging angiogenesis and osteogenesis [27,43]. Except for the direct osteogenic effect, Li et al. reported that Mg degradation could trigger the CGRP/VEGF/FAK pathway to promote H-type vessel formation and bone formation in sequence [15]. Furthermore, in this study, we found that Mg2+ could promote tube formation with increasing dosage. In summary, the mixed degradation products could promote bone formation, while the Mg2+ could promote angiogenesis.
There were some limitations in this study. Firstly, we did not perform a mechanical test because the wire always slipped from the fixation kit. Secondly, the bioeffect of Zn and Gd was not evaluated. This is mainly because the dosages of Zn and Gd could not reach the level of biological potential according to the published studies [37,38]. Thirdly, the wire was not scanned by an atomic force microscope to observe the surface topology. This is mainly because the wire was implanted into tissue by catheter guidance or drilling holes. The surface topology did not affect the implanting process. Furthermore, the wire degraded after implantation and the surface topology changed dynamically. Fourthly, no precise quantification data in SEM/EDS were obtained. SEM/EDS is not the optimal method to quantify the low wt.% element in alloys. To collect effective data, the working voltage should be higher than 15 kV. Based on this study’s results, more in vivo studies using the ZG21 wire will be carried out in implantation and trauma repair models.
4. Conclusions
The Mg-Zn-Gd wire was feasible as a surgical suture. Its degradation process was stable, and the degradation products were safe. As a bioactive material, the degradation products could promote osteogenic and angiogenic potential. These findings present a promising medical translation potential in future wound suturing, fracture fixation, soft tissue repair, and spine fusion (Figure 6).
Figure 6.
Clinical translation of Mg-Zn-Gd wire.
5. Materials and Methods
5.1. Materials Preparation
5.1.1. Preparation of ZG21 Wire
High-purity Mg (99.99%), Mg−30 wt.% Gd master alloy (760 °C), and high-purity Zn (99.995%, 700 °C) were melted by a resistance furnace at Shanghai Jiao Tong University. A mixed atmosphere (1 vol% SF6 and 99 vol% CO2) was used to protect the melting process. After molding, the ingots were machined into billets with a 60 mm diameter and height of 60 mm. After two-stage extrusion, 1.5 mm wires were processed for further drawing (330–430 °C). After repeated drawing and annealing, 0.15 mm -diameter wires were finally prepared (Figure 7).
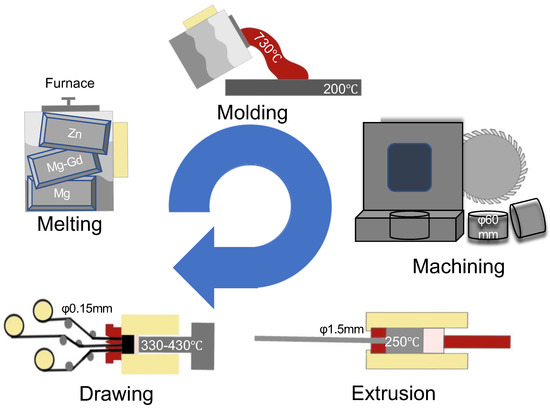
Figure 7.
The preparation of the Mg-Zn-Gd alloy.
5.1.2. Extracts Preparation
According to ISO10993-5, the ZG21 wire was immersed into Modified Eagle’s Medium α (MEM α) (Gibco, Thermo Scientific, Waltham, MA, USA) with 10% Fetal Bovine Serum (FBS) (Gibco, Thermo Scientific, Waltham, MA, USA). The volume of the extraction medium was calculated as 1.25 mL/cm2. Then, the extraction system was incubated at 37 °C, 100% humidity, and 5% CO2 for 72 h. The collected specimens were diluted as 100%, 50%, and 10%. The different concentrations of Mg2+, Zn2+, and Gd3+ were tested.
5.2. Degradation and Cytotoxicity
5.2.1. In Vitro Degradation Protocol
The ZG21 alloy sample was immersed in Hank’s solution (1.25 cm2/mL, 37 ℃, 220 rpm). The immersion period lasted for 14 days without immersion buffer refreshing. The pH value was measured daily for 14 continuous days after immersion. The initial pH value was set to 7.4. At days 3, 7, and 14, the weight (after washing by NH4Cl (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)) was recorded, and the ratio to initial weight was recorded.
5.2.2. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium Bromide (MTT) Tests
The pre-osteoblast cell line MC3T3−E1, fibroblast cell line L929, and endothelial cell line MS−1were cultured with the Modified Eagle’s Medium α (MEM α) (Gibco, Thermo Scientific, Waltham, MA, USA) with 10% Fetal Bovine Serum (FBS) (Gibco, Thermo Scientific, Waltham, MA, USA) and seeded in a 96-well plate with 500 cells with 100 mL of medium in each well. At the test time point (days 1, 3, and 5), 50 mL of MTT solution (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was mixed with each sample and incubated for 4 h. Then, 200 mL of dimethyl sulfoxide (DMSO (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)) was used to refresh the well. After the reaction, the absorbance value was read at the 570 nm spectrum. Group of 10% extracts, 50% extracts, or 100% extracts; 1 mM, 2.5 mM, 5 mM, 10 mM, 12.5 mM, 15 mM, 20 mM, and 25 mM Mg2+; 0.001 mM, 0.01 mM, and 0.1 mM Zn2+; 10−5 mM, 10−4 mM, and 10−3 mM Gd3+ were tested separately.
5.3. Spectroscopy and Microscopic Investigations
5.3.1. Scanning Electron Microscope–Energy-Dispersive X-ray Spectroscopy (SEM/EDS)
The wire was polished and etched with acetic acid and nitric acid solution. After dehydration, the wires were scanned by SEM. After immersion for 72 h, the corrosive interface was scanned by SEM/EDS (S3400N, HITACHI, Japan).
5.3.2. Osteogenic Differentiation Protocol
Around 1000 pre-osteoblast cells (MC3T3-E1) for each well were seeded in a 24-well plate cultured with osteogenic medium (DMEM (Gibco, Thermo Scientific, MA, USA), 10−8 M Dexamethasone (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), 5mM b-glycerophosphate (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), 50 mM, ascorbic acid (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)). Three groups were set: the control group, the Mg ions group (2.5 mM), and the 10% metal extracts group. The induction process was terminated after 21 days of induction. The calcium nodes were stained with Alizarin Red (ARS) (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany).
5.3.3. Angiogenic Tube Formation
Around 2.5 × 104 endothelial cells (MS−1) per dish were seeded into 96-well plates coated with Matrigel (Gibco, Thermo Scientific, Waltham, MA, USA). Tube formation was measured using an inverted phase-contrast microscope at 4 h with and without Mg ions (2.5 mM and 5 mM and 7.5 mM) treatment. The total branch length and junction number were measured by ImageJ software (NIH, USA).
5.4. Statistics
Data were analyzed using SPSS (IBM, NYS, USA) and GraphPad Prism (GraphPad Software, CA, USA). The t-test was used to compare the difference between the control group and each intervention group in the MTT test. The one-way ANOVA was used for multi-group comparison if only one time point was used. The Tukey test was used as post hoc for both one-way ANOVA and the multi-comparison method. A p-value less than 0.05 was considered statistically significant for all tests.
5.5. Ethic Statement
The MC3T3−E1 cell line was obtained from Dr. Xuan He, who provided ATCC, CRL2694. The L929 cell line was obtained from Dr. Haiyue Zu, who provided ATCC, CCL1. The MS−1 cell line was obtained from Dr. Ye Li, who provided ATCC, CRL2460.
Author Contributions
Conceptualization, X.H. and H.Z.; Data curation, J.S.; methodology, X.H. and Y.L.; software, X.H.; validation, X.H., Y.L. and H.Z.; formal analysis, X.H.; investigation, X.H. and Y.L.; resources, H.M.; data curation, X.H.; writing—original draft preparation, X.H.; writing—review and editing, M.T.Y.O.; visualization, Y.L.; supervision W.L.; project administration, W.L.; funding acquisition X.H., M.T.Y.O. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the theme-based research scheme of Hong Kong Research Grant Council (RGC Ref: T13-402/17-N).
Acknowledgments
We especially appreciate the support from Guangyin Yuan, Ling Qin, Patrick Shu-hang Yung, Xiaowei Zhang, Han Li, and Hua Duan.
Conflicts of Interest
We declare that we have no conflicts of interest in the authorship or publication of this contribution.
References
- Manam, N.S.; Harun, W.; Shri, D.; Ghani, S.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Reith, G.; Schmitz-Greven, V.; Hensel, K.O.; Schneider, M.M.; Tinschmann, T.; Bouillon, B.; Probst, C. Metal implant removal: Benefits and drawbacks—A patient survey. BMC Surg. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Pavlicek, W.P.; Peter, M.B.; Spangehl, M.J.; Roberts, C.C.; Paden, R.G. Metal artifact reduction image reconstruction algorithm for CT of implanted metal orthopedic devices: A work in progress. Skelet. Radiol. 2009, 38, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research status of biodegradable metals designed for oral and maxillofacial applications: A review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Asthana, N.; Aljabali, A.A.; Bhardwaj, S.K.; Kralj, S.; Penkova, A.; Thomas, S.; Zaheer, T.; Gomes de Souza, F. A critical review on multifunctional smart materials ‘nanographene’ emerging avenue: Nano-imaging and biosensor applications. Crit. Rev. Solid State Mater. Sci. 2021, 47, 691–707. [Google Scholar] [CrossRef]
- Pal, K. Bio-Manufactured Nanomaterials; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Guo, Y.; Wang, M.; Ge, J.; Niu, W.; Chen, M.; Cheng, W.; Lei, B. Bioactive biodegradable polycitrate nanoclusters enhances the myoblast differentiation and in vivo skeletal muscle regeneration via p38 MAPK signaling pathway. Bioact. Mater. 2020, 5, 486–495. [Google Scholar] [CrossRef]
- Cai, Y.A.; Zhu, J.Y.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Santhosh, K.S.; Sarojini, S.; Umesh, M. Anti-Biofilm Activities of Nanocomposites: Current Scopes and Limitations. In Bio-Manufactured Nanomaterials; Springer: Cham, Switzerland, 2021; pp. 83–94. [Google Scholar]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Transl. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. BioMedical Eng. OnLine 2013, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, Y.; Li, H.; Liu, Y.; Bai, X.; Chau, W.; Zheng, Y.; Qin, L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials 2018, 157, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Han, P.; Zhao, C.; Zhang, S.; Wu, H.; Ni, J.; Hou, P.; Zhang, Y.; Liu, J.; Xu, H. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials 2016, 81, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Mi, J.; He, X.; Pan, Q.; Zheng, L.; Zu, H.; Chen, Z.; Dai, B.; Li, X. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials 2021, 275, 120984. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Xu, J.; Ye, C.R.; Chang, L.; Wang, X.; Yao, H.; Wang, J.; Zhang, R.; Xue, Q.; Tang, N. Magnesium facilitates the healing of atypical femoral fractures: A single-cell transcriptomic study. Mater. Today 2022, 52, 43–62. [Google Scholar] [CrossRef]
- Berend, K.R.; Lombardi, A.V. Intraoperative femur fracture is associated with stem and instrument design in primary total hip arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 2377–2381. [Google Scholar] [CrossRef]
- Bogie, R.; Arts, J.J.; Koole, S.N.; Rhijn, L.W.V.; Willems, P.C. The Use of Metal Sublaminar Wires in Modern Growth-Guidance Scoliosis Surgery: A Report of 4 Cases and Literature Review. Int. J. Spine Surg. 2020, 14, 182–188. [Google Scholar] [CrossRef]
- Zumstein, M.; Jacob, H.A.; Schneeberger, A.G. In vitro comparison of standard and knotless metal suture anchors. Arthrosc. J. Arthrosc. Relat. Surg. 2004, 20, 517–520. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Miao, H.; Huang, H.; Fan, S.; Tan, J.; Wang, Z.; Ding, W.; Yuan, G. Nanoscale precipitations in deformed dilute alloying Mg-Zn-Gd alloy. Mater. Des. 2020, 196, 109122. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, D.; Chen, C.; Zhang, L.; Pei, J.; Su, Y.; Huang, H.; Wang, Z.; Kang, B.; Ding, W. Research on Biodegradable Mg–Zn–Gd Alloys for Potential Orthopedic Implants: In Vitro and in Vivo Evaluations. ACS Biomater. Sci. Eng. 2019, 5, 1623–1634. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-Z.; Wang, J.-L.; Xu, J.-K.; Zhang, X.-T.; Liu, B.-Y.; Huang, L.; Zhang, R.; Zu, H.-Y.; He, X.; Mi, J. Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model. Biomaterials 2020, 238, 119828. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Guo, Y.; Xue, Y.; Wang, M.; Chen, M.; Winston, D.D.; Cheng, W.; Lei, B. Biodegradable multifunctional bioactive Eu-Gd-Si-Ca glass nanoplatform for integrative imaging-targeted tumor therapy-recurrence inhibition-tissue repair. Nano Today 2021, 38, 101137. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Klausen, H.L.; Yan, N.; Liu, J.; Chen, F.; Song, W.; Dong, M.; Zhang, Y. Growing vertical aligned mesoporous silica thin film on nanoporous substrate for enhanced degradation, drug delivery and bioactivity. Bioact. Mater. 2021, 6, 1452–1463. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Song, B.; Chow, D.H.; Yung, P.S.-H.; Qin, L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017, 63, 393–410. [Google Scholar] [CrossRef]
- Seelig, M. A study of magnesium wire as an absorbable suture and ligature material. Arch. Surg. 1924, 8, 669–680. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Xue, Y.; Wang, Z.; Yang, L. Biocorrosion behavior and cytotoxicity of a Mg–Gd–Zn–Zr alloy with long period stacking ordered structure. Mater. Lett. 2012, 86, 42–45. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Yin, W.; Lin, G. Mechanical properties, degradation performance and cytotoxicity of Mg–Zn–Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Li, R.G.; Li, H.R.; Pan, H.C.; Xie, D.S.; Zhang, H. Achieving exceptionally high strength in binary Mg-13Gd alloy by strong texture and substantial precipitates. Scr. Mater. 2021, 193, 142–146. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, S.; Wang, L.; Liu, Q.; Guan, S. The microstructure and properties of cyclic extrusion compression treated Mg-Zn-Y-Nd alloy for vascular stent application. J. Mech. Behav. Biomed. Mater. 2012, 8, 1–7. [Google Scholar] [CrossRef]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. Med. Device Diagn. Ind. 1998, 20, 96–98. [Google Scholar]
- ISO 10993-12; 2012 Biological Evaluation of Medical Devices—Part 12: Sample preparation and reference materials. International Standard Organization: Geneva, Switzerland, 2012.
- Fischer, J.; Pröfrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng. B 2011, 176, 1773–1777. [Google Scholar] [CrossRef]
- Nelson, K.L.; Gifford, L.M.; Lauber-Huber, C.; Gross, C.A.; Lasser, T.A. Clinical safety of gadopentetate dimeglumine. Radiology 1995, 196, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Murata, N.; Murata, K.; Gonzalez-Cuyar, L.F.; Maravilla, K.R. Gadolinium tissue deposition in brain and bone. Magn. Reson. Imaging 2016, 34, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Fu, W.; Cheng, W.; Chan, K.; Yung, P.S.-H.; Qin, L. Biodegradable magnesium screws accelerate fibrous tissue mineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit. Sci. Rep. 2017, 7, 40369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Hung, C.-C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef]
- de Oca, M.; Guerrero, F.; Martinez-Moreno, J.M.; Madueno, J.A.; Herencia, C.; Peralta, A.; Almaden, Y.; Lopez, I.; EAguilera-Tejero, K. Gundlach, Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS ONE 2014, 9, e89525. [Google Scholar]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).