Sonochemical Synthesis of Nanostructured Ni-Fe-C System and Its Catalytic Activity Based on Decolorization of Reactive Black 5 Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Sonochemical Synthesis of Ni-Fe Samples
2.2. Characterization Tools
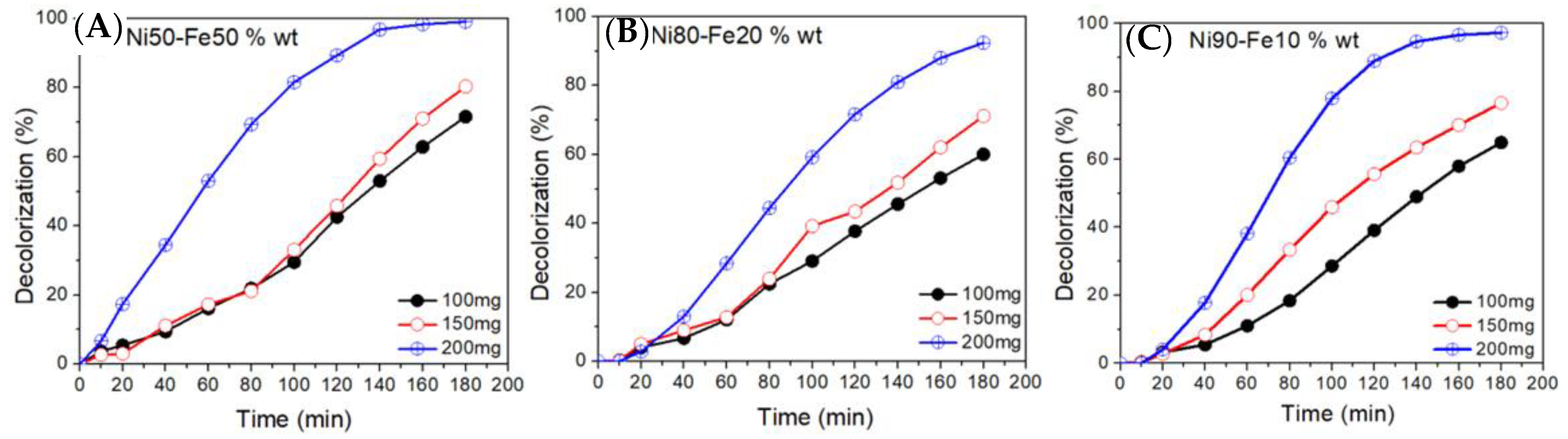
2.3. Dye Decolorization Evaluation
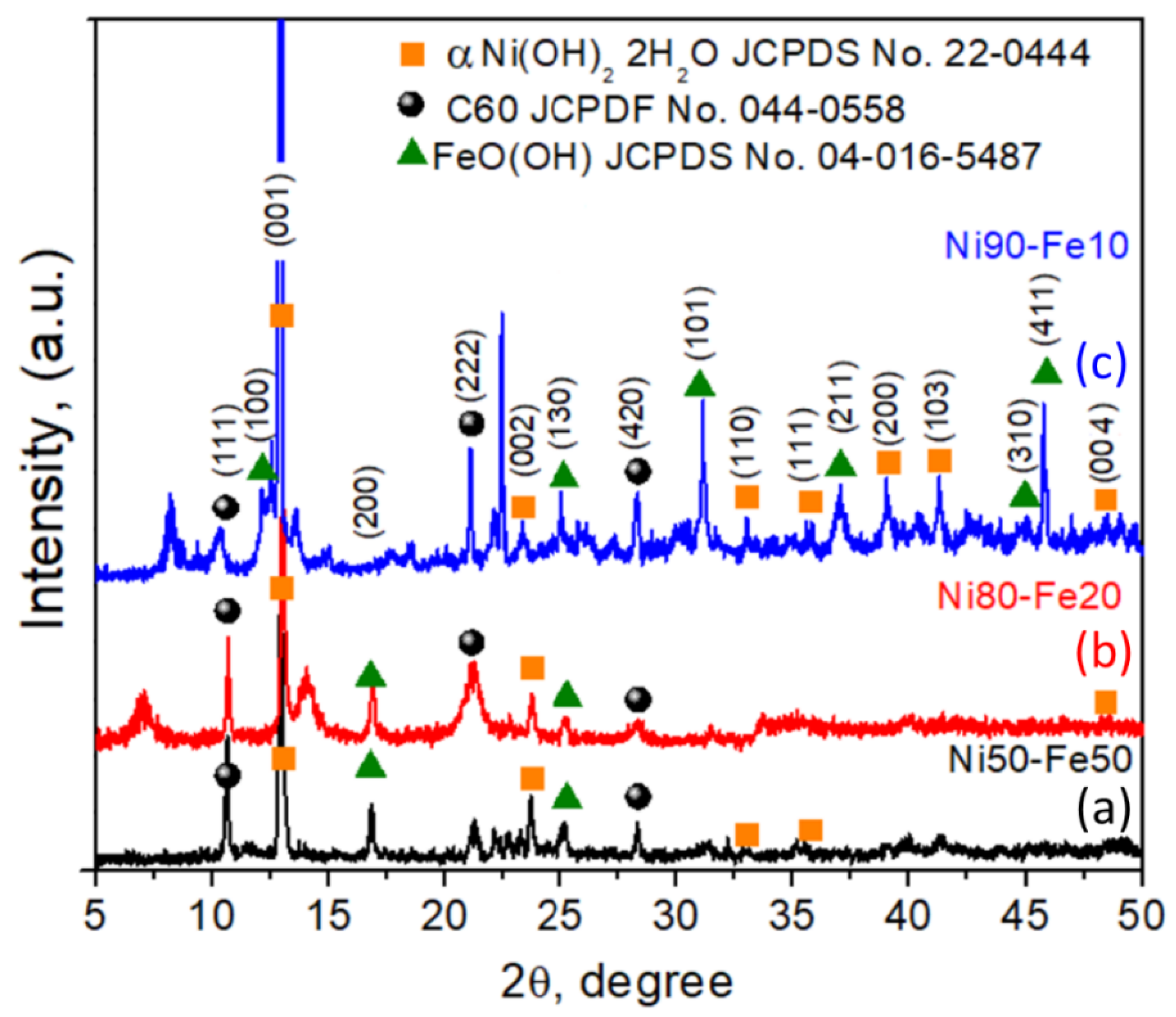
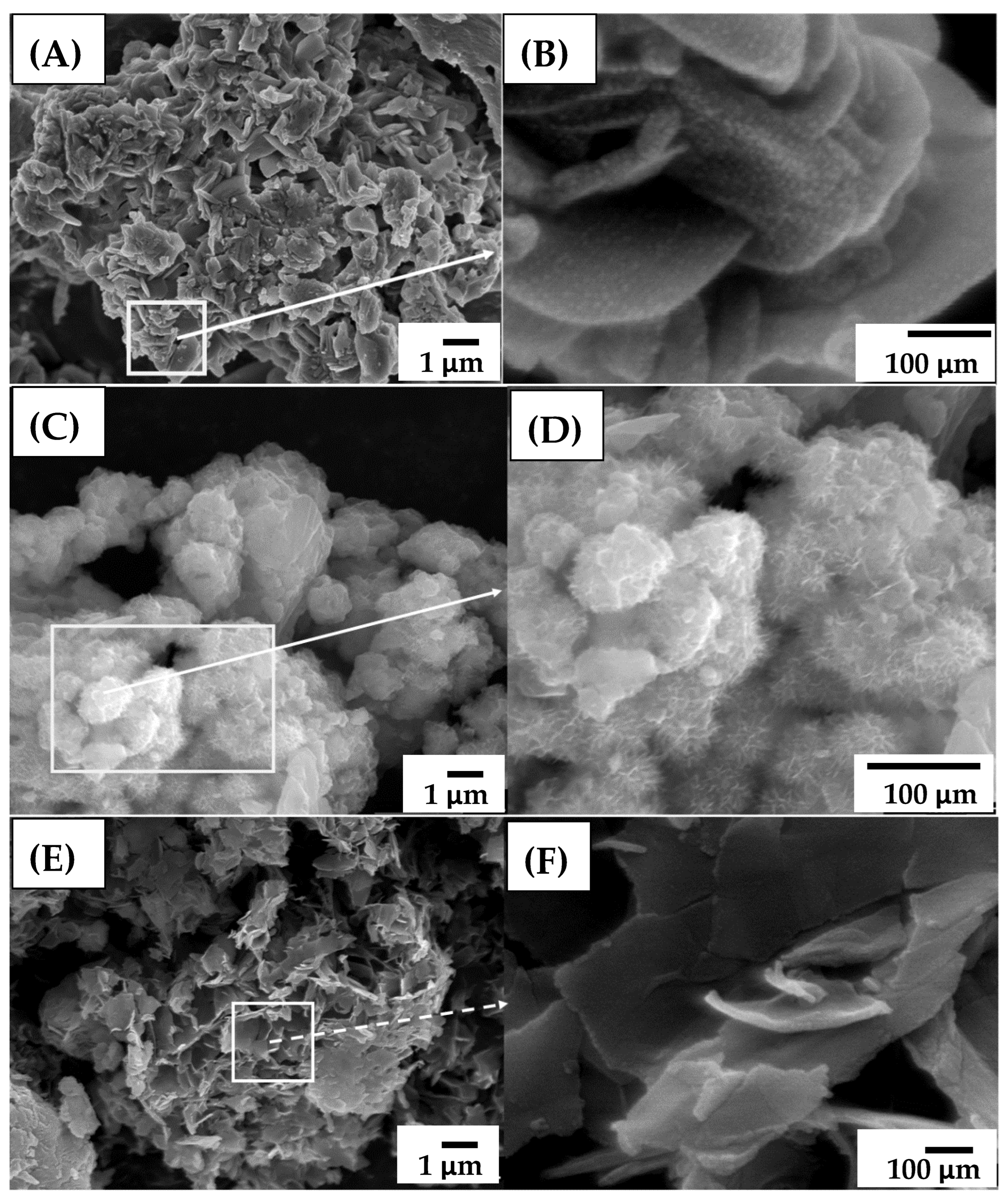
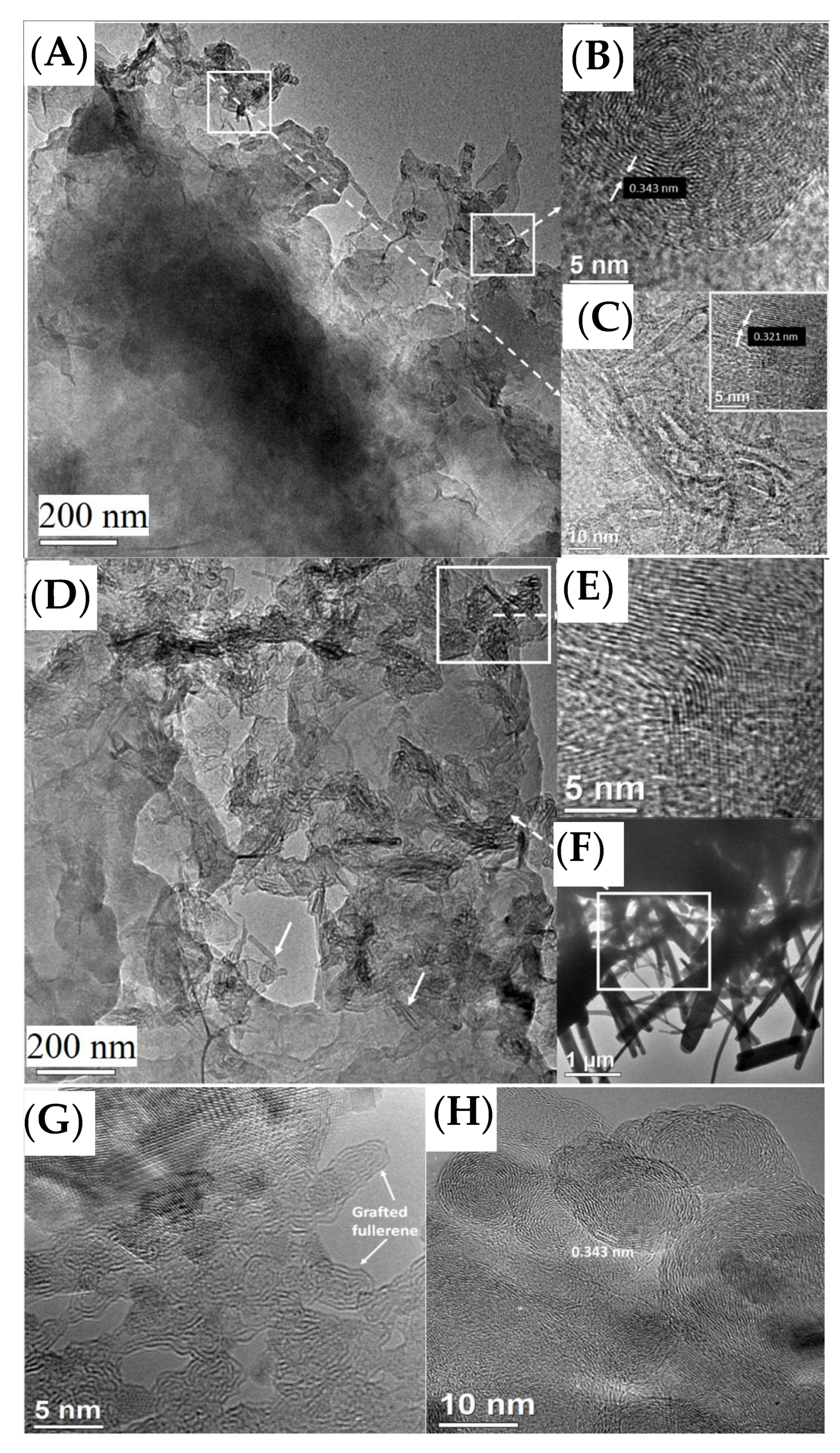
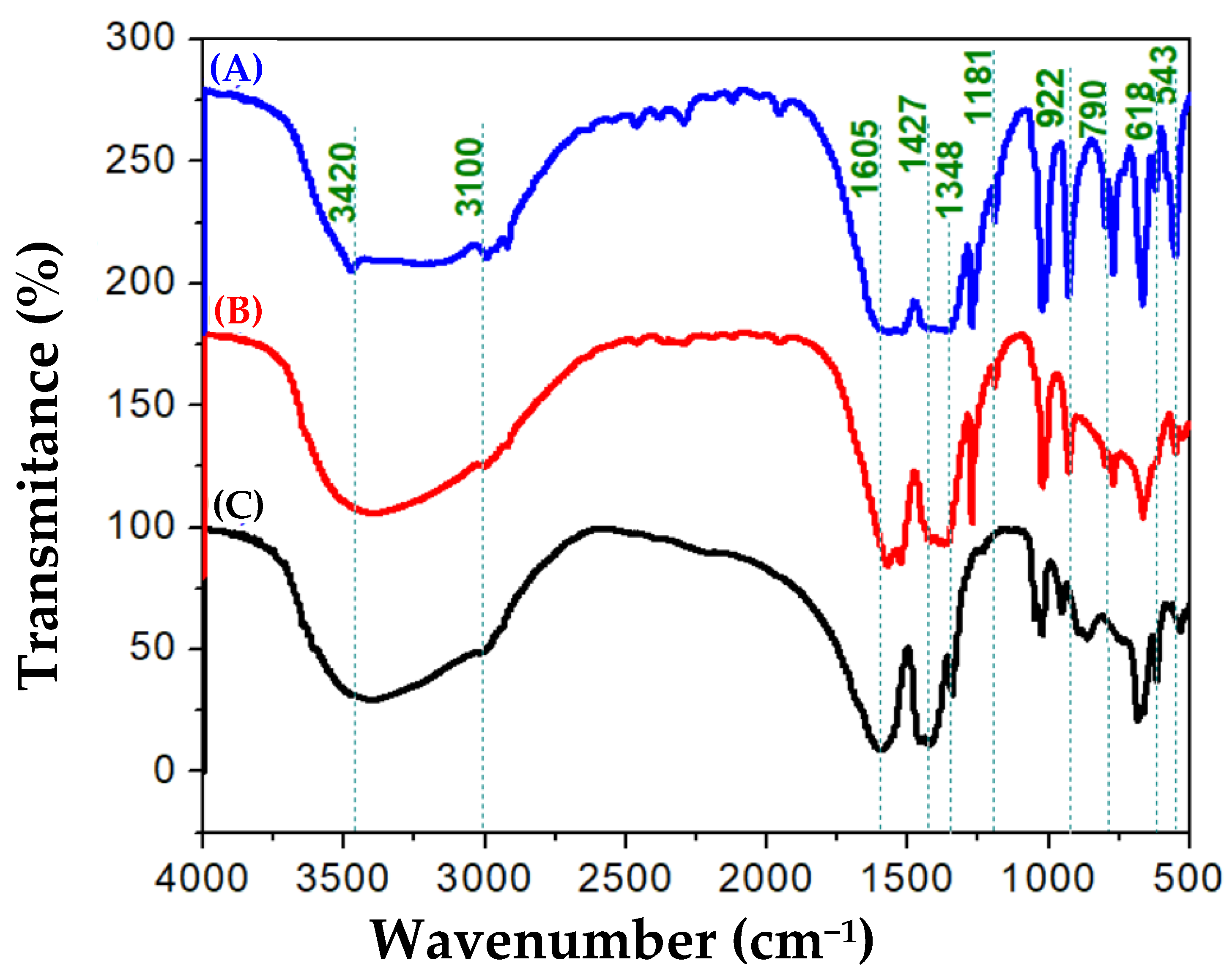
3. Results and Discussion
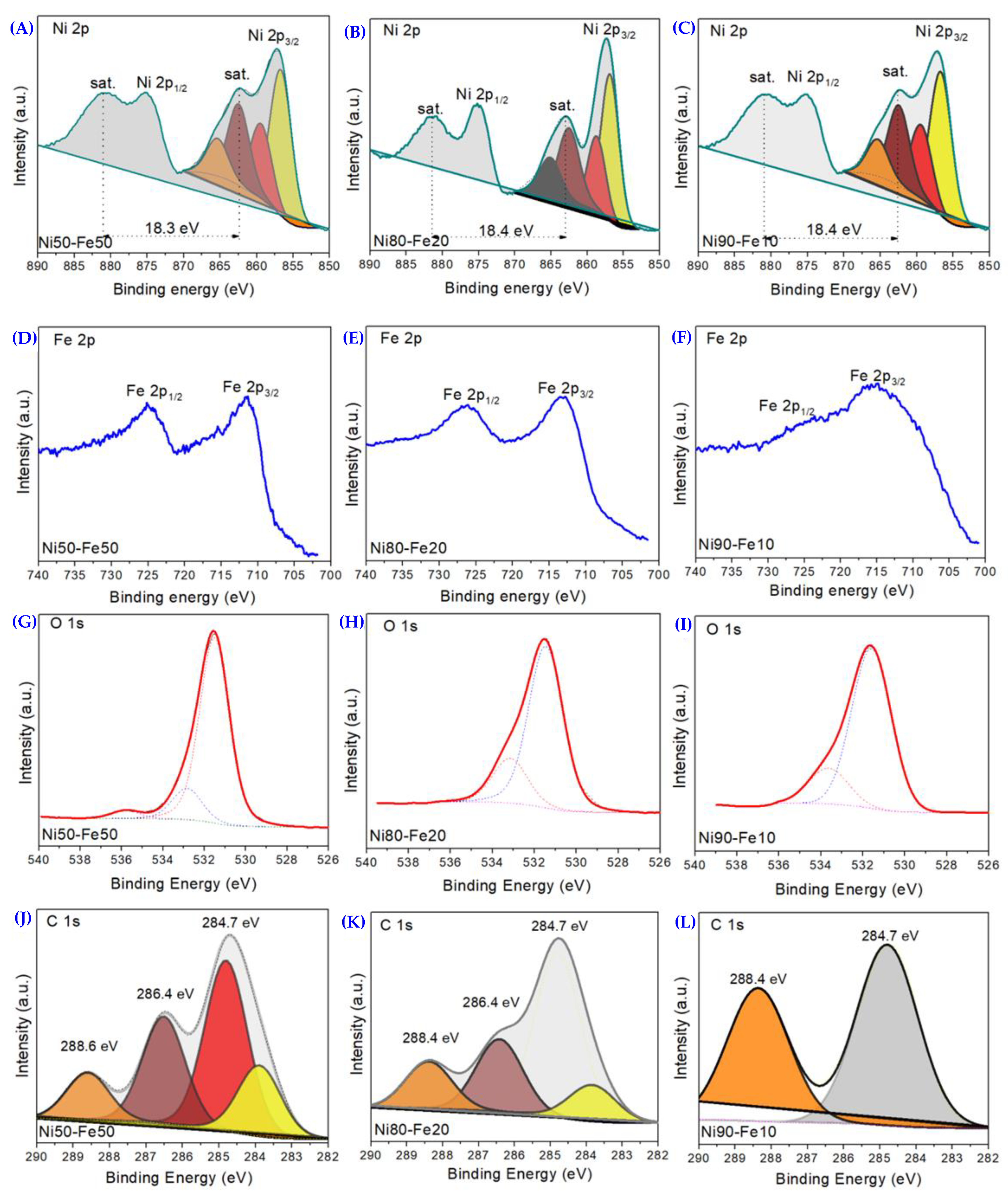
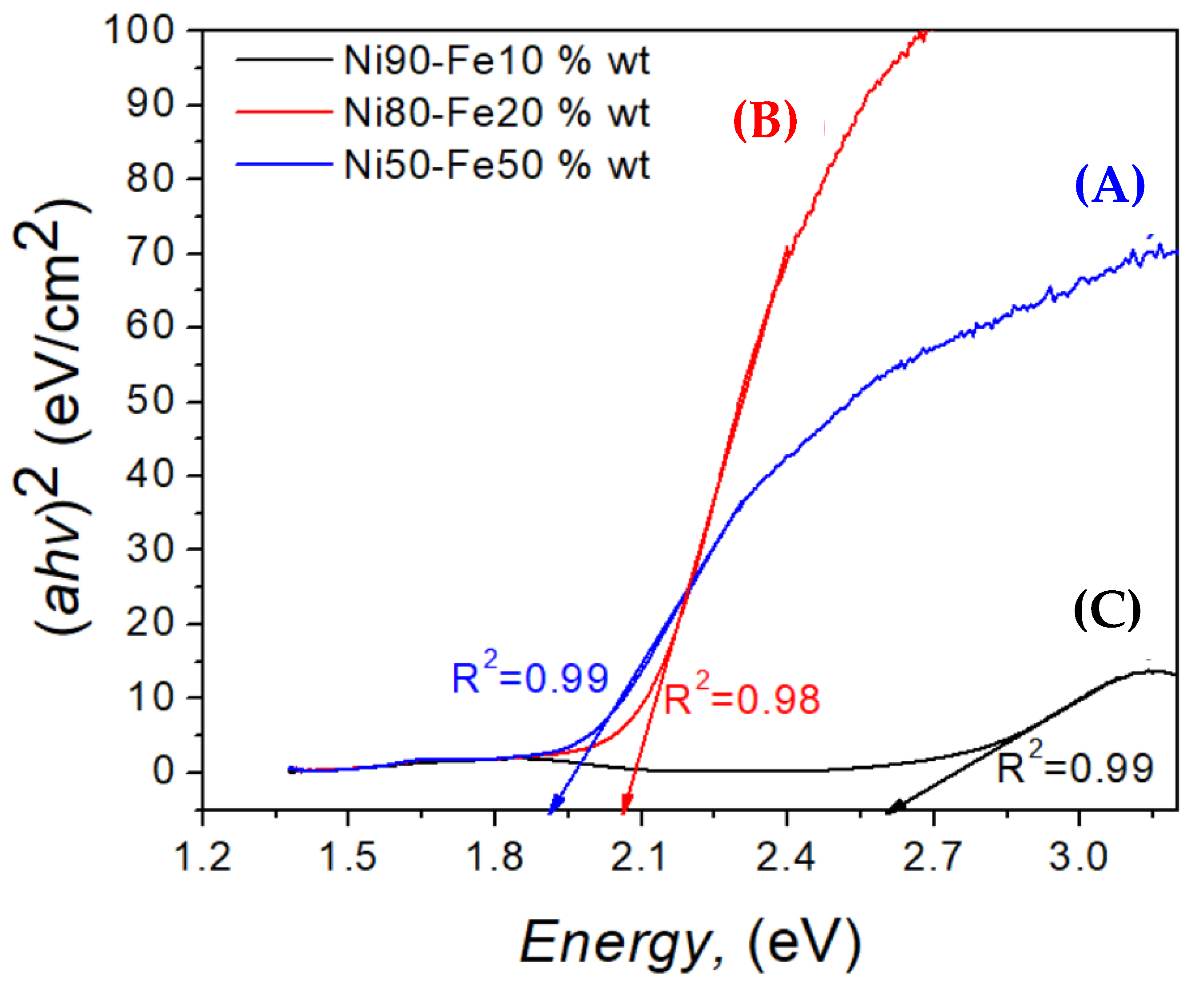
3.1. Ni50-Fe50 wt% Sample
3.2. Ni80-Fe20 wt% Sample
3.3. Ni90-Fe10 wt% Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, M.; Wang, Q.; Zhang, X.; Bao, J.; Gong, X.; Liu, Y. Two-Dimensional Transition Metal Oxide and Hydroxide-Based Hierarchical Architectures for Advanced Supercapacitor Materials. Front. Chem. 2020, 8, 390. [Google Scholar] [CrossRef]
- Hu, Z.; Xiao, X.; Jin, H.; Li, T.; Chen, M.; Liang, Z.; Guo, Z.; Li, J.; Wan, J.; Huang, L.; et al. Rapid mass production of two-dimensional metal oxides and hydroxides via the molten salts method. Nat. Commun. 2017, 8, 15630. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xu, J.; Bian, Z.; Yang, Y.; Zhang, L.; Wang, H. BiVO4 plate with Fe and Ni oxyhydroxide cocatalysts for the photodegradation of sulfadimethoxine antibiotics under visible-light irradiation. Chem. Eng. J. 2019, 389, 123426. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Youn, D.H.; Kang, K.; Lee, J.S. Highly Conformal Deposition of an Ultrathin FeOOH Layer on a Hematite Nanostructure for Efficient Solar Water Splitting. Angew. Chem. Int. Ed. 2016, 55, 10854–10858. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.I.; Serov, A.; Atanassov, P. Metal oxides/CNT nano-composite catalysts for oxygen reduction/oxygen evolu-tion in alkaline media. Appl. Catal. B Environ. 2015, 163, 623–627. [Google Scholar] [CrossRef]
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-schematic water splitting into H2 and O2 using metal sulfide as a hy-drogen-evolving photocatalyst and reduced graphene oxide as a solid-state electron mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Perez, I.; Maubert, A.M.; Rendón, L.; Santiago, P.; Herrera-Hernández, H.; Díaz-Barriga Arceo, L.; Garibay Febles, V.; González, E.P.; González-Reyes, L. Ultrasonic synthesis: Structural, optical and electrical cor-relation of TiO2 nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 8832–8847. [Google Scholar]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Ye, K.-H.; Wang, Z.; Gu, J.; Xiao, S.; Yuan, Y.; Zhu, Y.; Zhang, Y.; Mai, W.; Yang, S. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy Environ. Sci. 2017, 10, 772–779. [Google Scholar] [CrossRef]
- Chowdhury, M.; Ntiribinyange, M.; Nyamayaro, K.; Fester, V. Photocatalytic activities of ultra-small β-FeOOH and TiO2 heterojunction structure under simulated solar irradiation. Mater. Res. Bull. 2015, 68, 133–141. [Google Scholar] [CrossRef]
- Rawal, S.B.; Chakraborty, A.K.; Lee, W.I. Heterojunction of FeOOH and TiO2 for the formation of visible light photo-catalyst. Bull. Kor. Chem. Soc. 2009, 30, 2613–2616. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.M.; Pan, T.; Zhao, Y.L.; Zhang, J.Q.; Cao, C.N. The structure and electrochemical performance of spherical Al-substituted α-Ni(OH)2 for alkaline rechargeable batteries. J. Power Sour. 2005, 143, 243–255. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, Y.; He, Z.; Wang, J.; Xu, J.; Cai, J.; Zhang, N.; Zhou, H.; Fan, H.; Shao, H.; et al. Surfactant-assisted photochemical deposition of three-dimensional nanoporous nickel oxyhydroxide films and their energy storage and conversion properties. J. Mater. Chem. A 2013, 1, 4277. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Seethya, B.N.H.; Ganganagappa, N.; Eshwaraswamy, K.B.; Kakarla, R.R. Enhanced Photocatalytic Activity and Biosensing of Gadolinium Substituted BiFeO3 Nanoparticles. ChemistrySelect 2018, 3, 9025–9033. [Google Scholar] [CrossRef]
- Abdellah, A.R.; Abdelhamid, H.N.; El-Adasy, A.-B.A.; Atalla, A.A.; Aly, K.I. One-pot synthesis of hierarchical porous covalent organic frameworks and two-dimensional nanomaterials for selective removal of anionic dyes. J. Environ. Chem. Eng. 2020, 8, 104054. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Suarez-Martinez, I.; Grobert, N.; Ewels, C.P. Nomenclature of sp2 carbon nanoforms. Carbon 2012, 50, 741–747. [Google Scholar] [CrossRef]
- Quintana, M.; Grzelczak, M.; Spyrou, K.; Kooi, B.; Bals, S.; Van Tendeloo, G.; Rudolf, P.; Prato, M. Production of large graphene sheets by exfoliation of graphite under high power ultrasound in the presence of tiopronin. Chem. Commun. 2012, 48, 12159–12161. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, M.; Quintana, M.; Rudolf, P.; Zerbetto, F.; Prato, M. Rolling up a Graphene Sheet. ChemPhysChem 2013, 14, 3447–3453. [Google Scholar] [CrossRef]
- Quintana, M.; Grzelczak, M.; Spyrou, K.; Calvaresi, M.; Bals, S.; Kooi, B.; Van Tendeloo, G.; Rudolf, P.; Zerbetto, F.; Prato, M. A Simple Road for the Transformation of Few-Layer Graphene into MWNTs. J. Am. Chem. Soc. 2012, 134, 13310–13315. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Margine, E.R.; Mukai, M.; Robertson, A.W.; Giustino, F.; Kirkland, A.I. Dislocation-Driven Deformations in Graphene. Science 2012, 337, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Chuvilin, A.; Bichoutskaia, E.; Lopez, M.D.C.G.; Chamberlain, T.; Rance, G.; Kuganathan, N.; Biskupek, J.; Kaiser, U.; Khlobystov, A. Self-assembly of a sulphur-terminated graphene nanoribbon within a single-walled carbon nanotube. Nat. Mater. 2011, 10, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Chuvilin, A.; Kaiser, U.; Bichoutskaia, E.; Besley, N.; Khlobystov, A. Direct transformation of graphene to fullerene. Nat. Chem. 2010, 2, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, L.A.L.; Bao, Q.; Lin, M.; Deng, S.; Goh, B.M.; Loh, K.P. Room-Temperature Synthesis of Soluble Carbon Nanotubes by the Sonication of Graphene Oxide Nanosheets. J. Am. Chem. Soc. 2009, 131, 16832–16837. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Guan, J.; Zhang, Q. Goethite hierarchical nanostructures: Glucose-assisted synthesis, chemical conversion into hematite with excellent photocatalytic properties. Mater. Chem. Phys. 2011, 127, 371–378. [Google Scholar] [CrossRef]
- Kang, S.; Wang, G.; Fang, M.; Wang, H.; Wang, X.; Cai, W. Water bath synthesis and enhanced photocatalytic performances of urchin-like micro/nanostructured α-FeOOH. J. Mater. Res. 2015, 30, 1629–1638. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Pinto, P.; Lanza, G.; Ardisson, J.; Lago, R. Controlled Dehydration of Fe(OH)3 to Fe2O3: Developing Mesopores with Complexing Iron Species for the Adsorption of β-Lactam Antibiotics. J. Braz. Chem. Soc. 2018, 30, 307–371. [Google Scholar] [CrossRef]
- Cheng, M.-Y.; Hwang, B.-J. Control of uniform nanostructured α-Ni(OH)2 with self-assembly sodium dodecyl sulfate templates. J. Colloid Interface Sci. 2009, 337, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Saghatforoush, L.A.; Hasanzadeh, M.; Sanati, S.; Mehdizadeh, R. Ni(OH)2and NiO Nanostructures: Synthesis, Characterization and Electrochemical Performance. Bull. Korean Chem. Soc. 2012, 33, 2613–2618. [Google Scholar] [CrossRef]
- Vidotti, M.; Salvador, R.P.; Ponzio, E.; de Torresi, S.C. Mixed Ni/Co hydroxide nanoparticles synthesized by sonochemical method. J. Nanosci. Nanotechnol. 2007, 7, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Bantignies, J.L.; Deabate, S.; Righi, A.; Rols, S.; Hermet, P.; Sauvajol, J.L.; Henn, F. New Insight into the Vibrational Behavior of Nickel Hydroxide and Oxyhydroxide Using Inelastic Neutron Scattering, Far/Mid-Infrared and Raman Spectroscopies. J. Phys. Chem. C 2008, 112, 2193–2201. [Google Scholar] [CrossRef]
- Cataldo, F.; García-Hernández, D.A.; Manchado, A. Sonochemical Synthesis of Fullerene C60/Anthracene Diels-Alder Mono and Bis-adducts. Full-Nanotub. Carbon Nanostruct. 2014, 22, 565–574. [Google Scholar] [CrossRef]
- Hare, J.P.; Dennis, T.J.; Kroto, H.W.; Taylor, R.; Allaf, A.W.; Balm, S.; Walton, D.R.M. The IR spectra of fullerene-60 and -70. J. Chem. Soc., Chem. Commun. 1991, 6, 412–413. [Google Scholar] [CrossRef]
- Alves, G.C.; Ladeira, L.O.; Righi, A.; Krambrock, K.; Calado, H.D.; Gil, R.P.D.F.; Pinheiro, M. Synthesis of C60(OH)18-20 in aqueous alkaline solution under O2-atmosphere. J. Braz. Chem. Soc. 2006, 17, 1186–1190. [Google Scholar] [CrossRef][Green Version]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Lee, J.W.; Ahn, T.; Soundararajan, D.; Ko, J.M.; Kim, J.-D. Non-aqueous approach to the preparation of reduced graphene oxide/α-Ni(OH)2 hybrid composites and their high capacitance behavior. Chem. Commun. 2011, 47, 6305–6307. [Google Scholar] [CrossRef]
- Abdel-Samad, H.; Watson, P.R. An XPS study of the adsorption of lead on goethite (α-FeOOH). Appl. Surf. Sci. 1998, 136, 46–54. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Li, C. Beta-FeOOH-supported graphitic carbon nitride as an efficient visible light photocatalyst. J. Mol. Catal. A Chem. 2016, 423, 463–471. [Google Scholar] [CrossRef]
- Chagas, P.; Da Silva, A.C.; Passamani, E.C.; Ardisson, J.D.; De Oliveira, L.C.A.; Fabris, J.D.; Paniago, R.M.; Monteiro, D.S.; Pereira, M.C. δ-FeOOH: A superparamagnetic material for controlled heat release under AC magnetic field. J. Nanoparticle Res. 2013, 15, 1544. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, Y.; Wang, T.; Zhu, P.-L.; Yu, S.; Yu, Y.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Electrochemical fabrication of Ni(OH)2/Ni 3D porous composite films as integrated capacitive electrodes. RSC Adv. 2015, 5, 12931–12936. [Google Scholar] [CrossRef]
- Sherman, D.M. Electronic structures of iron(III) and manganese(IV) (hydr)oxide minerals: Thermodynamics of photochemical reductive dissolution in aquatic environments. Geochim. Cosmochim. Acta 2005, 69, 3249–3255. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.; Shi, X.; Zeng, H.; Xia, H. Amorphous FeOOH Quantum Dots Assembled Mesoporous Film Anchored on Graphene Nanosheets with Superior Electrochemical Performance for Supercapacitors. Adv. Funct. Mater. 2015, 26, 919–930. [Google Scholar] [CrossRef]
- Cao, T.; Huang, P.; Zhang, K.; Sun, Z.; Zhu, K.; Yuan, L.; Chen, K.; Chen, N.; Li, Y. Interfacial engineering via inserting functionalized water-soluble fullerene derivative interlayers for enhancing the performance of perovskite solar cells. J. Mater. Chem. A 2018, 6, 3435–3443. [Google Scholar] [CrossRef]
- Zheng, J.-P.; Zhen, M.-M.; Ge, J.-C.; Liu, Q.-L.; Jiang, F.; Shu, C.-Y.; Alhadlaq, H.A.; Wang, C.-R. Multifunctional gadofulleride nanoprobe for magnetic resonance imaging/fluorescent dual modality molecular imaging and free radical scavenging. Carbon 2013, 65, 175–180. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Zeng, J.; Wang, T.; Ali, A.; Zeng, H. Boosting the photocatalytic H2 evolution activity of Fe2O3 polymorphs (α-, γ- and β-Fe2O3) by fullerene [C60]-modification and dye-sensitization under visible light irradiation. RSC Adv. 2017, 7, 29184–29192. [Google Scholar] [CrossRef]
- Shaheen, N.; Yousuf, M.A.; Shakir, I.; Zulfiqar, S.; Agboola, P.O.; Warsi, M.F. Wet chemical route synthesis of spinel oxide nano-catalysts for photocatalytic applications. Phys. B Condens. Matter 2019, 580, 411820. [Google Scholar] [CrossRef]
- Yu, C.; Li, G.; Wei, L.; Fan, Q.; Shu, Q.; Yu, J. Fabrication, characterization of β-MnO2 microrod catalysts and their performance in rapid degradation of dyes of high concentration. Catal. Today 2014, 224, 154–162. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Reddy, K.R.; Jaesool, S.; Yoo, K. Template-free synthesis of tetragonal Co-doped ZrO2 nanoparticles for applications in electrochemical energy storage and water treatment. Electrochim. Acta 2019, 317, 416–426. [Google Scholar] [CrossRef]
- Warsi, M.F.; Shaheen, N.; Sarwar, M.I.; Agboola, P.O.; Shakir, I.; Zulfiqar, S. A comparative study on photocatalytic activities of various transition metal oxides nanoparticles synthesized by wet chemical route. Desalination Water Treat 2021, 211, 181–195. [Google Scholar] [CrossRef]
- Rauf, M.; Meetani, M.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.; Al-Mur, B.A.; Alseroury, F.A.; Eniola, J. Photocatalytic degradation of cefoxitin sodium antibiotic using novel BN/CdAl2O4 composite. J. Clean. Prod. 2019, 246, 119076. [Google Scholar] [CrossRef]
- Potle, V.D.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K. Sonochemical preparation of ternary rGO-ZnO-TiO2 nanocomposite photocatalyst for efficient degradation of crystal violet dye. Optik 2020, 208, 164555. [Google Scholar] [CrossRef]
- Tabatabaeinejad, S.M.; Amiri, O.; Ghanbari, M.; Salavati-Niasari, M. Dy2Cu2O5 nanostructures: Sonochemical fabrication, characterization, and investigation of photocatalytic ability for elimination of organic contaminants. J. Mol. Liq. 2021, 344, 117883. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, J.; Rani, P.; Kaur, N.; Kumar, S.; Rawat, M. A novel and one-pot synthesis of Punica granatum mediated copper oxide having flower-like morphology as an efficient visible-light driven photocatalyst for degradation of textile dyes in waste water. J. Mol. Liq. 2022, 355, 118966. [Google Scholar] [CrossRef]
- Tabatabaeinejad, S.M.; Ghanbari, M.; Najm, Z.M.; Abdul-Fattah, M.N.; Hameed, N.M.; Salavati-Niasari, M. Facile sonochemical preparation of La2Cu2O5 nanostructures, characterization, the evaluation of performance, mechanism, and kinetics of photocatalytic reactions for the removal of toxic pollutants. J. Mol. Liq. 2022, 362, 119718. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Baladi, M.; Salavati-Niasari, M. Sono-synthesis of MnWO4 ceramic nanomaterials as highly efficient photocatalysts for the decomposition of toxic pollutants. Ceram. Int. 2021, 47, 30178–30187. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Karimi, F.; Kazemdehdashti, H.; Kavoshi, L. Fast sonochemically-assisted synthesis of pure and doped zinc sulfide quantum dots and their applicability in organic dye removal from aqueous media. J. Photochem. Photobiol. B Biol. 2018, 181, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zinatloo-Ajabshir, S.; Mortazavi-Derazkola, S.; Salavati-Niasari, M. Simple sonochemical synthesis of Ho2O3-SiO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminant. Ultrason. Sonochem. 2017, 39, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Shende, T.; Bhanvase, B.; Rathod, A.; Pinjari, D.; Sonawane, S. Sonochemical synthesis of Graphene-Ce-TiO2 and Graphene-Fe-TiO2 ternary hybrid photocatalyst nanocomposite and its application in degradation of crystal violet dye. Ultrason. Sonochem. 2017, 41, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Talebzadeh, Z.; Masjedi-Arani, M.; Amiri, O.; Salavati-Niasari, M. La2Sn2O7/g-C3N4 Nanocomposites: Rapid and Green Sonochemical Fabrication and Photo-Degradation Performance for Removal of Dye Contaminations. Ultrason. Sonochem. 2021, 77, 105678. [Google Scholar] [CrossRef]
- Singh, A.R.; Dhumal, P.S.; Bhakare, M.A.; Lokhande, K.D.; Bondarde, M.P.; Some, S. In-situ synthesis of metal oxide and polymer decorated activated carbon-based photocatalyst for organic pollutants degradation. Sep. Purif. Technol. 2022, 286, 120380. [Google Scholar] [CrossRef]
- Al Kausor, M.; Chakrabortty, D. Graphene oxide based semiconductor photocatalysts for degradation of organic dye in waste water: A review on fabrication, performance enhancement and challenges. Inorg. Chem. Commun. 2021, 129, 108630. [Google Scholar] [CrossRef]
- Mahdavi, K.; Salavati-Niasari, M.; Amiri, O.; Ghanbari, M. Synthesis of La9.33Si6O26 nano-photocatalysts by ultrasonically accelerated method for comparing water treatment efficiency with changing conditions. Arab. J. Chem. 2021, 15, 103481. [Google Scholar] [CrossRef]
- Abkar, E.; Izadi, E.; Amiri, O.; Ghanbari, M.; Salavati-Niasari, M. Sonochemical synthesis and characterization of Cu2HgI4 nanostructures photocatalyst with enhanced visible light photocatalytic ability. Arab. J. Chem. 2021, 15, 103536. [Google Scholar] [CrossRef]
- Uma, K.; KrishnaKumar, B.; Pan, G.-T.; Yang, T.C.-K.; Lin, J.-H. Enriched silver plasmon resonance activity on the sonochemical synthesis of ZnO flowers with α-Fe2O3 as an efficient catalyst for photo-Fenton reaction and photo-oxidation of ethanol. J. Water Process Eng. 2019, 34, 101089. [Google Scholar] [CrossRef]
- Nur, A.S.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Purkayastha, M.D.; Sil, S.; Singh, N.; Ray, P.P.; Darbha, G.K.; Bhattacharyya, S.; Mallick, A.I.; Majumder, T.P. Sonochemical synthesis of nanospherical TiO2 within graphene oxide nanosheets and its application as a photocatalyst and a Schottky diode. FlatChem. 2020, 22, 100180. [Google Scholar] [CrossRef]
- Dhivya, A.; Rakhi Yadav Stella Packiam, C. An Eco-approach synthesis of undoped and Mn doped ZnO nano-photocatalyst for prompt decoloration of methylene blue dye. Mater. Today Proc. 2021, 48, 494–501. [Google Scholar] [CrossRef]
- Karuppasamy, P.; Nisha, N.R.N.; Pugazhendhi, A.; Kandasamy, S.; Pitchaimuthu, S. An investigation of transition metal doped TiO2 photocatalysts for the enhanced photocatalytic decoloration of methylene blue dye under visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105254. [Google Scholar] [CrossRef]
- Lerici, L.C.; Marchena, C.L.; Torres, C.C.; Campos, C.H.; Pierella, L.B. Fe-doped Al2O3 nanoplatforms as efficient and recyclable photocatalyst for the dyes remediation. J. Photochem. Photobiol. A Chem. 2021, 426, 113733. [Google Scholar] [CrossRef]
- Goto, T.; Cho, S.H.; Ohtsuki, C.; Sekino, T. Selective adsorption of dyes on TiO2-modified hydroxyapatite photocatalysts morphologically controlled by solvothermal synthesis. J. Environ. Chem. Eng. 2021, 9, 105738. [Google Scholar] [CrossRef]
- Kara, G.K.; Moshari, M.; Rabbani, M.; Rahimi, R. A novel and green heterogeneous photocatalytic system (Ca0.01Fe2.99O4/CaTiO3 nanocomposite): Protocol synthesis, characterization, and study of photo-decoloration activity. Mater. Chem. Phys. 2020, 259, 124062. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kumar, K.; Singh, K.; Bhattacharya, S. Fast decolorization of rhodamine-B dye using novel V2O5-rGO photocatalyst under solar irradiation. Synth. Met. 2021, 283, 116981. [Google Scholar] [CrossRef]
- Rajaitha, P.; Hajra, S.; Sahu, M.; Mistewicz, K.; Toroń, B.; Abolhassani, R.; Panda, S.; Mishra, Y.; Kim, H. Unraveling highly efficient nanomaterial photocatalyst for pollutant removal: A comprehensive review and future progress. Mater. Today Chem. 2021, 23, 100692. [Google Scholar] [CrossRef]
- Monsef, R.; Ghiyasiyan-Arani, M.; Amiri, O.; Salavati-Niasari, M. Sonochemical synthesis, characterization and application of PrVO4 nanostructures as an effective photocatalyst for discoloration of organic dye contaminants in wastewater. Ultrason. Sonochemistry 2019, 61, 104822. [Google Scholar] [CrossRef]
- Haghighi, P.; Alijani, S.; Bazyari, A.; Thompson, L.T. Visible light dye degradation over fluorinated mesoporous TiO2− WO3− Bi2O3/SiO2 nanocomposite photocatalyst-adsorbent using immersion well reactor. J. Photochem. Photobiol. A Chem. 2022, 426, 113790. [Google Scholar] [CrossRef]
- Deepak, N.; Pandey, A.; Shukla, S.; Saxena, S. Siloxene: A novel 2D photocatalyst for degradation of dye molecules. Nano-Struct. Nano-Objects 2021, 26, 100721. [Google Scholar] [CrossRef]
- Krishnegowda, J.; Ramesh, A.M.; Gangadhar, A.; Shivanna, S. Hydrothermal processing of interfacial BiCeO3/MWCNTs photocatalyst for rapid dye degradation and its biological interest. J. Environ. Chem. Eng. 2021, 9, 105774. [Google Scholar] [CrossRef]
- Ge, Y.; Luo, H.; Huang, J.; Zhang, Z. Visible-light-active TiO2 photocatalyst for efficient photodegradation of organic dyes. Opt. Mater. 2021, 115, 111058. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Yi, C. Preparation of photo-Fenton heterogeneous catalyst (Fe-TS-1 zeolite) and its application in typical azo dye decoloration. J. Photochem. Photobiol. A Chem. 2018, 356, 138–149. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W.-C. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 12, 5759. [Google Scholar] [CrossRef]
- Kumar, M.R.A.; Abebe, B.; Nagaswarupa, H.P.; Murthy, H.C.A.; Ravikumar, C.R.; Sabir, F.K. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: Its applications in dye decolorization and as supercapacitors. Sci. Rep. 2020, 10, 1249. [Google Scholar] [CrossRef]
- Biglar, F.; Talaiekhozani, A.; Aminsharei, F.; Park, J.; Barghi, A.; Rezania, S. Application of ZnO-Nd Nano-Photocatalyst for the Reactive Red 198 Dye Decolorization in the Falling-Film Photocatalytic Reactor. Toxics 2021, 9, 254. [Google Scholar] [CrossRef]
- Raj, S.; Singh, H.; Trivedi, R.; Soni, V. Biogenic synthesis of AgNPs employing Terminalia arjuna leaf extract and its efficacy towards catalytic degradation of organic dyes. Sci. Rep. 2020, 10, 9616. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Jia, Y.; Zhou, G. Kinetics of the acid orange 7 degradation in the photocatalytic system of UV/H2O2/TS-1. J. Water Process Eng. 2017, 19, 106–111. [Google Scholar] [CrossRef]
- Monsef, R.; Soofivand, F.; Alshamsi, H.A.; Al-Nayili, A.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Sonochemical synthesis and characterization of PrVO4/CdO nanocomposite and their application as photocatalysts for removal of organic dyes in water. J. Mol. Liq. 2021, 336, 116339. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Ravikumar, S.; Pandiyan, V.; Nithya, V.; Sylvestre, S.; Sivakumar, P.; Surya, C.; John, N.A.A.; Sobral, A.J. Synthesis, characterization of porphyrin and CdS modified spherical shaped SiO2 for Reactive Red 120 degradation under direct sunlight. J. Mol. Struct. 2020, 1210, 128021. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Hariharan, R.; Pandiyan, V.; Aguiar, A.; Abilio, S.J.F.N. Gelatin-assisted g-TiO2/BiOI heterostructure nanocomposites for azo dye degradation under visible light. J. Environ. Chem. Eng. 2018, 6, 4282–4288. [Google Scholar] [CrossRef]
- Alghamdi, Y.G.; Krishnakumar, B.; Malik, M.A.; Alhayyani, S. Design and Preparation of Biomass-Derived Activated Carbon Loaded TiO2 Photocatalyst for Photocatalytic Degradation of Reactive Red 120 and Ofloxacin. Polymers 2022, 14, 880. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, B.; Swaminathan, M. Solar Photocatalytic Degradation of Acid Black 1 with ZnO. Indian J. Chem. 2010, 49, 1035–1040. [Google Scholar] [CrossRef]


| Material | Synthesis | Morphology | Dye | Ref. |
|---|---|---|---|---|
| Ni-Fe-C | Sonochemical | Laminar morphology | RB5 | PW |
| rGO-ZnO-TiO2 | Sonochemical | Nanocomposite particles | MV | [56] |
| Dy2Cu2O5 | Sonochemical | Nanostructures | PR | [57] |
| Copper oxide | Green synthesis | Flowers | RO4, RY86 | [58] |
| La2Cu2O5 | Sonochemical | Spherical Porous nanostructures | AB | [59] |
| MnWO4 | Sonochemical | Nanostructures | AY23, MV | [60] |
| ZnS QDs Cu2+, Mn2+, Ag+ dopped | Sonochemical | Sphere | VBR | [61] |
| Ho2O3-SiO2 nanocomposites | Sonochemical | Nanocomposites | MB | [62] |
| Graphene-Ce-TiO2, Graphene-Fe-TiO2 | Sonochemical | Nanosheets | MV | [63] |
| La2Sn2O7/g-C3N4 | Sonochemical | Nanostructures | E, MV | [64] |
| ZnO NPs, activated carbon and polypyrrole (ZCP) | In situ synthesis | Nanocomposite | MB | [65] |
| Graphene oxide based semiconductor | Several methods | Nanostructures | Several dyes | [66] |
| La9.33Si6O26 | Sonochemical | Nanoparticles | AR14 | [67] |
| Cu2HgI4 | Sonochemical | Nanostructures | AB1, MO, EO, MB, MV, RB | [68] |
| ZnO-α-Fe2O3 | Sonochemical | Flowers | MB | [69] |
| Metal doped TiO2 | Several methods | Several shape | Several dyes | [70] |
| graphene oxide- TiO2 | Sonochemical | Nanosheets + nanospheres | MO, CR, PN | [71] |
| Mn doped ZnO | Green synthesis | Spherical flakes | MB | [72] |
| Transition metal doped TiO2 | Sol-gel | Several shapes | MB | [73] |
| Fe-doped Al2O3 | Hydrothermal | Nanoplatforms | MO | [74] |
| TiO2-modified hydroxyapatite | Solvothermal | Needle-shaped structures | MB, AF | [75] |
| Ca0.01Fe2.99O4/ CaTiO3 (CF/CT NC) | Solvothermal | Nanocomposite, nanospheres, rectangular prism | MB | [76] |
| V2O5-rGO | Hydrothermal | Nanocomposite | RB | [77] |
| Metal oxides, chalcogenides, and chalcohalides, Perovskite materials, Carbon based materials, Metal-Organic Frameworks (MOFs) | Several methods | Several shapes | Several dyes | [78] |
| PrVO4 | Several methods | Nanoparticles | EBT, E, MV | [79] |
| TiO2 − WO3 − Bi2O3/SiO2 | Hydrothermal | Sphere-like mesoporous nanoparticles | RB | [80] |
| siloxene | Chemical exfoliation | Nanosheets | MB, AA, AB, MG | [81] |
| BiCeO3/MWCNTs (BCM) | Hydrothermal | Nanotubes | MR | [82] |
| TiO2 | Solvothermal | Nanostructures | RB, MB, EY | [83] |
| Fe-TS-1 zeolite | Quick synthesis | Nanospheres | AO7 | [84] |
| CdS/TiO2 | Precipitation | Spherical nanoclusters | AB | [85] |
| TiO2-Fe2O3 | Green synthesis | Nanospheres | TY, MO | [86] |
| ZnO-Nd | Solution combustion | Nanoporous particles | RR198 | [87] |
| AgNPs | Green synthesis | Nanospheres | MO, MB, CR, 4N | [88] |
| UV/H2O2/TS-1 | Quick synthesis | Powder | AO7 | [89] |
| PrVO4/CdO | Sonochemical | Nanostructures | MB, E | [90] |
| CdS/SiO2 composite | Sol-gel | Nanospheres | RR120 | [91] |
| g-TiO2/BiOI | Sol-gel | Nanospheres | AB 1 | [92] |
| AC-TiO2 | Sol-gel | Nanostructures | RR120 | [93] |
| ZnO | Purchsed Merck | Microstructures | AB 1 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garibay Febles, V.; Melo-Máximo, D.V.; Hernández Perez, I.; Suárez Parra, R.; Espinoza-Tapia, J.C.; Luna Paz, R.; Diaz-Barriga Arceo, L.; Rivera Olvera, J.N.; Melo-Máximo, L.; González-Reyes, L. Sonochemical Synthesis of Nanostructured Ni-Fe-C System and Its Catalytic Activity Based on Decolorization of Reactive Black 5 Dye. Crystals 2022, 12, 1123. https://doi.org/10.3390/cryst12081123
Garibay Febles V, Melo-Máximo DV, Hernández Perez I, Suárez Parra R, Espinoza-Tapia JC, Luna Paz R, Diaz-Barriga Arceo L, Rivera Olvera JN, Melo-Máximo L, González-Reyes L. Sonochemical Synthesis of Nanostructured Ni-Fe-C System and Its Catalytic Activity Based on Decolorization of Reactive Black 5 Dye. Crystals. 2022; 12(8):1123. https://doi.org/10.3390/cryst12081123
Chicago/Turabian StyleGaribay Febles, Vicente, Dulce Viridiana Melo-Máximo, Isaías Hernández Perez, Raúl Suárez Parra, Julio César Espinoza-Tapia, Ricardo Luna Paz, Lucia Diaz-Barriga Arceo, Jesús Noé Rivera Olvera, Lizbeth Melo-Máximo, and Leonardo González-Reyes. 2022. "Sonochemical Synthesis of Nanostructured Ni-Fe-C System and Its Catalytic Activity Based on Decolorization of Reactive Black 5 Dye" Crystals 12, no. 8: 1123. https://doi.org/10.3390/cryst12081123
APA StyleGaribay Febles, V., Melo-Máximo, D. V., Hernández Perez, I., Suárez Parra, R., Espinoza-Tapia, J. C., Luna Paz, R., Diaz-Barriga Arceo, L., Rivera Olvera, J. N., Melo-Máximo, L., & González-Reyes, L. (2022). Sonochemical Synthesis of Nanostructured Ni-Fe-C System and Its Catalytic Activity Based on Decolorization of Reactive Black 5 Dye. Crystals, 12(8), 1123. https://doi.org/10.3390/cryst12081123





