Synthesis and Structures of the Capped and Sandwiched Cobalt Heptamolybdate Polyoxometalate (NH4)6{[Co(H2O)5][Mo7O24][Co(H2O)2][Mo7O24][Co(H2O)5]} · 6 H2O and Its Extended Structure (NH4)6{[Co(H2O)2]3[Mo7O24]2}
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Crystallization
2.2. Crystallographic Studies
2.3. UV–Vis–NIR Spectroscopy
2.4. Scanning Electron Microscopy and Energy Dispersive X-ray
3. Results and Discussion
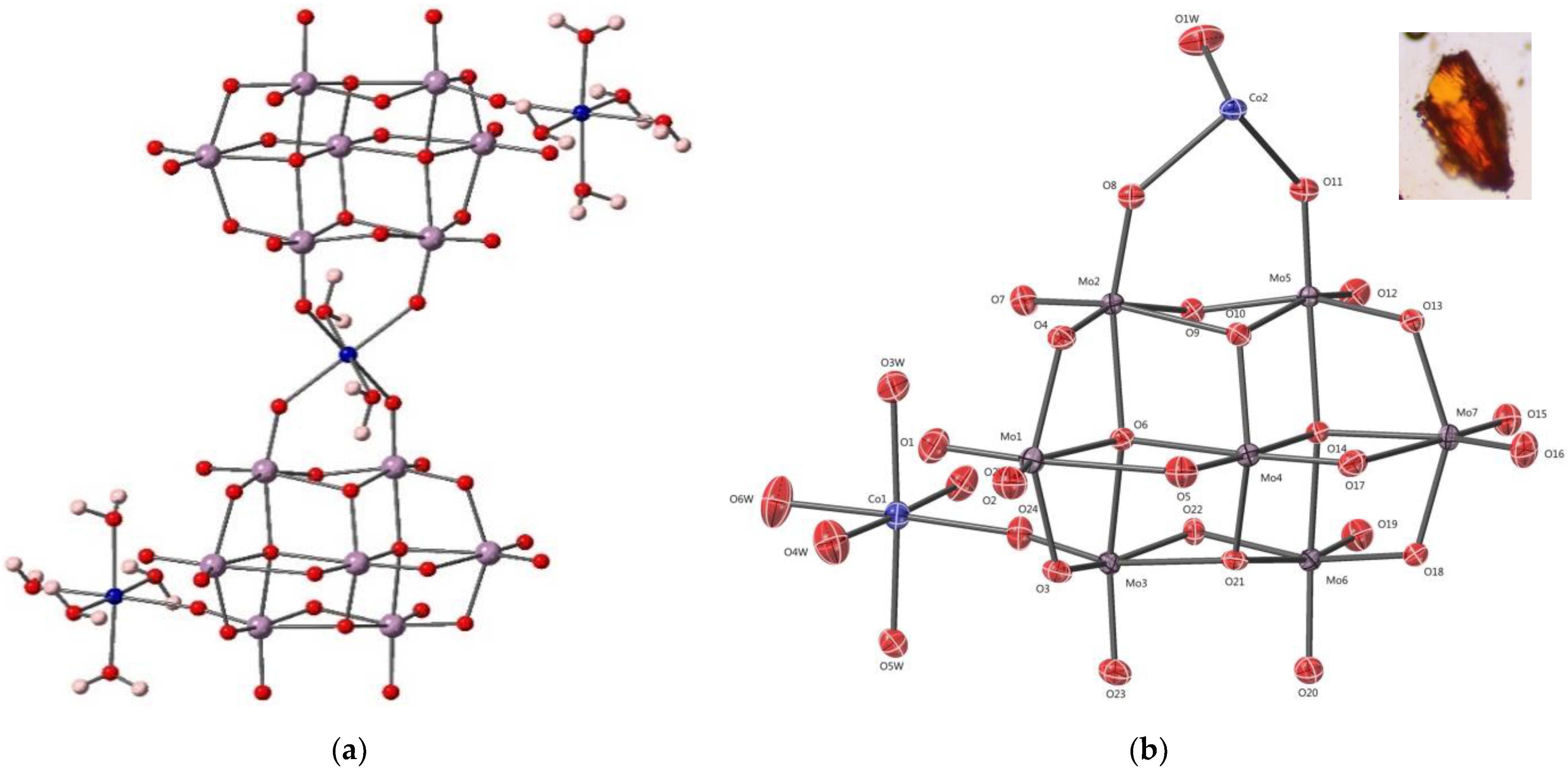
3.1. Crystal Structure of Co3Mo14-POM
3.2. Structural Comparison of Cobalt-Capped Heptamolybdates
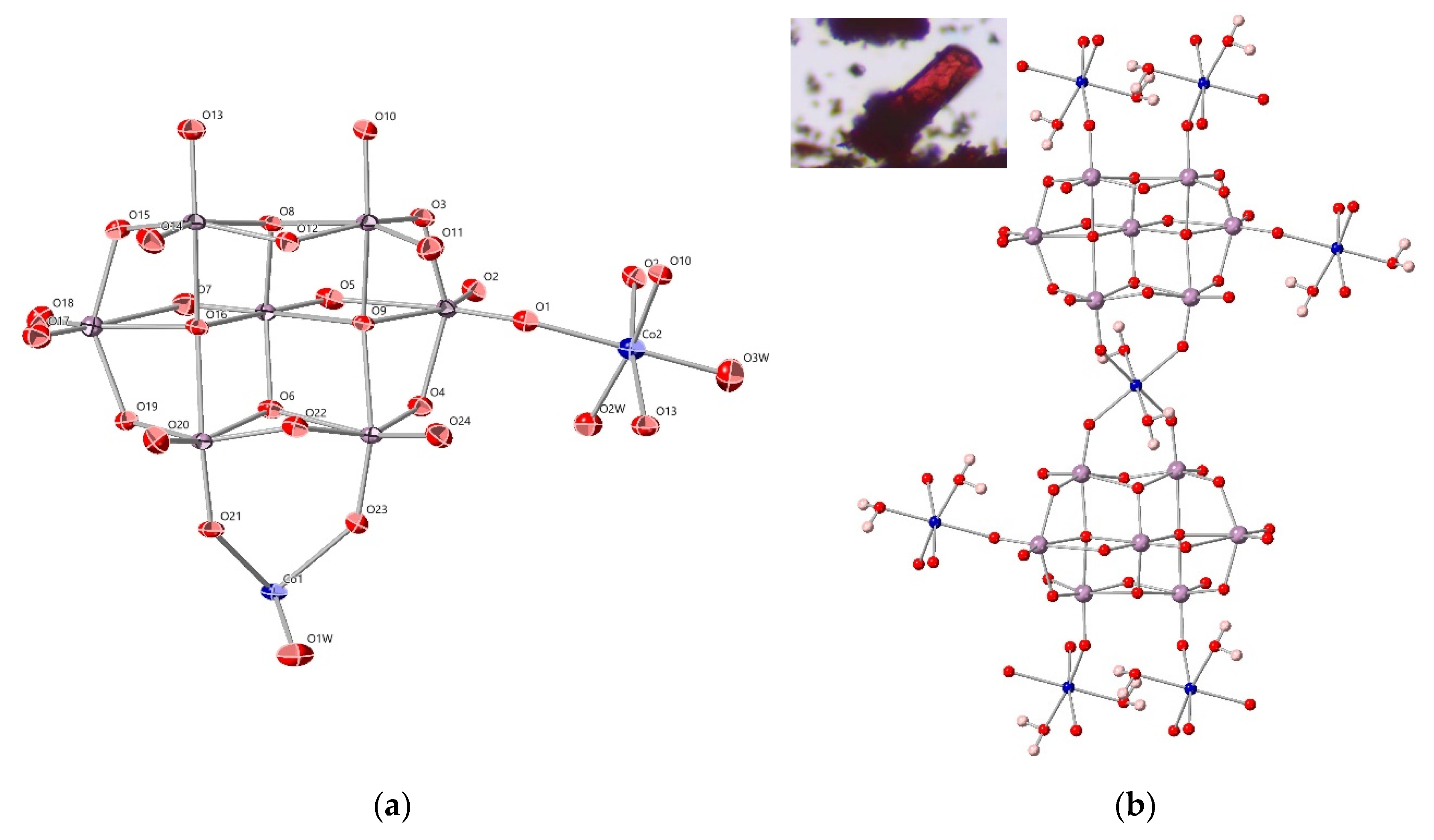
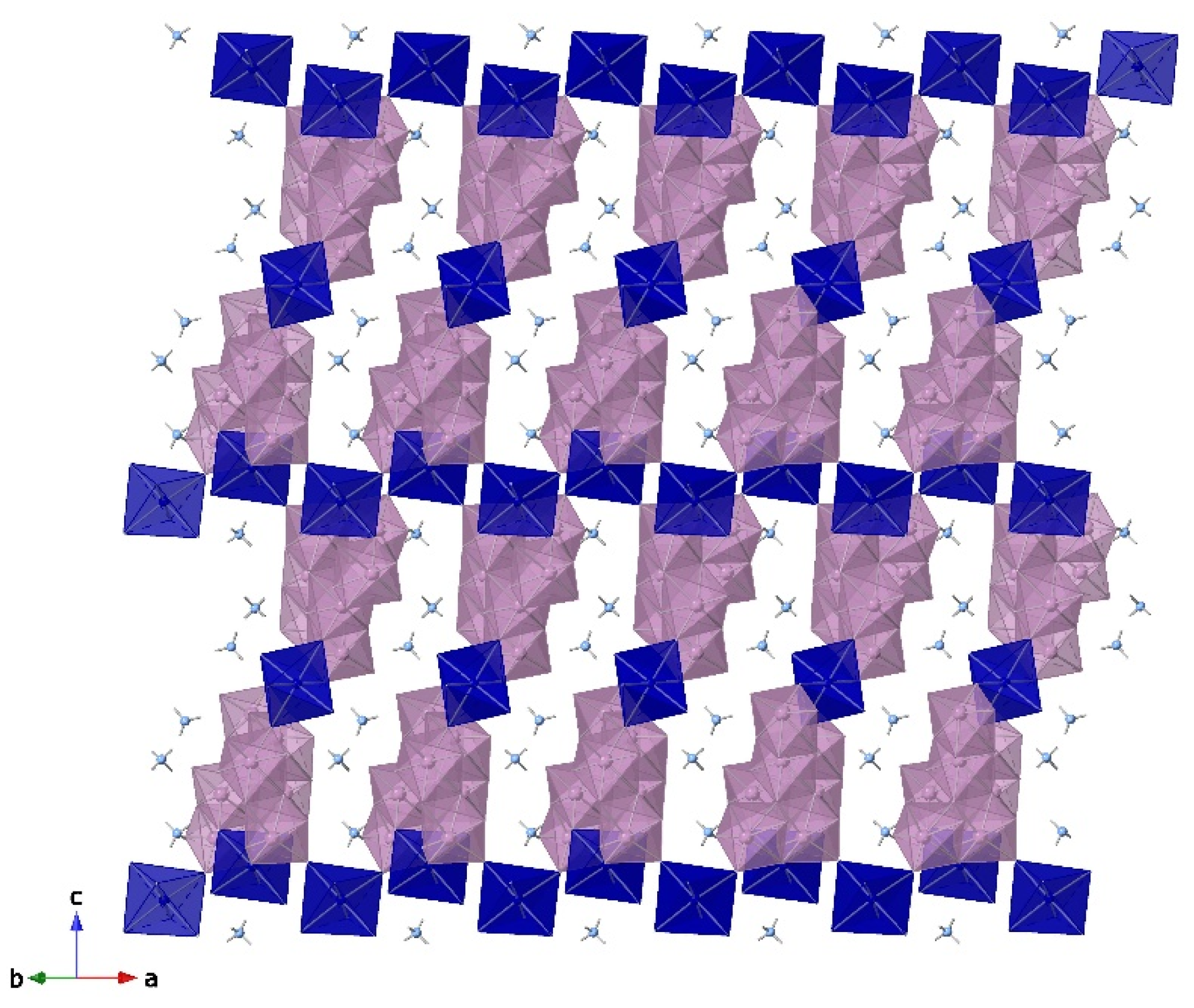
3.3. Crystal Structure of Co3Mo14-ES
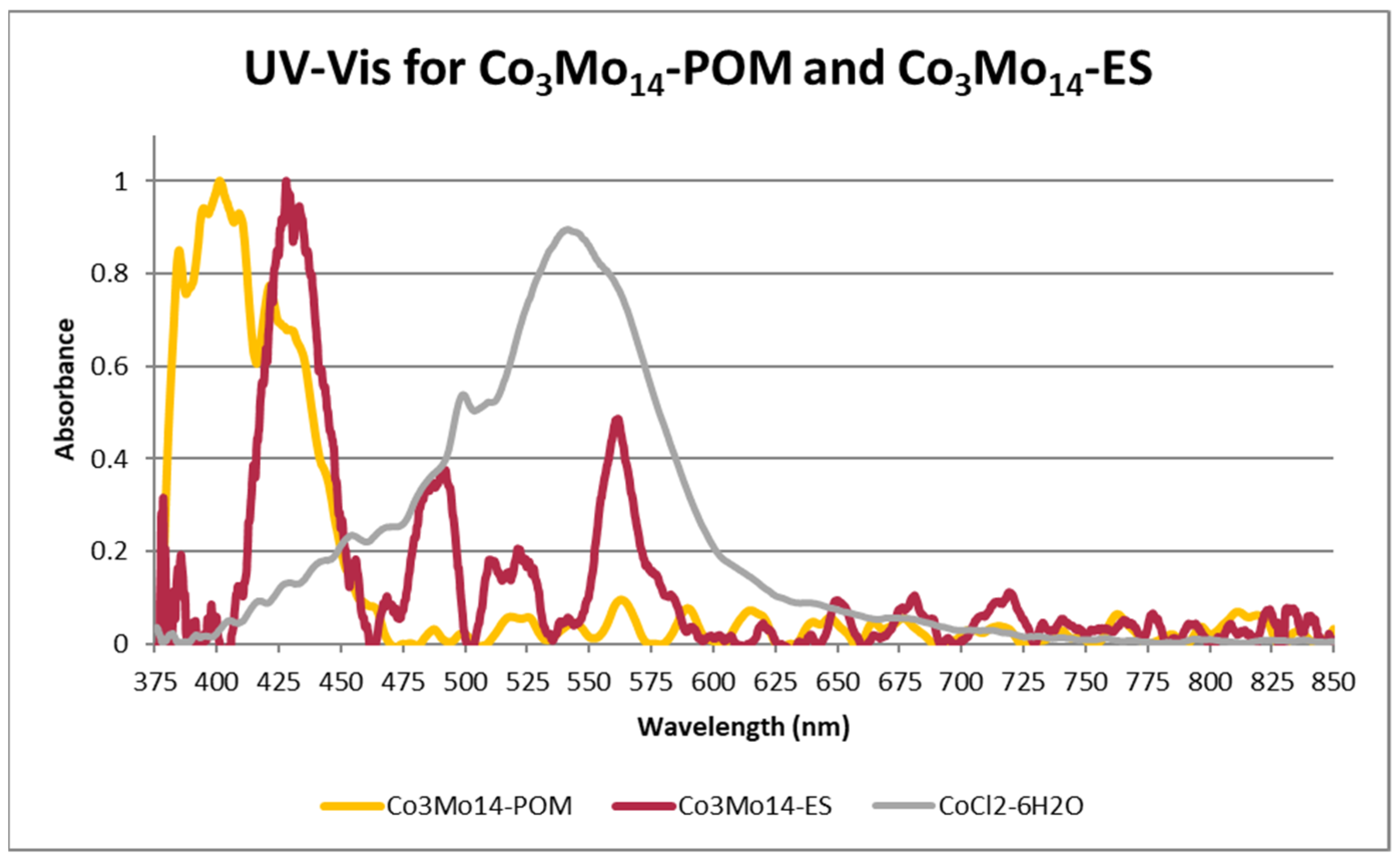
3.4. Solid-State UV–Vis–NIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keggin, J.F. The Structure and Formula of 12-Phosphotungstic Acid. Proc. R. Soc. A Math. Phys. Eng. Sci. 1934, 144, 75–100. [Google Scholar] [CrossRef]
- Anderson, J.S. Constitution of the Poly-Acids. Nature 1937, 140, 850. [Google Scholar] [CrossRef]
- Lindqvist, I. The Structure of the Hexaniobate Ion in 7Na2O·6Nb2O5·32H2O. Ark. Kemi 1953, 5, 247–250. [Google Scholar]
- Kepert, D.L. The Structures of Polyanions. Inorg. Chem. 1969, 1389, 1966–1968. [Google Scholar] [CrossRef]
- Graeber, E.J.; Morosin, B. The Molecular Configuration of the Decaniobate Ion (Nb10O286−). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 2137–2143. [Google Scholar] [CrossRef]
- Howarth, O.W.; Pettersson, L.; Andersson, I. Aqueous Peroxoisopolyoxometalates. In Polyoxometalate Chemistry; Pope, M.T., Muller, A., Eds.; Klumer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 145–159. [Google Scholar]
- Müller, A.; Beckmann, E.; Bögge, H.; Schmidtmann, M.; Dress, A. Inorganic Chemistry Goes Protein Size: A Mo368 Nano-Hedgehog Initiating Nanochemistry by Symmetry Breaking. Angew. Chem. Int. Ed. Engl. 2002, 41, 1162–1167. [Google Scholar] [CrossRef]
- Gouzerh, P.; Che, M. From Scheele and Berzelius to Müller: Polyoxometalates (POMs) Revisited and the Missing Link between the Bottom up and Top down Approaches. Actual. Chim. 2006, 298, 9–22. [Google Scholar]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building Blocks for Functional Nanoscale Systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef]
- Müller, A.; Gouzerh, P. From Linking of Metal-Oxide Building Blocks in a Dynamic Library to Giant Clusters with Unique Properties and towards Adaptive Chemistry. Chem. Soc. Rev. 2012, 41, 7431–7463. [Google Scholar] [CrossRef]
- Berzelius, J.J. Contribution to Further Knowledge of the Molybdenum Solution. Poggend. Ann. Phys. Chem. 1826, 6, 369–392. [Google Scholar] [CrossRef]
- Kehrmann, F. On the Understanding of Complex Inorganic Acids. Zeitschrift für Anorg. Chemie 1904, 39, 98–107. [Google Scholar]
- Rosenheim, V.A.; Jaenicke, V.J. The Knowledge of Iso- and Hetero-Poly Acids XV Announcement-Crital Experiments on the Constitution of Hetero-Poly Acids. Z. Für Anorg. Allg. Chem. 1917, 100, 304–354. [Google Scholar] [CrossRef]
- Pauling, L. The Molecular Structure of the Tungstosilicates and Related Compounds. J. Am. Chem. Soc. 1929, 51, 2868–2880. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-Based Molecular Materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef]
- Hasenknopf, B.; Micoine, K.; Lacôte, E.; Thorimbert, S.; Malacria, M.; Thouvenot, R. Chirality in Polyoxometalate Chemistry. Eur. J. Inorg. Chem. 2008, 2008, 5001–5013. [Google Scholar] [CrossRef]
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of Polyoxometalates: Towards Advanced Applications in Catalysis and Materials Science. Chem. Commun. 2008, 1837–1852. [Google Scholar] [CrossRef]
- Kortz, U.; Müller, A.; van Slageren, J.; Schnack, J.; Dalal, N.S.; Dressel, M. Polyoxometalates: Fascinating Structures, Unique Magnetic Properties. Coord. Chem. Rev. 2009, 253, 2315–2327. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic Polyoxometalates: From Molecular Magnetism to Molecular Spintronics and Quantum Computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef]
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble Metals in Polyoxometalates. Angew. Chem.-Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate Water Oxidation Catalysts and the Production of Green Fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef] [PubMed]
- Miras, H.N.; Vilà-Nadal, L.; Cronin, L. Polyoxometalate Based Open-Frameworks (POM-OFs). Chem. Soc. Rev. 2014, 43, 5679–5699. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, A.; Rompel, A. The Anderson–Evans Polyoxometalate: From Inorganic Building Blocks via Hybrid Organic–Inorganic Structures to Tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Y.; Yang, D.; Qin, Z.; Guo, D.; Bian, L.; Sang, X.; Sun, X.; Liu, X. Redox Poly-Counterion Doped Conducting Polymers for Pseudocapacitive Energy Storage. Adv. Funct. Mater. 2021, 31, 2006203. [Google Scholar] [CrossRef]
- Khenkin, A.M.; Neumann, R. Aerobic Oxidation of Vicinal Diols Catalyzed by an Anderson-Type Polyoxometalate, [IMo6O24]5−. Adv. Synth. Catal. 2002, 344, 1017–1021. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Li, X.; Pei, F.; Li, Y.; Lei, H. The Gadolinium Complexes with Polyoxometalates as Potential MRI Contrast Agents. Magn. Reson. Imaging 2007, 25, 412–417. [Google Scholar] [CrossRef]
- Long, D.-L.; Burkholder, E.; Cronin, L. Polyoxometalate Clusters, Nanostructures and Materials: From Self Assembly to Designer Materials and Devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Damjanović, V.; Kuzman, D.; Vrdoljak, V.; Muratović, S.; Žilić, D.; Stilinović, V.; Cindrić, M. Hydrothermal Reactions of [CoIII(C2O4)(NH3)4]+ and Polyoxomolybdates: Depolymerization of Polyoxomolybdates and in Situ Reduction of Cobalt. Cryst. Growth Des. 2019, 19, 6763–6773. [Google Scholar] [CrossRef]
- Damjanović, V.; Pisk, J.; Kuzman, D.; Agustin, D.; Vrdoljak, V.; Stilinović, V.; Cindrić, M. The Synthesis, Structure and Catalytic Properties of the [Mo7O24(μ-Mo8O26)Mo7O24]16− Anion Formed via Two Intermediate Heptamolybdates [Co(En)3]2[NaMo7O24]Cl·nH2O and (H3O)[Co(En)3]2[Mo7O24]Cl·9H2O. Dalton Trans. 2019, 48, 9974–9983. [Google Scholar] [CrossRef] [PubMed]
- Kuzman, D.; Damjanović, V.; Vrdoljak, V.; Stilinović, V.; Cindrić, M. Directing Role of the Synthetic Route on the Self-Assembly Process of MoO42− Units to Mo7O242− or Mo22O7416− Ions. Inorg. Chim. Acta 2020, 510, 119765. [Google Scholar] [CrossRef]
- Han, Z.; Ma, H.; Peng, J.; Chen, Y.; Wang, E.; Hu, N. The first polyoxometalate polymer constructed by assembly of the heptamolybdic anion and copper coordination groups. Inorg. Chem. Commun. 2004, 7, 182–185. [Google Scholar] [CrossRef]
- Long, D.-L.; Kögerler, P.; Farrugia, L.J.; Cronin, L. Reactions of a {Mo16}-type polyoxometalate cluster with electrophiles: A synthetic, theoretical and magnetic investigation. Dalton Trans. 2005, 54, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ke, Y.; Collins, D.J.; Lorigan, G.A.; Zhou, H.-C. Construction of Robust Open Metal−Organic Frameworks with Chiral Channels and Permanent Porosity. Inorg. Chem. 2007, 46, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-B. Heptaguanidinium sodium bis(pentaaquatetracosaoxoheptamolybdocobaltate) octahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m1911–m1912. [Google Scholar] [CrossRef]
- Hill, C.L.; Delannoy, L.; Duncan, D.C.; Weinstock, I.A.; Renneke, R.F.; Reiner, R.S.; Atalla, R.H.; Han, J.W.; Hillesheim, D.A.; Cao, R.; et al. Complex catalysts from self-repairing ensembles to highly reactive air-based oxidation systems. Comptes Rendus. Chim. 2007, 10, 305–312. [Google Scholar] [CrossRef]
- Li, T.; Lü, J.; Gao, S.; Cao, R. A new heptamolybdate-based supramolecular compound with an alternating organic and inorganic layer structure: Synthesis, crystal structure and properties of (Hapy)4[Co(H2O)5Mo7O24]·9H2O. Inorg. Chem. Commun. 2007, 10, 1342–1346. [Google Scholar] [CrossRef]
- Liu, F.; Marchal-Roch, C.; Dambournet, D.; Acker, A.; Marrot, J.; Sécheresse, F. From Molecular to Two-Dimensional Anderson Polyoxomolybdate: Synthesis, Crystal Structure, and Thermal Behavior of [{Ni(H2O)4}2{Ni(OH)6Mo6O18}]·4H2O and [Ni(H2O)6][Ag2{Ni(OH)6Mo6O18}]·8H2O. Eur. J. Inorg. Chem. 2008, 2008, 2191–2198. [Google Scholar] [CrossRef]
- Arumuganathan, T.; Rao, A.S.; Das, S.K. Polyoxometalate Supported Transition Metal Complexes: Synthesis, Crystal Structures, and Supramolecular Chemistry. Cryst. Growth Des. 2010, 10, 4272–4284. [Google Scholar] [CrossRef]
- Casan-Pastor, N.; Bas-Serra, J.; Coronado, E.; Pourroy, G.; Baker, L.C.W. First ferromagnetic interaction in a heteropoly complex: [CoII4O14(H2O)2(PW9O27)2]10−. Experiment and theory for intramolecular anisotropic exchange involving the four Co(II) atoms. J. Am. Chem. Soc. 1992, 114, 10380–10383. [Google Scholar] [CrossRef]
- Li, L.; Shen, Q.; Xue, G.; Xu, H.; Hu, H.; Feng, F.; Wang, J. Two sandwich arsenomolybdates based on the new building block As(iii)Mo7O279−: [Cr2(AsMo7O27)2]12− and [Cu2(AsMo7O27)2]14−. Dalton Trans. 2008, 2, 5698–5700. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Yamaguchi, S.; Tsuchida, K.; Nakagawa, Y.; Uehara, K.; Yamaguchi, K.; Mizuno, N. Synthesis and Catalysis of Di- and Tetranuclear Metal Sandwich-Type Silicotungstates [(γ-SiW10O36)2M2(μ-OH)2]10− and [(γ-SiW10O36)2M4(M4-O)(μ-OH)6]8− (M = Zr or Hf). J. Am. Chem. Soc. 2008, 130, 5472–5478. [Google Scholar] [CrossRef]
- Keita, B.; Kortz, U.; Holzle, L.R.B.; Brown, S.; Nadjo, L. Efficient Hydrogen-Evolving Cathodes Based on Proton and Electron Reservoir Behaviors of the Phosphotungstate [H7P8W48O184]33− and the Co(II)-Containing Silicotungstates [Co6(H2O)30{Co9Cl2(OH)3(H2O)9(β-SiW8O31)3}]5− and [{Co3(B-β-SiW9O33(OH))(B-β-SiW8O29OH)2}2]22−. Langmuir 2007, 23, 9531–9534. [Google Scholar] [CrossRef]
- Sartorel, A.; Carraro, M.; Scorrano, G.; De Zorzi, R.; Geremia, S.; McDaniel, N.D.; Bernhard, S.; Bonchio, M. Polyoxometalate Embedding of a Tetraruthenium(IV)-oxo-core by Template-Directed Metalation of [γ-SiW10O36]8−: A Totally Inorganic Oxygen-Evolving Catalyst. J. Am. Chem. Soc. 2008, 130, 5006–5007. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, C.A.; Harley, S.J.; McAlpin, J.G.; Hocking, R.K.; Mercado, B.Q.; Johnson, R.L.; Villa, E.M.; Fidler, M.K.; Olmstead, M.M.; Spiccia, L.; et al. Rates of Water Exchange for Two Cobalt(II) Heteropolyoxotungstate Compounds in Aqueous Solution. Chem.–A Eur. J. 2011, 17, 4408–4417. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palmer, D.; Conley, M.; Parsonson, L.; Leila Rimmer, I.S. CrystalMaker 2020; CrystalMaker Software Ltd.: Oxford, UK, 2020. [Google Scholar]
- Spek, A.L. PLATONSQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction CrysAlis PRO 2021; Agilent Technologies Ltd.: Oxford, UK, 2021.
- Evans, H.T. Refined molecular structure of the heptamolybdate and hexamolybdotellurate ions. J. Am. Chem. Soc. 1968, 90, 3275–3276. [Google Scholar] [CrossRef]
- Evans, H.T.; Gatehouse, B.M.; Leverett, P. Crystal structure of the heptamolybdate(VI)(paramolybdate) ion, [Mo7O24]6–, in the ammonium and potassium tetrahydrate salts. J. Chem. Soc. Dalton Trans. 1975, 505–514. [Google Scholar] [CrossRef]
- Tian, C.-H.; Sun, Z.-G.; Li, J.; Zheng, X.-F.; Liang, H.-D.; Zhang, L.-C.; You, W.-S.; Zhu, Z.-M. Synthesis and crystal structures of two new inorganic–organic hybrid polyoxomolybdate complexes: [Himi]4[{Co(Imi)2(H2O)2}Mo7O24]·4H2O and [Zn(Imi)4]2[(Imi)2Mo8O26]·6H2O. Inorg. Chem. Commun. 2007, 10, 757–761. [Google Scholar] [CrossRef]
| Co3Mo14-POM | Co3Mo14-ES | |
|---|---|---|
| Chemical formula | (NH4)6[Co3H24Mo14O60•6(H2O)] | (NH4)6[Co3H12Mo14O54] |
| Habit, Color | Blocks, Orange-Brown | Prism, Red-Violet |
| Mr | 2720.49 | 2504.30 |
| Crystal system, Space group | Triclinic, P-1 | Triclinic, P-1 |
| Temperature (K) | 293 | 293 |
| a, b, c (Å) | 8.0045 (3), 11.2181 (4), 18.0591 (7) | 7.8945 (2), 9.9241 (3), 16.2957 (4) |
| α, β, γ (°) | 107.385 (4), 95.428 (3), 100.586 (3) | 96.765 (2), 94.010 (2), 110.520 (3) |
| V (Å3) | 1501.77 (10) | 1178.85 (6) |
| Z | 1 | 1 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 3.75 | 4.74 |
| Crystal size (mm) | 0.09 × 0.07 × 0.05 | 0.07 × 0.04 × 0.04 |
| Diffractometer | Dtrek-CrysAlis PRO-abstract goniometer imported rigaku-D*TREK images | Dtrek-CrysAlis PRO-abstract goniometer imported rigaku-D*TREK images |
| Absorption correction | Multi-scan CrysAlis PRO 1.171.38.46 Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. | Multi-scan CrysAlis PRO 1.171.40.53 Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Tmin, Tmax | 0.958, 1.000 | 0.976, 1.000 |
| No. of measured, independent and observed [I > 2σ (I)] reflections | 19,093, 9006, 6850 | 12,130, 5783, 4452 |
| Rint | 0.037 | 0.036 |
| (sin θ/λ)max (Å−1) | 0.714 | 0.667 |
| R [F2 > 2σ(F2)], Wr (F2), S | 0.038, 0.091, 1.07 | 0.042, 0.102, 1.10 |
| No. of reflections | 9006 | 5783 |
| No. of parameters | 467 | 388 |
| No. of restraints | 57 | 57 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 1.53, −1.26 | 4.04, −1.54 |
| Compound | μ2-Bridging Co-O | Avg. Terminal Co-O(Aqua) | Co-N |
|---|---|---|---|
| (Hapy)4[Co(H2O)5Mo7O24]·9H2O [39] | 2.099(3) Å [a] | 2.079(5) Å | N/A |
| (2.051–2.101) | |||
| (CH6N3)7Na[CoMo7O24(H2O)5]2·8H2O [37] | 2.082(7) Å [b] | 2.099(10) Å | N/A |
| (2.081–2.121) | |||
| [3-ampH]4[{Co(3-ampy)(H2O)4}Mo7O24]·4H2O [41] | 2.073(2) Å [a] | 2.099(3) Å | 2.122(3) Å |
| (2.071–2.123) | |||
| This Work—Co1 | 2.150(3) Å [c] | 2.087(4) Å | N/A |
| (2.064–2.109) | |||
| This Work—Co2 | 2.097(3) Å [b] | 2.038(5) Å | |
| (2.075–2.119) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spriet, M.R.; Polinski, M.J.; Mercado, B.Q.; Villa, E.M. Synthesis and Structures of the Capped and Sandwiched Cobalt Heptamolybdate Polyoxometalate (NH4)6{[Co(H2O)5][Mo7O24][Co(H2O)2][Mo7O24][Co(H2O)5]} · 6 H2O and Its Extended Structure (NH4)6{[Co(H2O)2]3[Mo7O24]2}. Crystals 2022, 12, 1201. https://doi.org/10.3390/cryst12091201
Spriet MR, Polinski MJ, Mercado BQ, Villa EM. Synthesis and Structures of the Capped and Sandwiched Cobalt Heptamolybdate Polyoxometalate (NH4)6{[Co(H2O)5][Mo7O24][Co(H2O)2][Mo7O24][Co(H2O)5]} · 6 H2O and Its Extended Structure (NH4)6{[Co(H2O)2]3[Mo7O24]2}. Crystals. 2022; 12(9):1201. https://doi.org/10.3390/cryst12091201
Chicago/Turabian StyleSpriet, Matthieu R., Matthew J. Polinski, Brandon Q. Mercado, and Eric M. Villa. 2022. "Synthesis and Structures of the Capped and Sandwiched Cobalt Heptamolybdate Polyoxometalate (NH4)6{[Co(H2O)5][Mo7O24][Co(H2O)2][Mo7O24][Co(H2O)5]} · 6 H2O and Its Extended Structure (NH4)6{[Co(H2O)2]3[Mo7O24]2}" Crystals 12, no. 9: 1201. https://doi.org/10.3390/cryst12091201
APA StyleSpriet, M. R., Polinski, M. J., Mercado, B. Q., & Villa, E. M. (2022). Synthesis and Structures of the Capped and Sandwiched Cobalt Heptamolybdate Polyoxometalate (NH4)6{[Co(H2O)5][Mo7O24][Co(H2O)2][Mo7O24][Co(H2O)5]} · 6 H2O and Its Extended Structure (NH4)6{[Co(H2O)2]3[Mo7O24]2}. Crystals, 12(9), 1201. https://doi.org/10.3390/cryst12091201






