Characterization and Gamma-ray Shielding Performance of Calcinated and Ball-Milled Calcinated Bentonite Clay Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Fabrication
2.2. Characterization
2.2.1. Chemical Composition
2.2.2. X-ray Diffraction Analysis
2.2.3. Particle Size Measurements
2.2.4. The Fabricated Samples Measurements
2.3. Radiation Shielding Evaluation
3. Results and Discussion
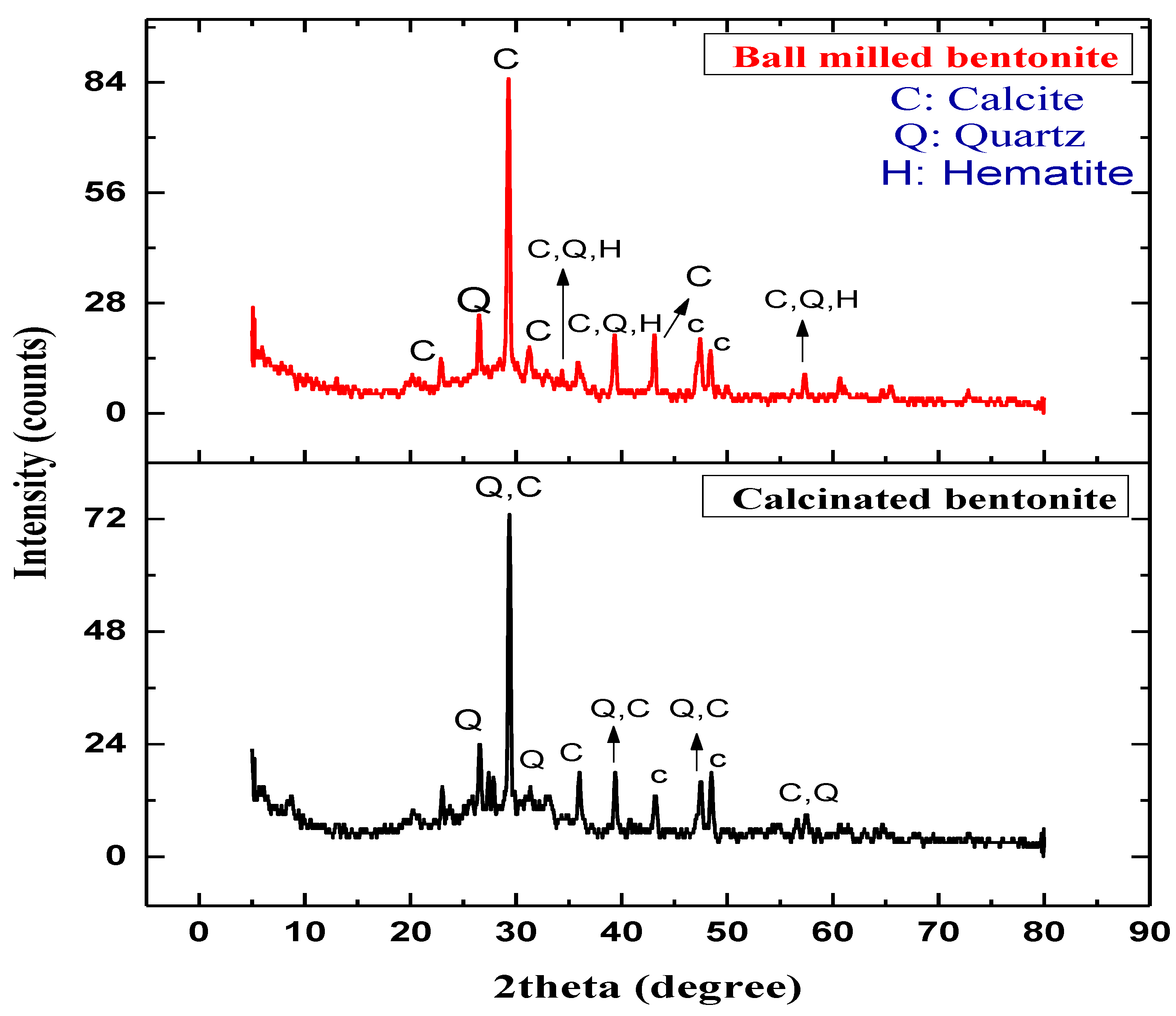
3.1. X-ray Diffraction
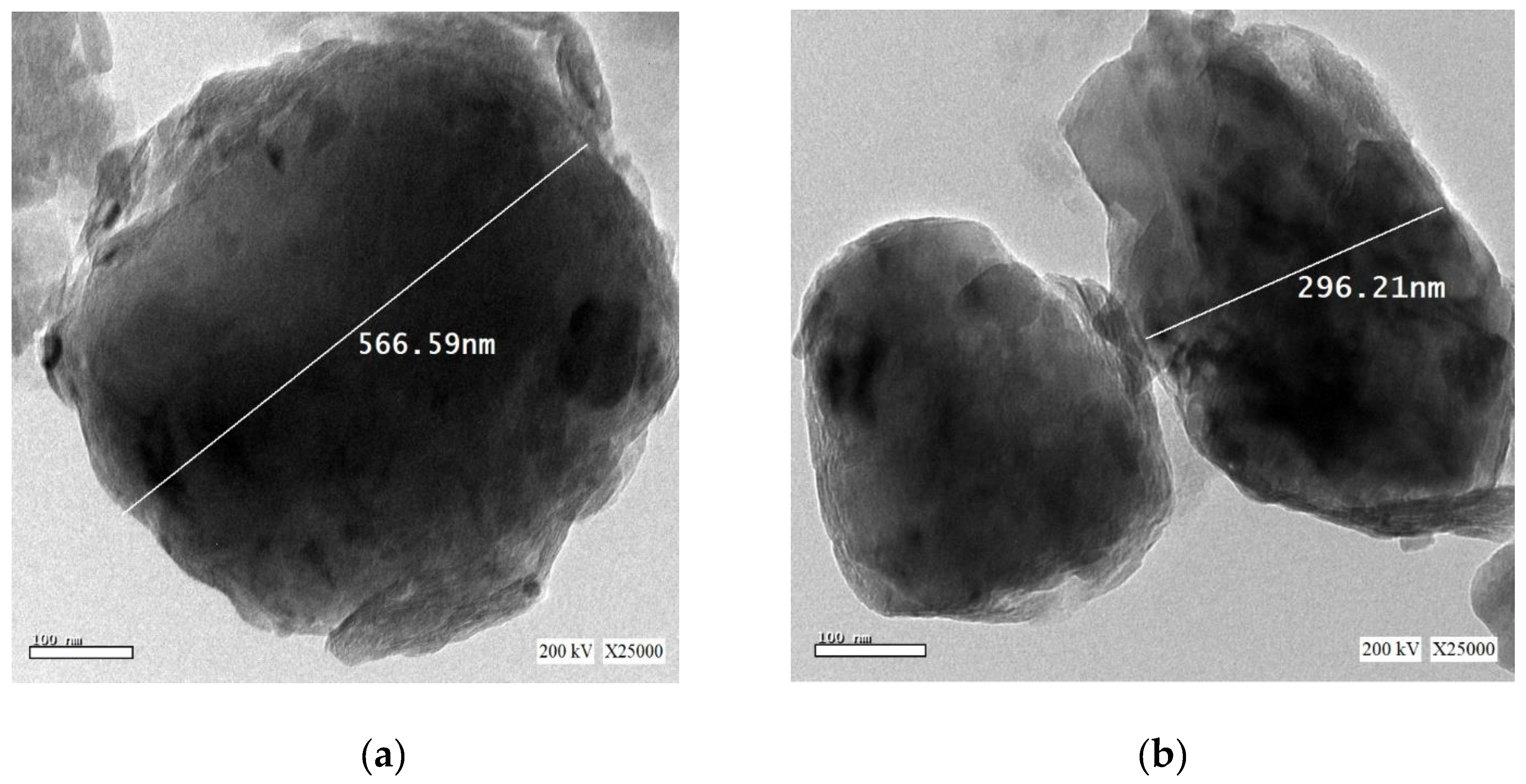
3.2. Particle Size Determination
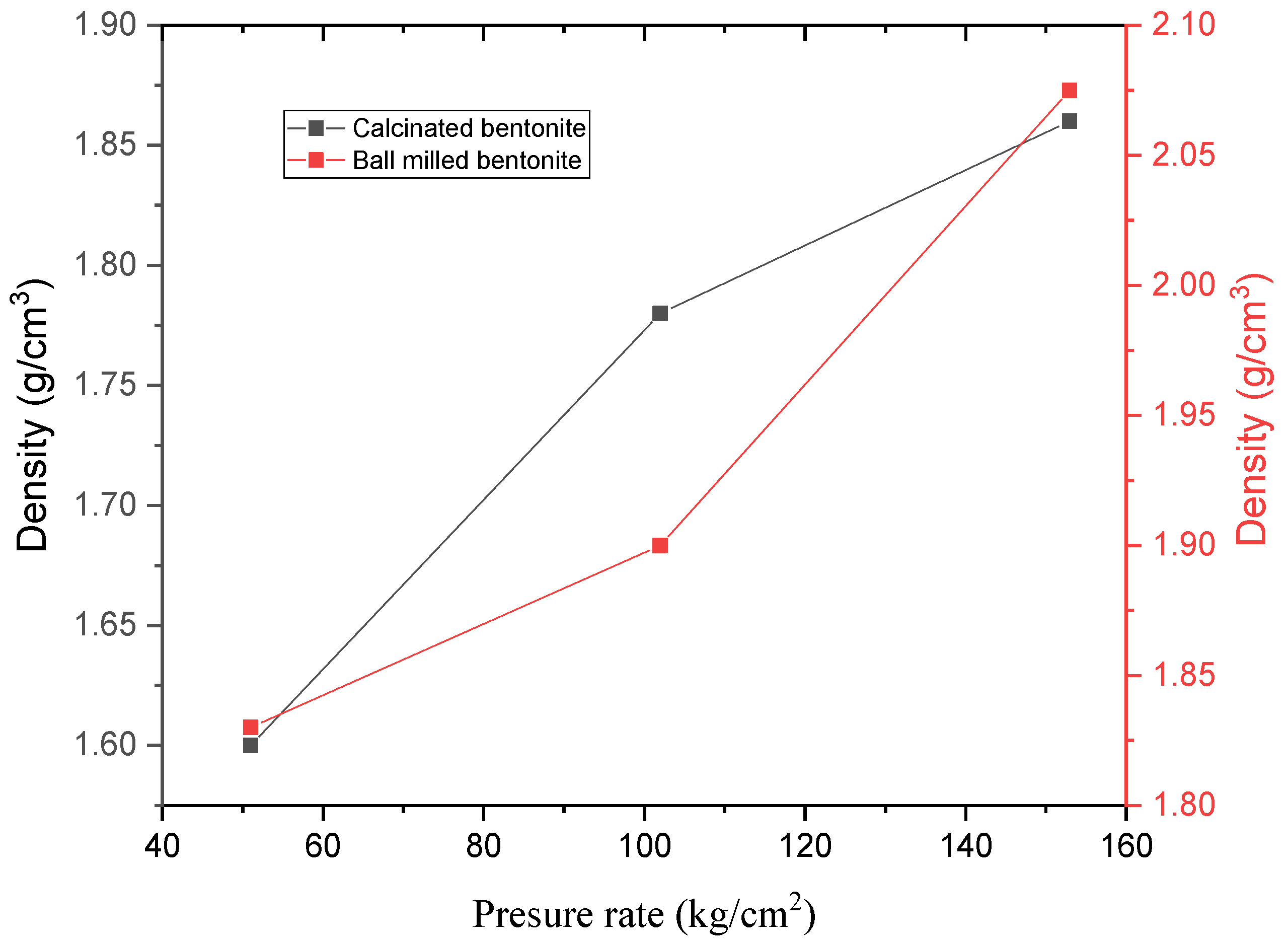
3.3. Density Measurement
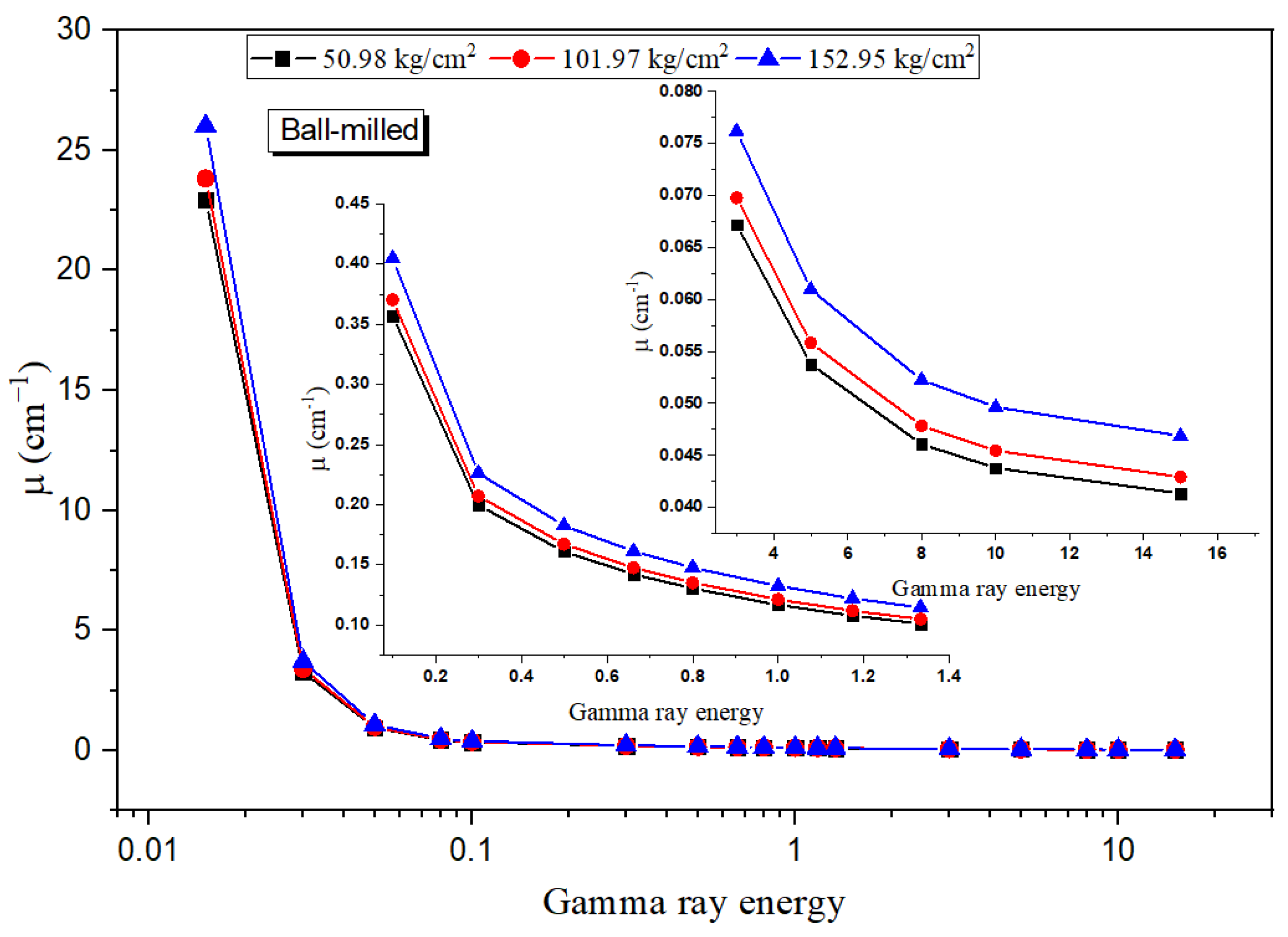
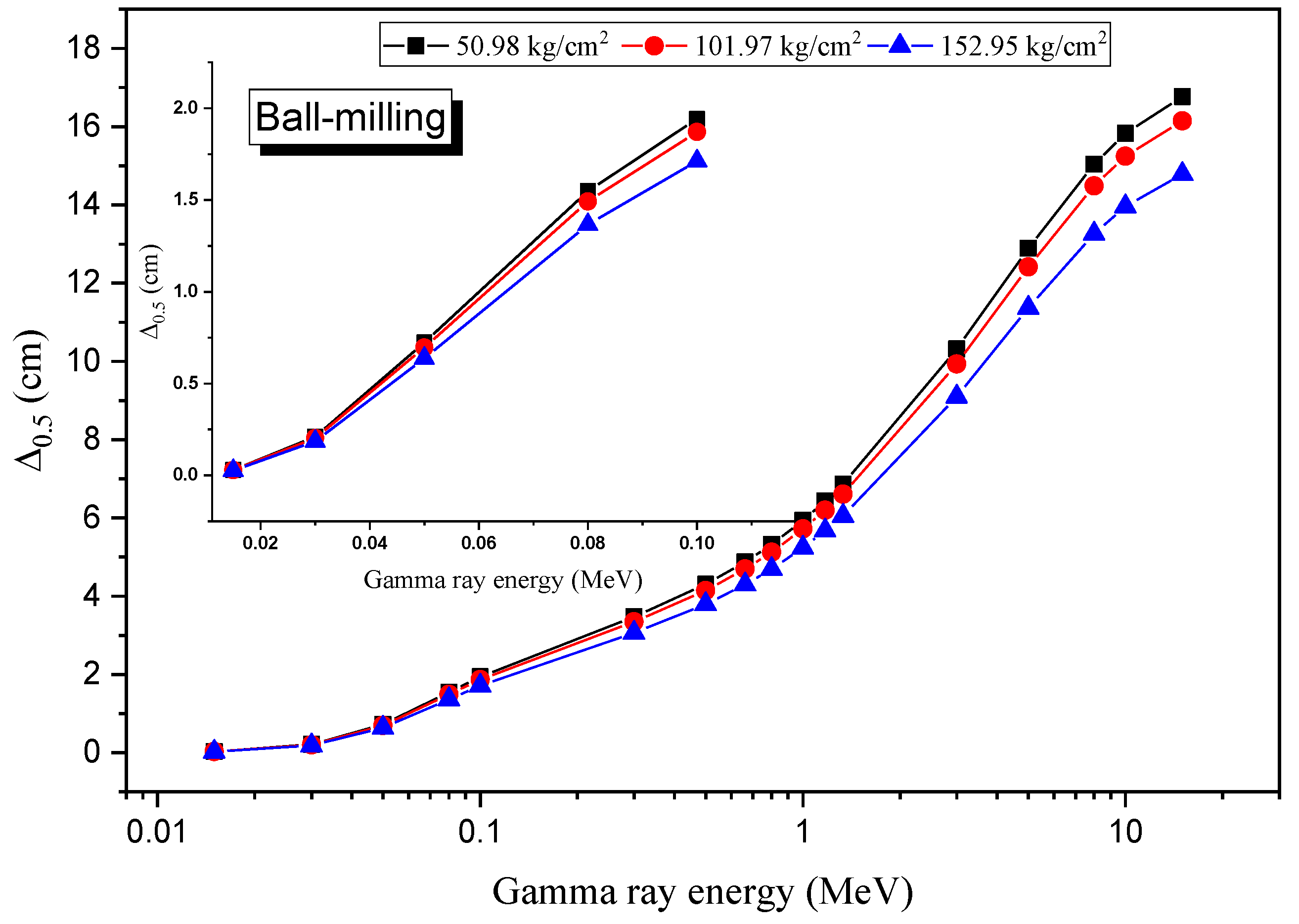
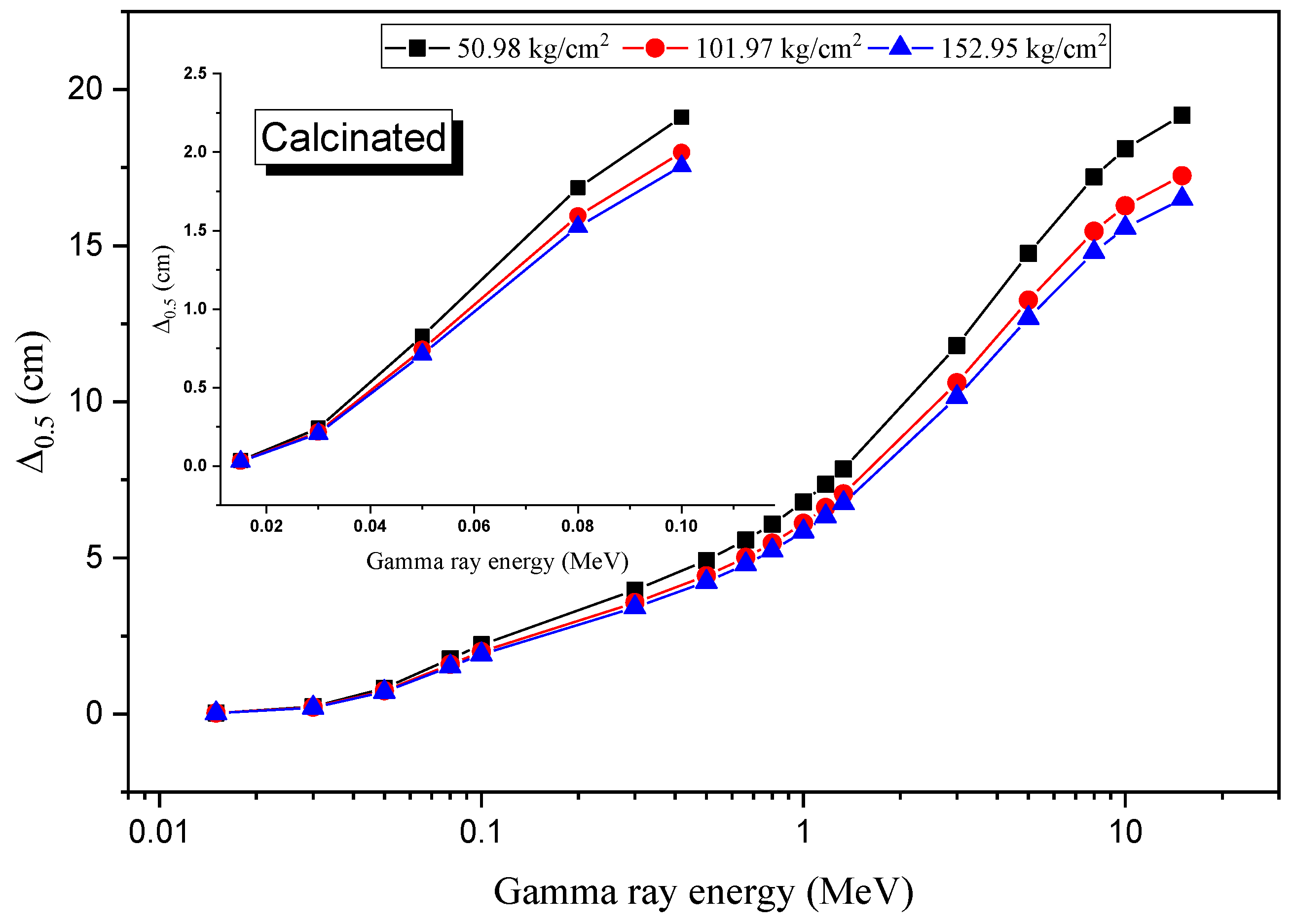
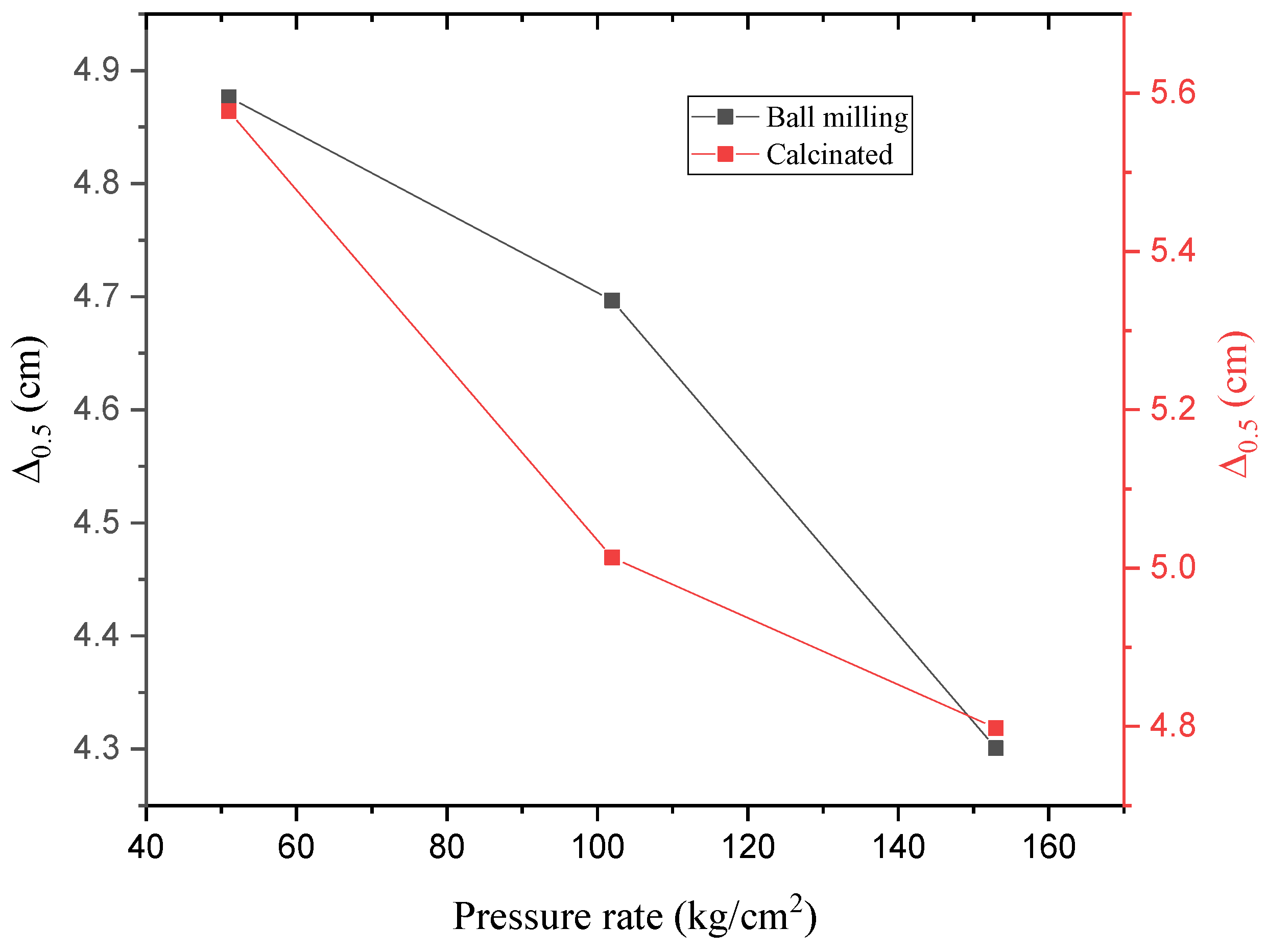
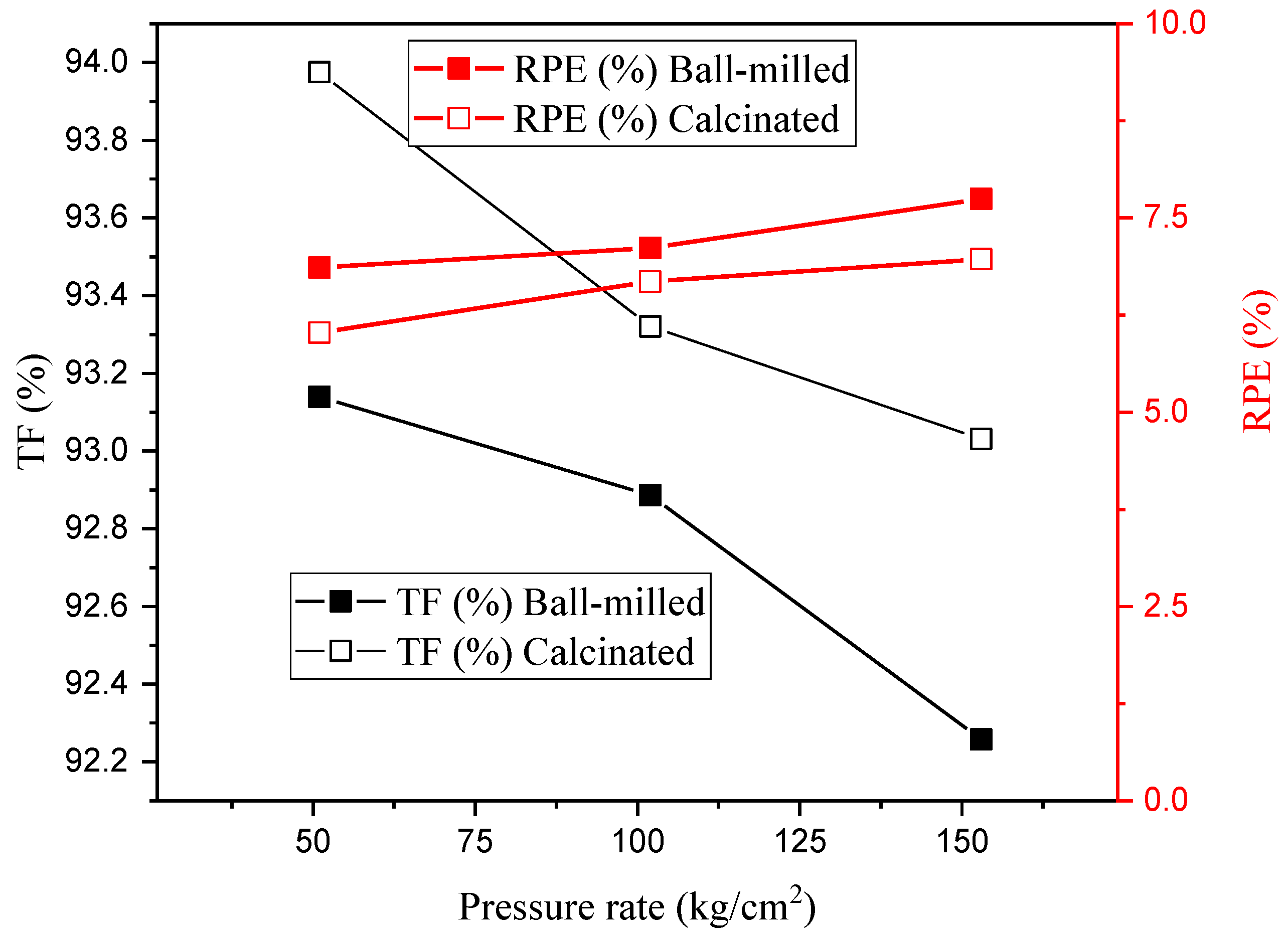
3.4. Radiation Shielding Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beir, V. Health Effects of Exposure to Low Levels of Ionizing Radiation; National Academies Press (US): Washington, DC, USA, 1990. [Google Scholar]
- Agar, O. Study on Gamma Ray Shielding Performance of Concretes Doped with Natural Sepiolite Mineral. Radiochim. Acta 2018, 106, 1009–1016. [Google Scholar] [CrossRef]
- González-Ortega, M.A.; Segura, I.; Cavalaro, S.H.P.; Toralles-Carbonari, B.; Aguado, A.; Andrello, A.C. Radiological Protection and Mechanical Properties of Concretes with EAF Steel Slags. Constr. Build. Mater. 2014, 51, 432–438. [Google Scholar] [CrossRef]
- Agar, O.; Tekin, H.O.; Sayyed, M.I.; Korkmaz, M.E.; Culfa, O.; Ertugay, C. Experimental Investigation of Photon Attenuation Behaviors for Concretes Including Natural Perlite Mineral. Results Phys. 2019, 12, 237–243. [Google Scholar] [CrossRef]
- Allam, E.A.; El-Sharkawy, R.M.; El-Taher, A.; Shaaban, E.R.; Massoud, E.E.S.; Mahmoud, M.E. Enhancement and Optimization of Gamma Radiation Shielding by Doped Nano HgO into Nanoscale Bentonite. Nucl. Eng. Technol. 2022, 54, 2253–2261. [Google Scholar] [CrossRef]
- Asal, S.; Erenturk, S.A.; Haciyakupoglu, S. Bentonite Based Ceramic Materials from a Perspective of Gamma-Ray Shielding: Preparation, Characterization and Performance Evaluation. Nucl. Eng. Technol. 2021, 53, 1634–1641. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Elsafi, M.; Almutiri, M.N.; Mahmoud, R.M.M.; Alzahrani, J.S.; Sayyed, M.I.; Abbas, M.I. Enhancement of Bentonite Materials with Cement for Gamma-Ray Shielding Capability. Materials 2021, 14, 4697. [Google Scholar] [CrossRef]
- Hasan, W.K.; Mahdi, J.T.; Hameed, A.S. Measurement Technique of Linear and Mass Attenuation Coefficients of Polyester—Bentonite Composite as Gamma Radiation Shielding Materials. AIP Conf. Proc. 2019, 2144, 030018. [Google Scholar] [CrossRef]
- Hager, I.Z.; Rammah, Y.S.; Othman, H.A.; Ibrahim, E.M.; Hassan, S.F.; Sallam, F.H. Nano-Structured Natural Bentonite Clay Coated by Polyvinyl Alcohol Polymer for Gamma Rays Attenuation. J. Theor. Appl. Phys. 2019, 13, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Orolínová, Z.; Mockovčiaková, A.; Dolinská, S.; Briančin, J. Uticaj termičke obrade na bentonitska svojstva. Arch. Tech. Sci. 2012, 4, 49–56. [Google Scholar] [CrossRef]
- Darweesh, M.; Nagieb, Z.A. Hydration of Calcined Bentonite Portland Blended Cement Pastes. Indian J. Chem. Technol. 2007, 14, 301–307. Available online: http://nopr.niscpr.res.in/handle/123456789/1124 (accessed on 1 July 2022).
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, Y.T.; Rao, K.V.; Kumar, V.S.S.; Kumari, B.S. X-Ray Analysis by Williamson-Hall and Size-Strain Plot Methods of ZnO Nanoparticles with Fuel Variation. World J. Nano Sci. Eng. 2014, 4, 21–28. [Google Scholar] [CrossRef]
- Sallam, F.H.; Ibrahim, E.M.; Hassan, S.F.; Omar, A. Enhanced Bentonite/PVA Matrix for Advanced Shielding Applications. Nucl. Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction; Springer: Boston, MA, USA, 1998; ISBN 978-1-4899-0150-7. [Google Scholar]
- X-5 Monte Carlo Team. MCNP—A General Monte Carlo N-Particle Transport Code, Version 5. La-Ur-03-1987 2003, 1–100. Available online: https://mcnp.lanl.gov/pdf_files/la-ur-03-1987.pdf (accessed on 1 July 2022).
- Mahmoud, K.A.; Sayyed, M.I.; Alhuthali, A.M.S.; Hanfi, M.Y. The Effect of CuO Additive on the Mechanical and Radiation Shielding Features of Li2B4O7–Pb2O3 Glass System. Boletín De La Soc. Española De Cerámica Y Vidr. 2022, 61, 275–283. [Google Scholar] [CrossRef]
- Kilic, G.; Ilik, E.; Mahmoud, K.A.; El-Agawany, F.I.; Alomairy, S.; Rammah, Y.S. The Role of B2O3 on the Structural, Thermal, and Radiation Protection Efficacy of Vanadium Phosphate Glasses. Appl. Phys. A Mater. Sci. Processing 2021, 127, 265. [Google Scholar] [CrossRef]
- Abu El-Soad, A.M.; Sayyed, M.I.; Mahmoud, K.A.; Şakar, E.; Kovaleva, E.G. Simulation Studies for Gamma Ray Shielding Properties of Halloysite Nanotubes Using MCNP-5 Code. Appl. Radiat. Isot. 2019, 154, 108882. [Google Scholar] [CrossRef]
- Boussaa, S.A.; Kheloufi, A.; Zaourar, N.B.; Kerkar, F. Valorization of Algerian Sand for Photovoltaic Application. Acta Phys. Pol. A 2016, 130, 133–137. [Google Scholar] [CrossRef]
- Barhoum, A.; Van Assche, G.; Makhlouf, A.S.H.; Terryn, H.; Baert, K.; Delplancke, M.-P.; El-Sheikh, S.M.; Rahier, H. A Green, Simple Chemical Route for the Synthesis of Pure Nanocalcite Crystals. Cryst. Growth Des. 2015, 15, 573–580. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, X.Y.; Hu, W.B.; Zhou, G.Y. Microwave-Assisted Hydrothermal Synthesis of Hexagonal Columnar-Shaped Calcium Carbonate. Adv. Mater. Res. 2011, 239, 1643–1648. [Google Scholar] [CrossRef]
- Alsaif, N.A.M.; Alotiby, M.; Hanfi, M.Y.; Mahmoud, K.A.; Al-Yousef, H.A.; Alotaibi, B.M.; Sayyed, M.I.; Al-Hadeethi, Y. Comprehensive Study of Radiation Shielding and Mechanical Features of Bi2O3-TeO2-B2O3-GeO2 Glasses. J. Aust. Ceram. Soc. 2021, 57, 1267–1274. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, A.; Sayyed, M.I.; Laariedh, F.; Mahmoud, K.A.; Nebhen, J.; Khandaker, M.U.; Faruque, M.R.I. Tailoring Bismuth Borate Glasses by Incorporating PbO/GeO2 for Protection against Nuclear Radiation. Sci. Rep. 2021, 11, 7784. [Google Scholar] [CrossRef] [PubMed]
- Sazirul, I.; Mahmoud, K.A.; Sayyed, M.I.; Alim, B.; Rahman, M.d.M.; Mollah, A.S. Study on the radiation attenuation properties of locally available bees-wax as a tissue equivalent bolus material in radiotherapy. Radiat. Phys. Chem. 2020, 172, 108559. [Google Scholar]
- Mahmoud, K.A.; Abu El-Soad, A.M.; Kovaleva, E.G.; Sayyed, M.I.; Tashlykov, O.L. Modeling a three-layer container based on halloysite nano-clay for radioactive waste disposal. Prog. Nucl. Energy 2022, 152, 104379. [Google Scholar] [CrossRef]





| Oxides (%) | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Na2O | K2O | P2O5 | TiO2 | L.O.I | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcinated | 29.60 | 13.70 | 34.20 | 4.20 | 3.80 | 1.90 | 0.810 | 0.54 | 0.45 | 8.67 | 97.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallem, F.H.; Sayyed, M.I.; Aloraini, D.A.; Almuqrin, A.H.; Mahmoud, K.A. Characterization and Gamma-ray Shielding Performance of Calcinated and Ball-Milled Calcinated Bentonite Clay Nanoparticles. Crystals 2022, 12, 1178. https://doi.org/10.3390/cryst12081178
Sallem FH, Sayyed MI, Aloraini DA, Almuqrin AH, Mahmoud KA. Characterization and Gamma-ray Shielding Performance of Calcinated and Ball-Milled Calcinated Bentonite Clay Nanoparticles. Crystals. 2022; 12(8):1178. https://doi.org/10.3390/cryst12081178
Chicago/Turabian StyleSallem, Fawzy H., M. I. Sayyed, Dalal Abdullah Aloraini, Aljawhara H. Almuqrin, and K. A. Mahmoud. 2022. "Characterization and Gamma-ray Shielding Performance of Calcinated and Ball-Milled Calcinated Bentonite Clay Nanoparticles" Crystals 12, no. 8: 1178. https://doi.org/10.3390/cryst12081178
APA StyleSallem, F. H., Sayyed, M. I., Aloraini, D. A., Almuqrin, A. H., & Mahmoud, K. A. (2022). Characterization and Gamma-ray Shielding Performance of Calcinated and Ball-Milled Calcinated Bentonite Clay Nanoparticles. Crystals, 12(8), 1178. https://doi.org/10.3390/cryst12081178







