An Overview on the Process Development and the Formation of Non-Dendritic Microstructure in Semi-Solid Processing of Metallic Materials
Abstract
1. Introduction
2. Process Development for Component Manufacturing
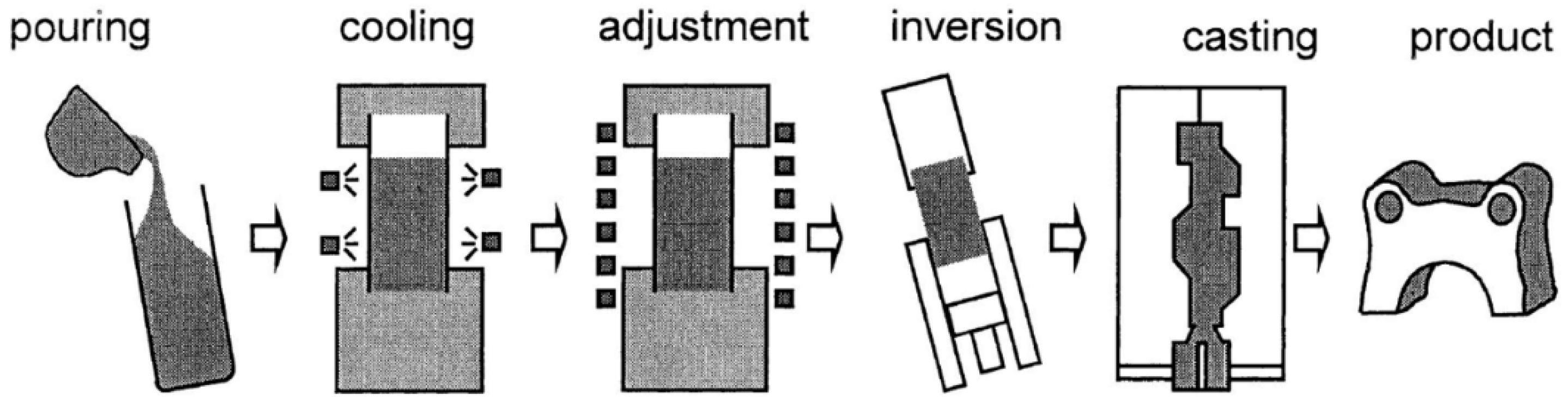
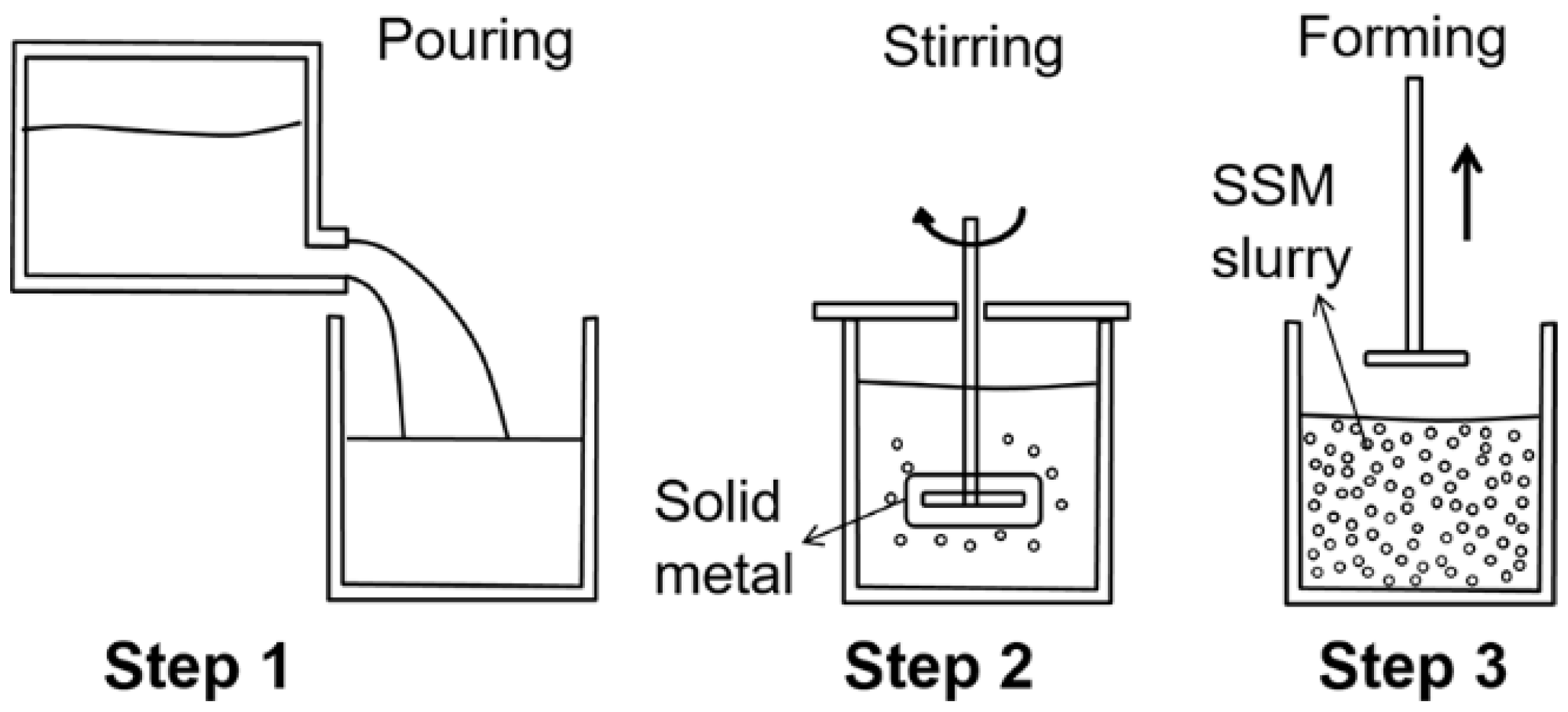
2.1. Rheocasting
2.2. Thixoforming
2.3. Rheomoulding
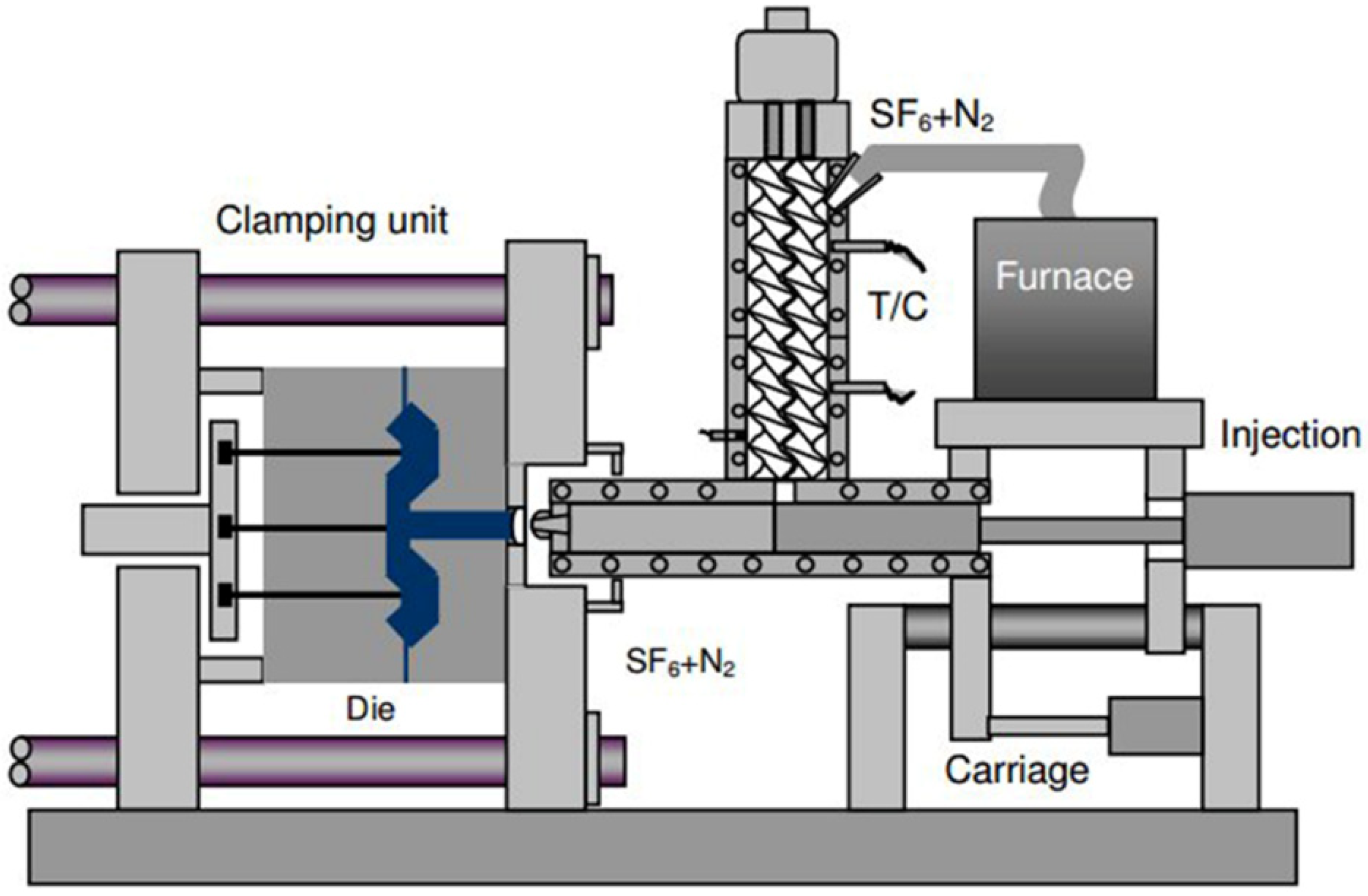
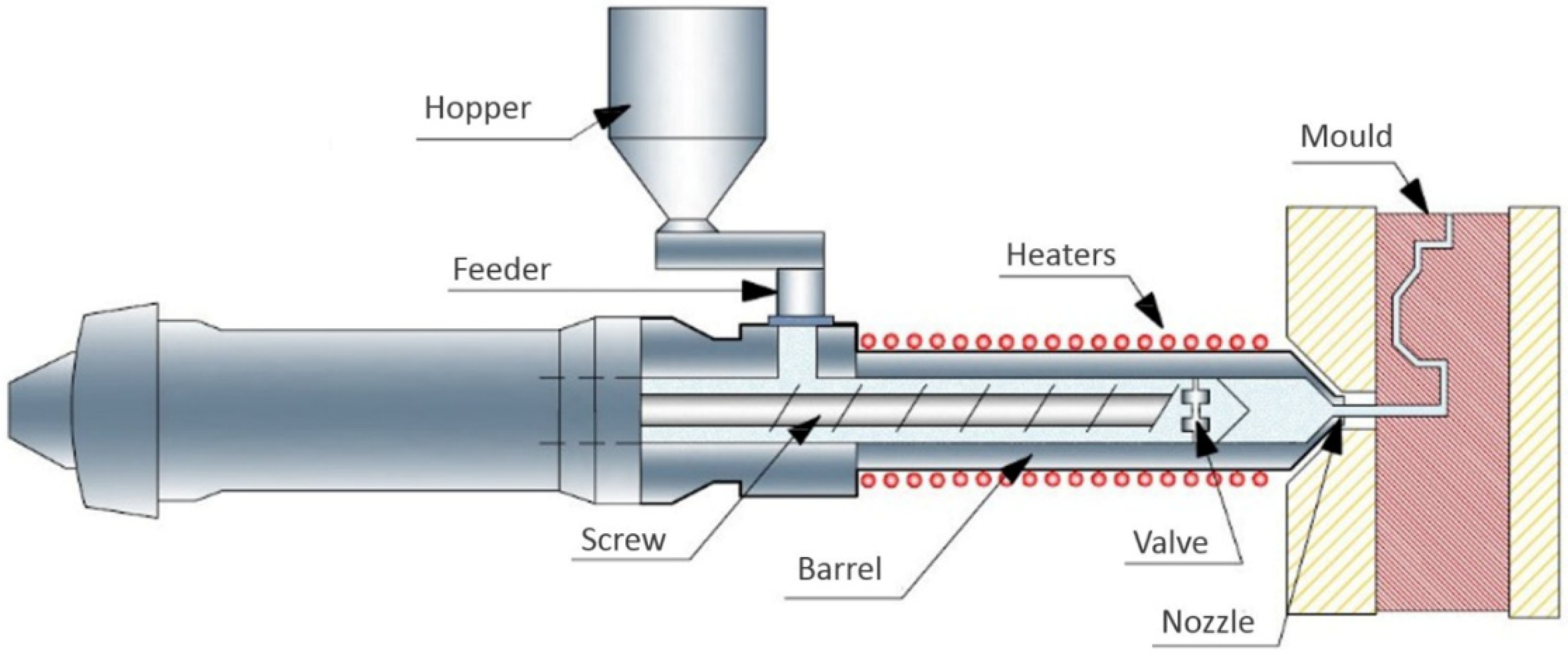
2.4. Thixomoulding
2.5. Thixojoining
2.6. Rheo-Extrusion and Thixo-Extrusion
2.7. Semi-Solid Twin Roll Casting
2.8. Rheo-Mixing
3. Manufacturing Method for Non-Dendritic Slurry and Feedstock
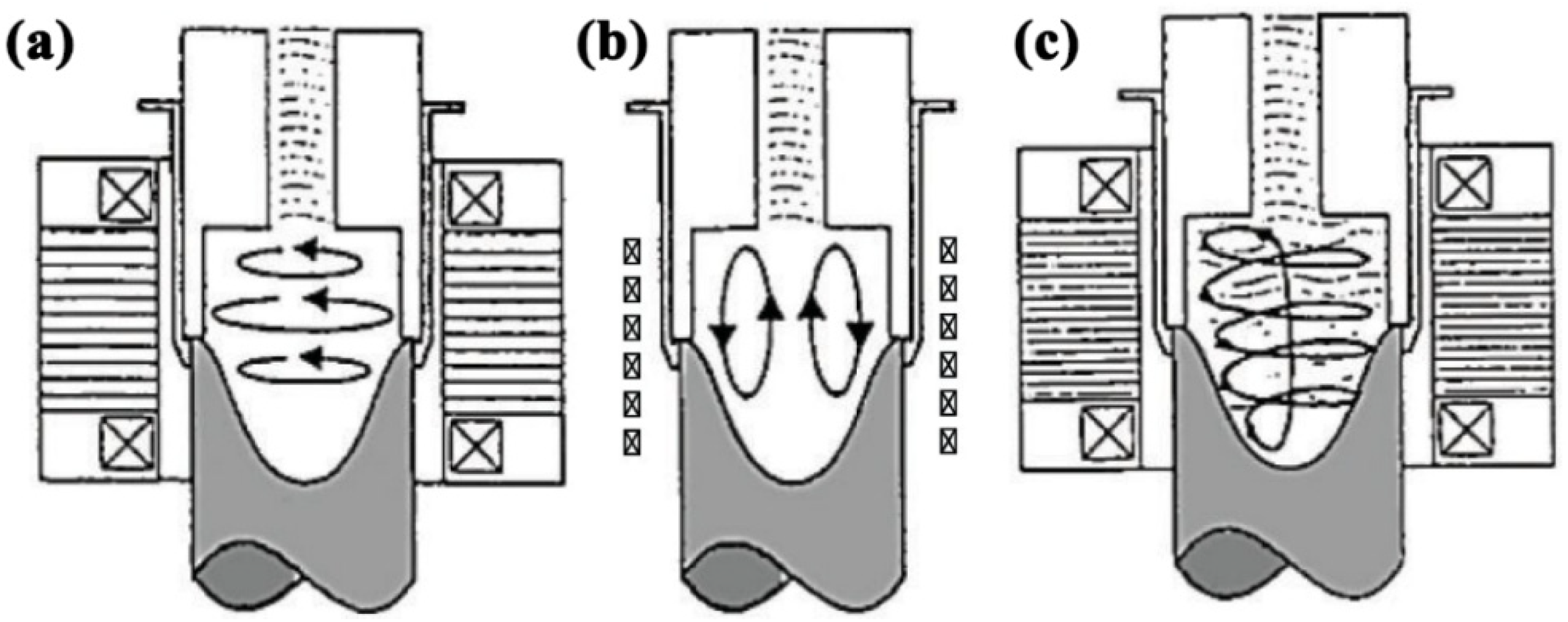
3.1. Mechanical Stirring
3.2. Magnetohydrodynamic Stirring
3.3. Slope Cooling Plate (SCP)
3.4. Continuous Rheoconversion Process (CRP)
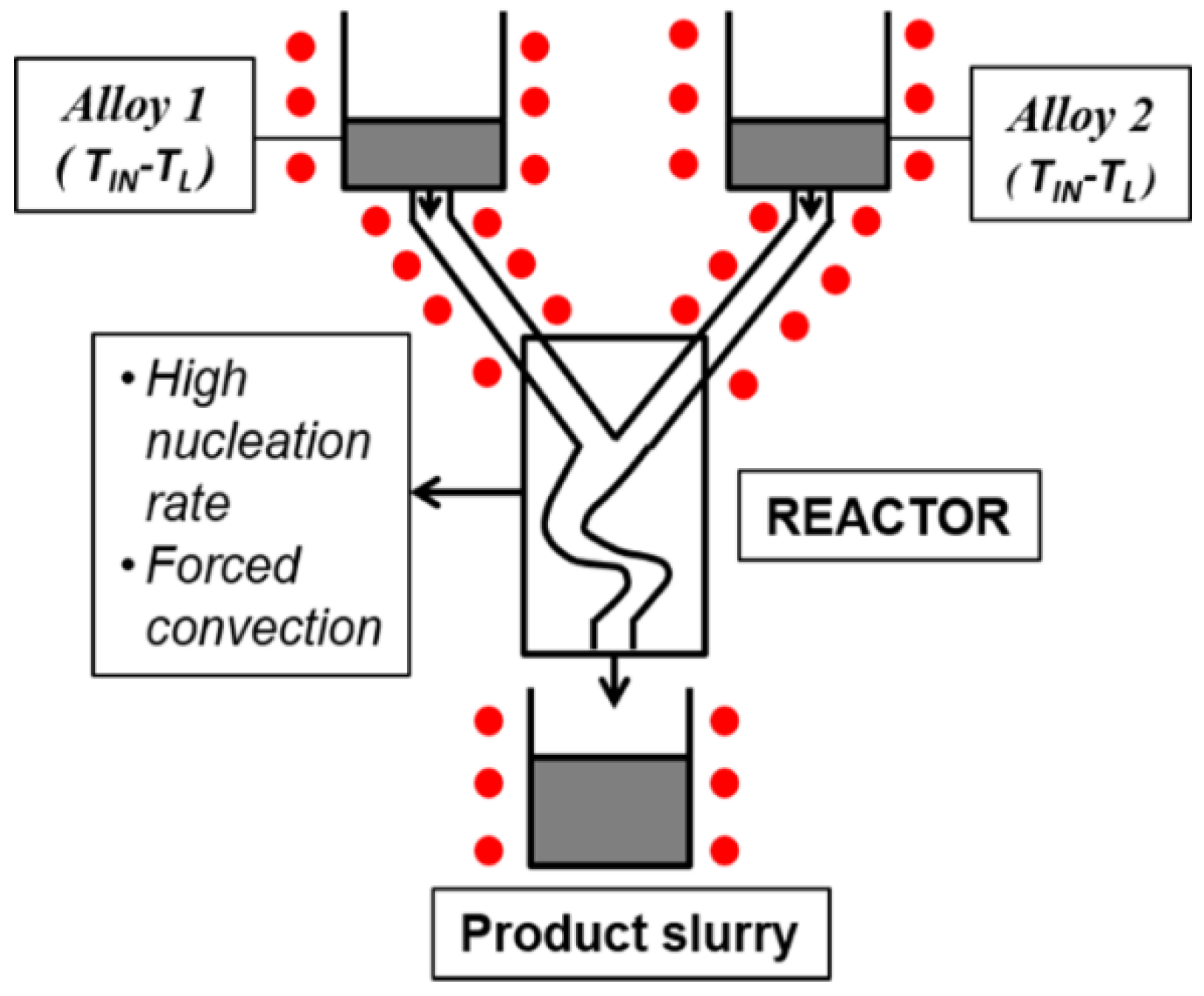
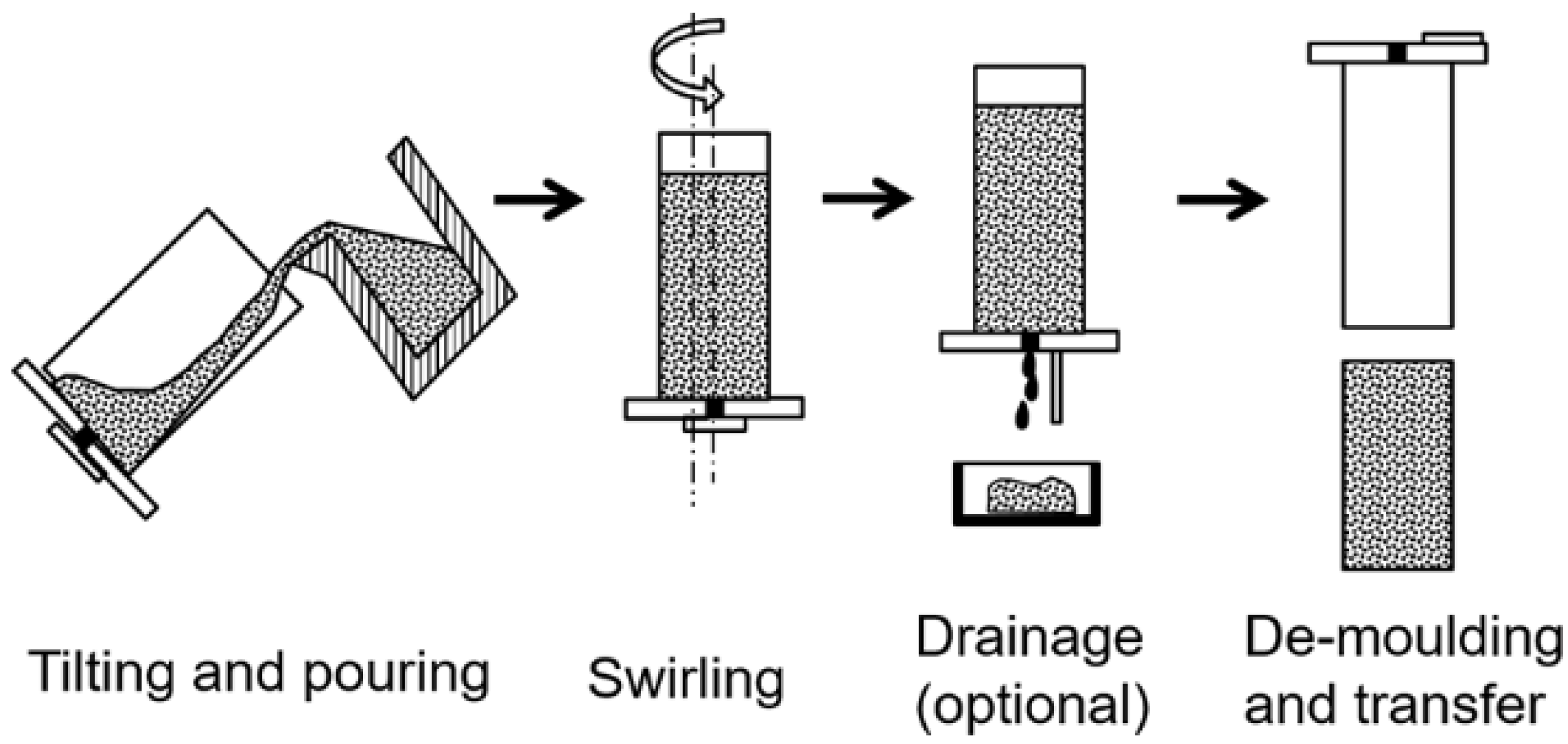
3.5. Semi-Solid Rheocasting (SSR)
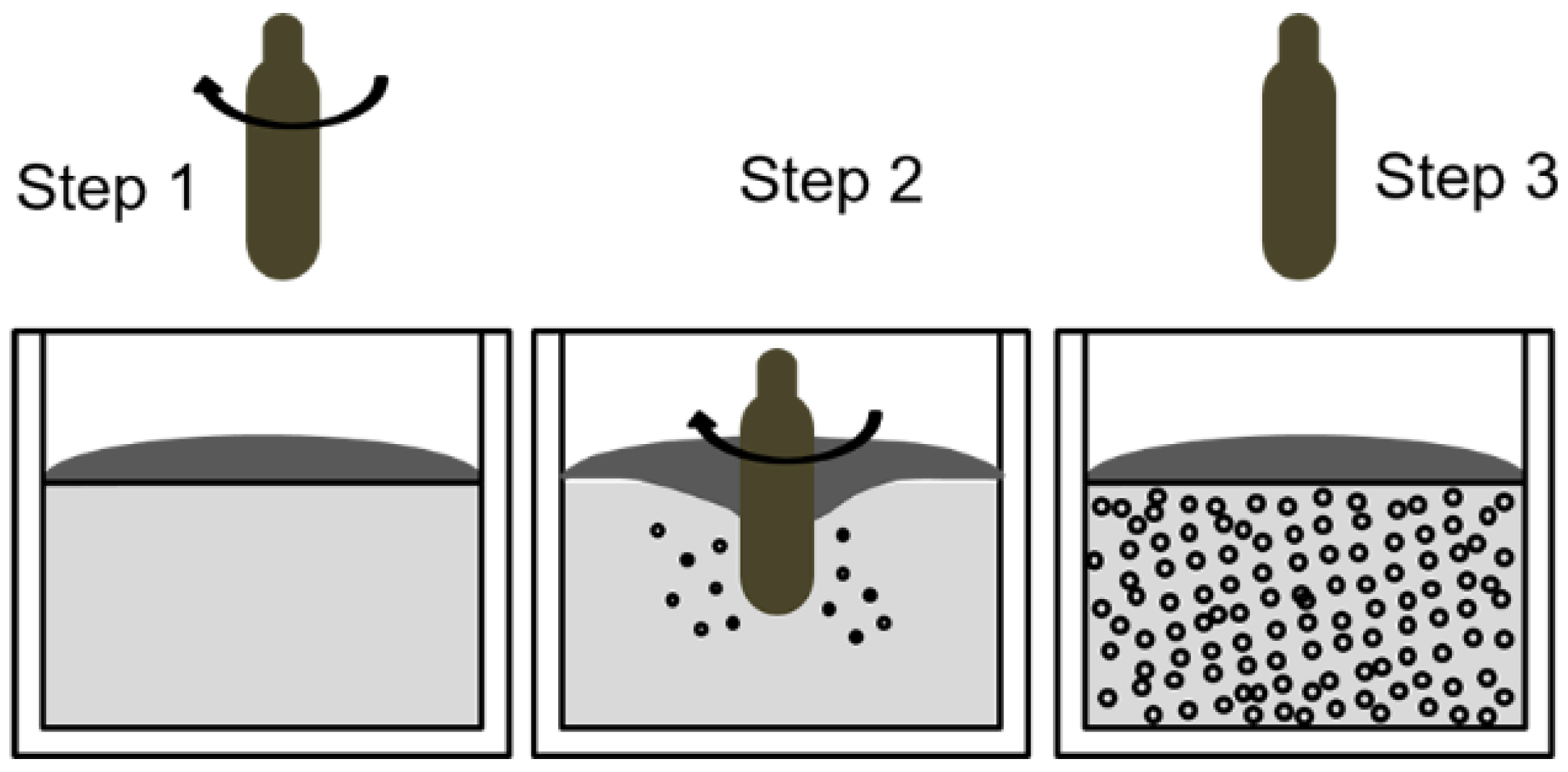
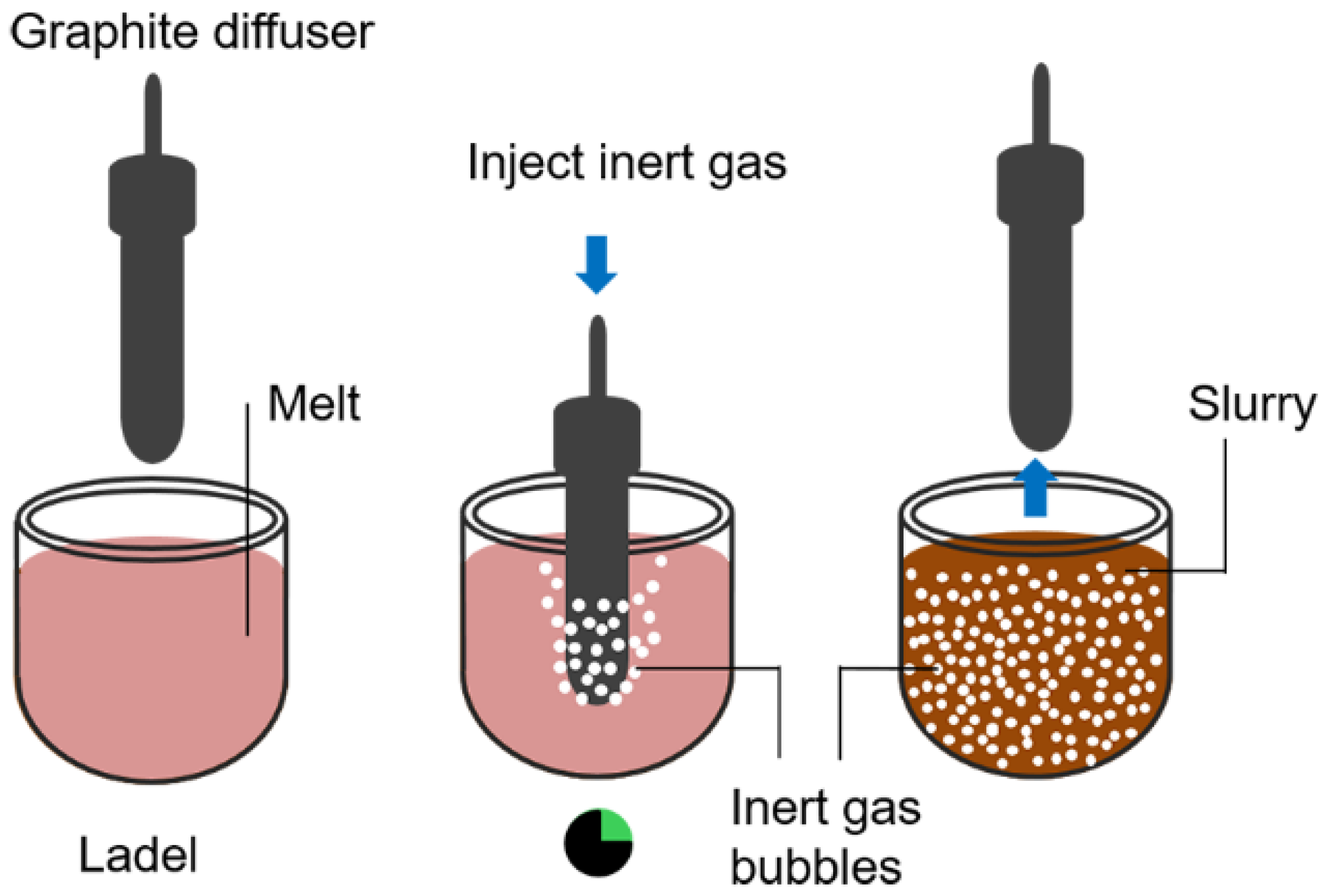
3.6. Gas-Induced Semi-Solid Process (GISS)
3.7. Swirled Enthalpy Equilibration Device Process (SEED)
3.8. Chemical Grain Refinement
3.9. Liquidus and/or Sub-Liquidus Casting
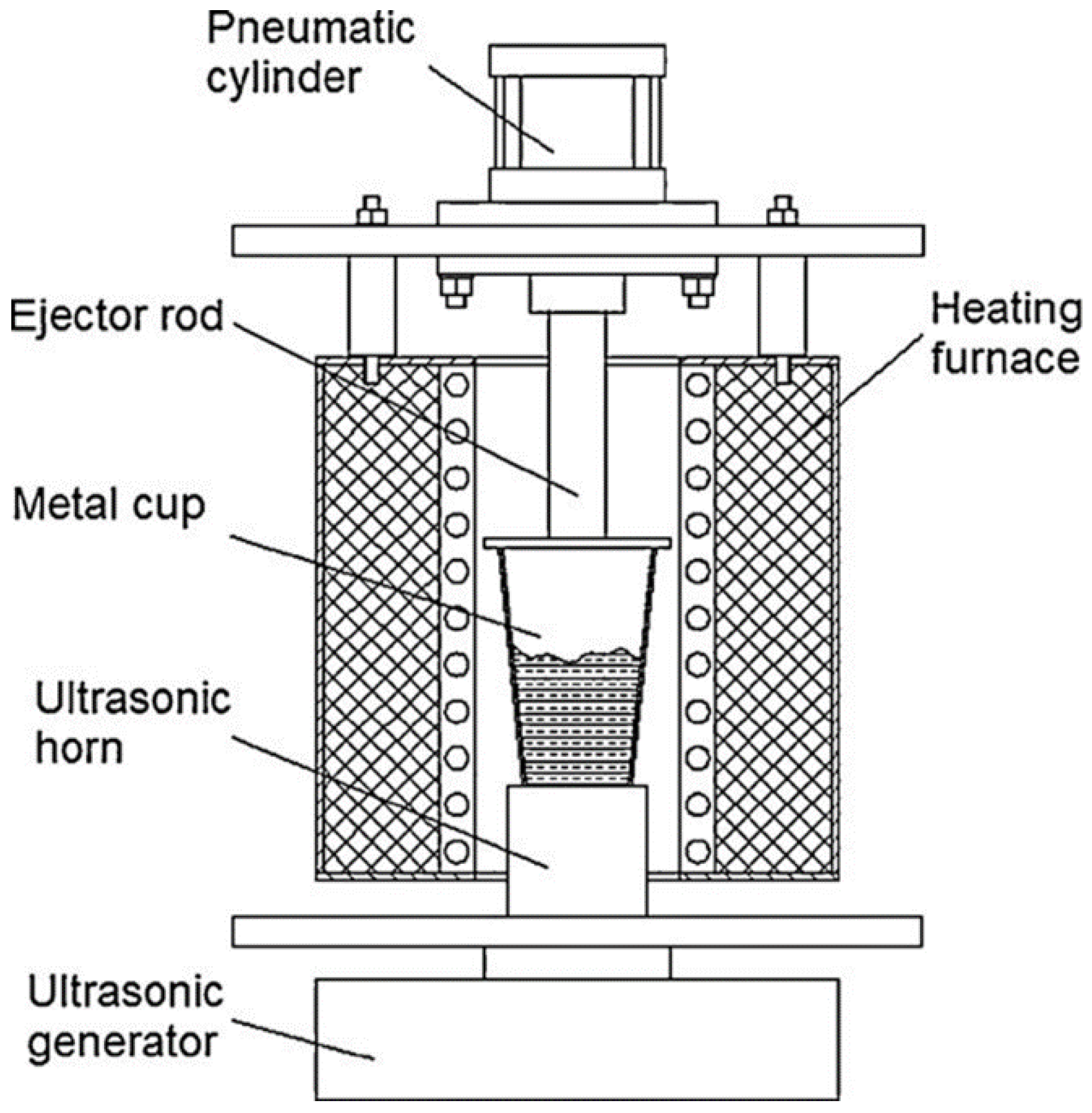
3.10. Ultrasonic Vibrations (UV)
3.11. Shearing-Cooling Roll (SCR)
3.12. Stress-Induced and Melt-Activated Process (SIMA) and Recrystallization and Partial Melting (RAP)
3.13. Spray Casting
3.14. Direct Thermal Method (DTM)
3.15. Direct Partial Re-Melting (DPRM)
3.16. Rapid Slurry Forming (RSF)
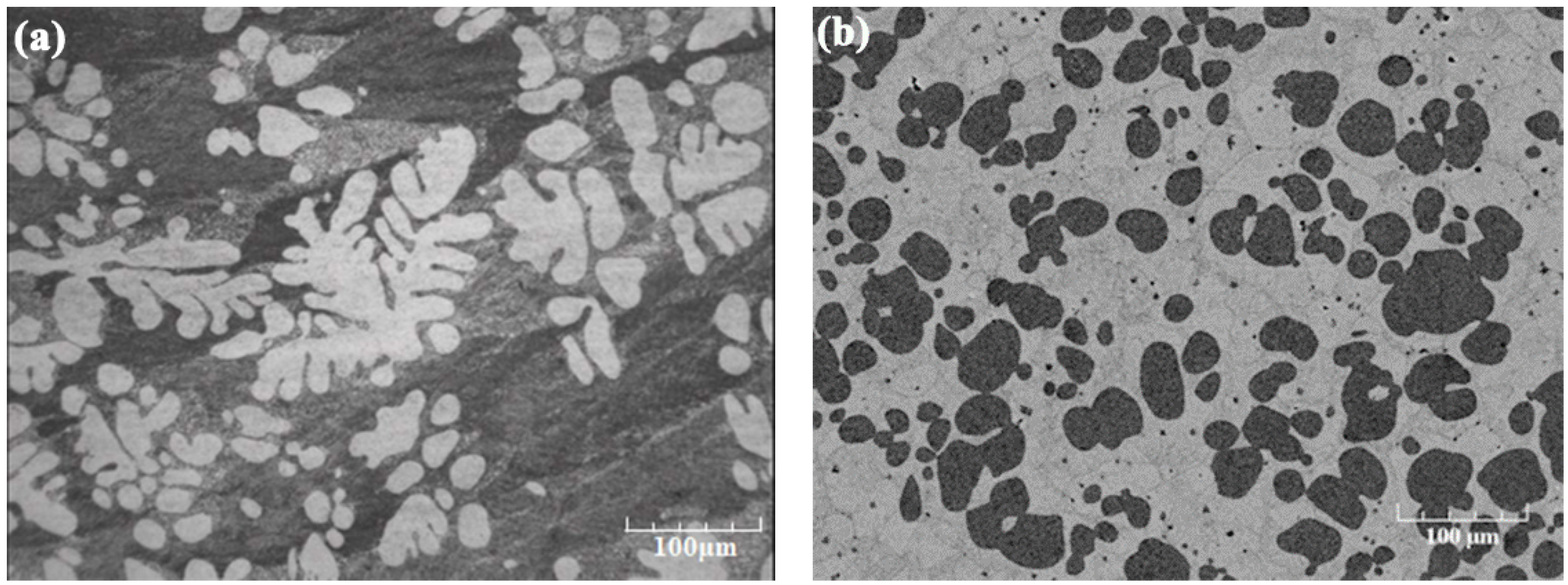
4. Evolution of Non-Dendritic Microstructural in Semi-Solid Processing
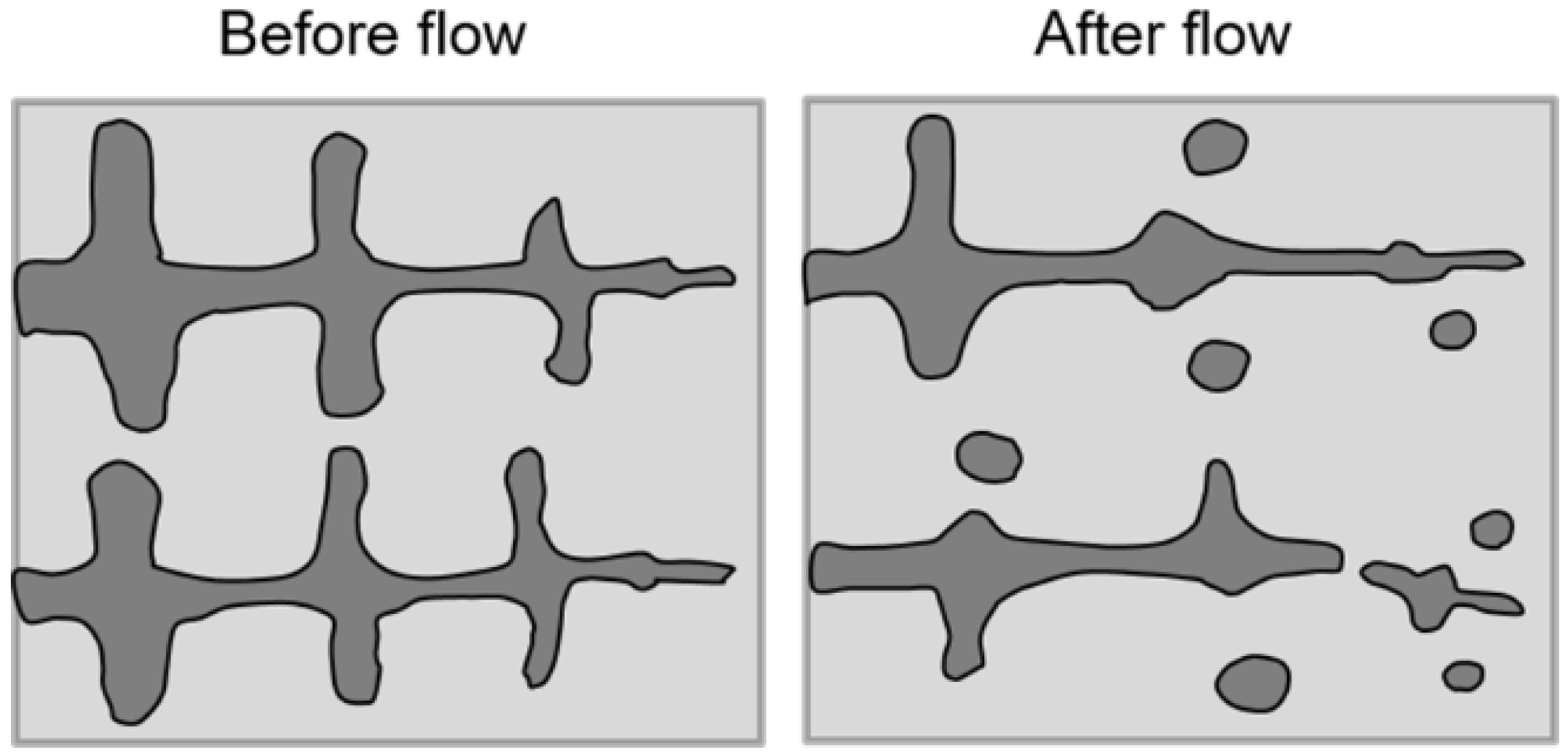
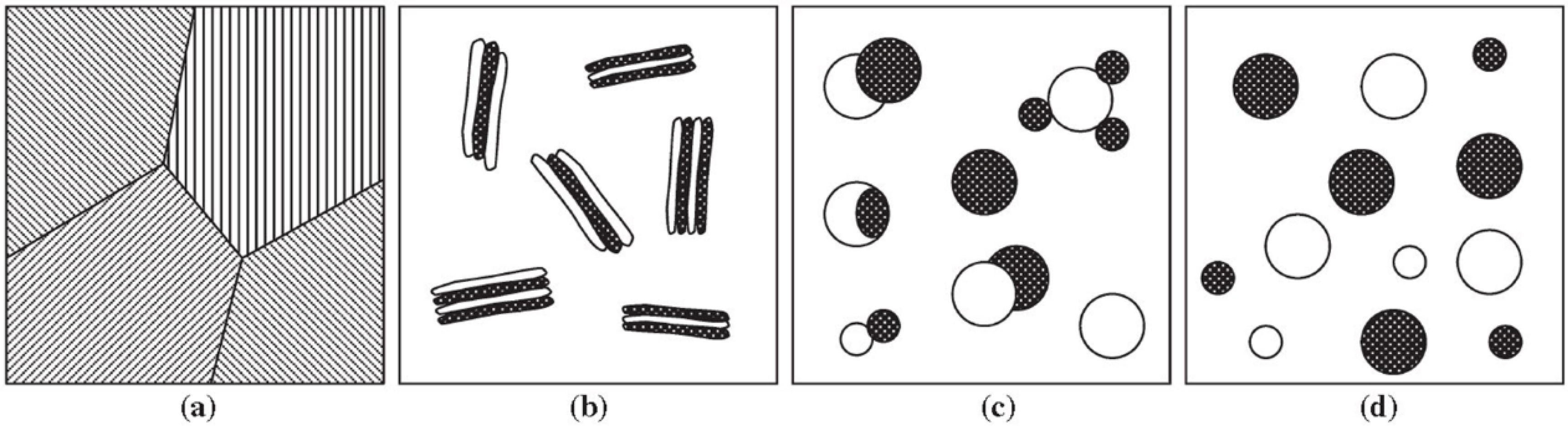
4.1. Microstructural Evolution under Shear (Forced Convection)
4.2. Microstructural Evolution by Grain Refinement
4.3. Microstructural Evolution during Partial Re-Melting
4.4. Microstructural Evolution in an Inclined Cooling Surface
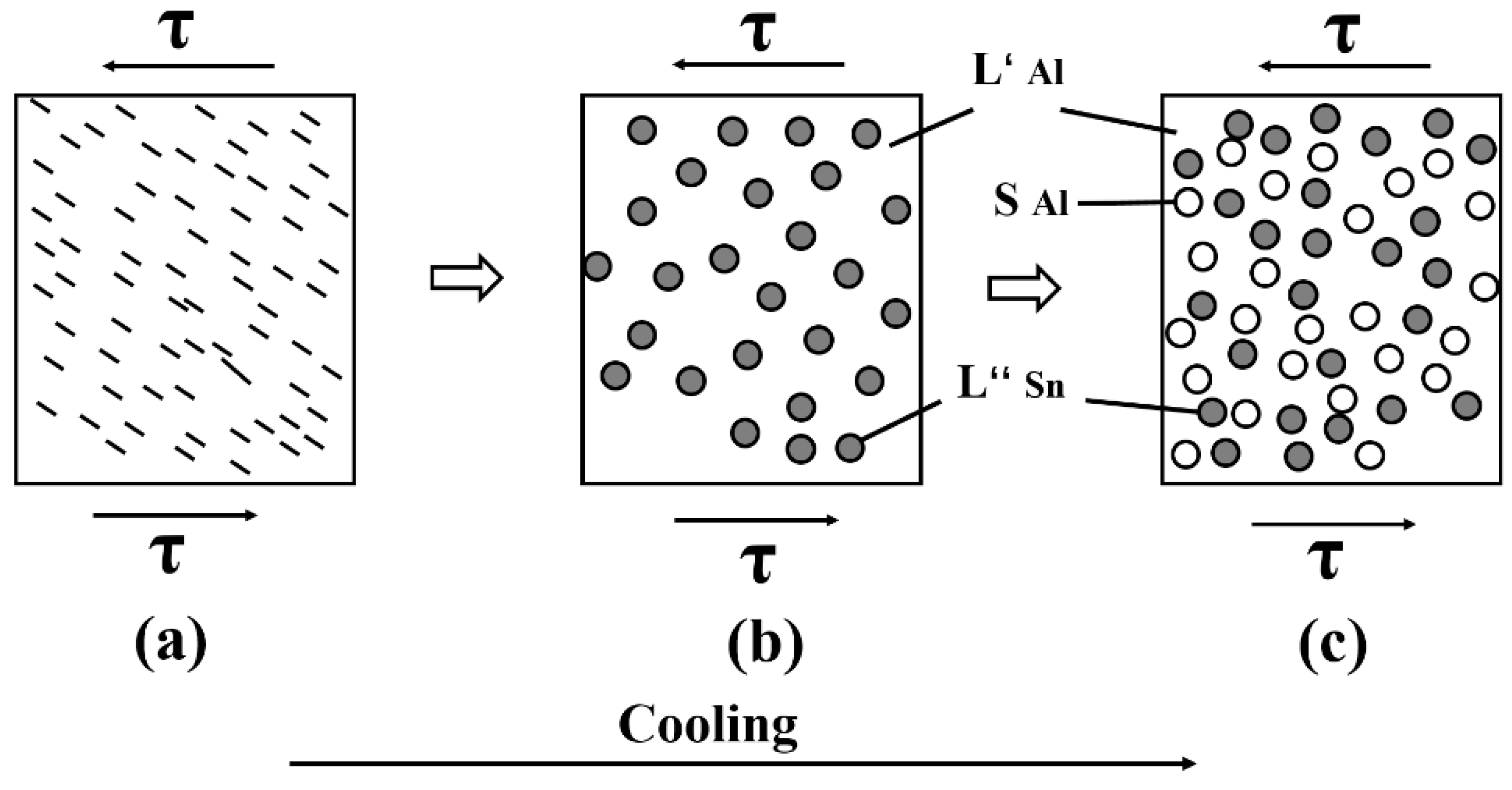
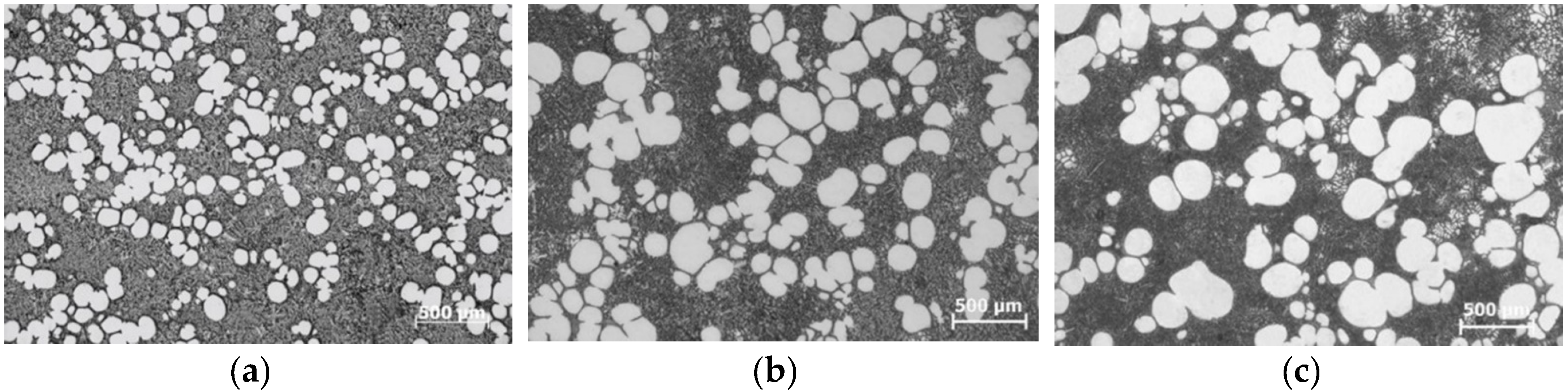
5. Microstructural Evolution of Binary Eutectic Alloy under Forced Convection
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flemings, M.C. Behavior of metal alloys in the semisolid state. Metall. Trans. A 1991, 22, 957–981. [Google Scholar] [CrossRef]
- Kirkwood, D.H. Semisolid metal processing. Int. Mater. Rev. 1994, 39, 173–189. [Google Scholar] [CrossRef]
- Fan, Z. Semisolid metal processing. Int. Mater. Rev. 2002, 47, 49–85. [Google Scholar] [CrossRef]
- Atkinson, H.V. Modelling the semisolid processing of metallic alloys. Prog. Mater. Sci. 2005, 50, 341–412. [Google Scholar] [CrossRef]
- Winklhofer, J. Semi-Solid Casting of Aluminium from an Industrial Point of View. Solid State Phenom. 2019, 285, 24–30. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, B.M.; Kang, C.G. Die life considering the deviation of the preheating billet temperature in hot forging process. Finite Elem. Anal. Des. 2005, 41, 1255–1269. [Google Scholar] [CrossRef]
- Flemings, M.C.; Riek, R.G.; Young, K.P. Rheocasting. Mater. Sci. Eng. 1976, 25, 103–117. [Google Scholar] [CrossRef]
- Jarfors, A.E.W. A Comparison Between Semisolid Casting Methods for Aluminium Alloys. Metals 2020, 10, 1368. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z.; Liu, G.; Fang, X.; Song, S. Twin-screw rheomoulding of AZ91D Mg-Alloy. In Proceedings of the 7th International Conference on Semi-Solid Processing of Alloys and Composites, Tsukuba, Japan, 25–27 September 2002. [Google Scholar]
- Czerwinski, F.; Zielinska-Lipiec, A.; Pinet, P.J.; Overbeeke, J. Correlating the microstructure and tensile properties of a thixomolded AZ91D magnesium alloy. Acta Mater. 2001, 49, 1225–1235. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z. Extruded microstructure of Zn–5 wt-%Al eutectic alloy processed by twin screw extrusion. Mater. Sci. Technol. 2012, 28, 1287–1294. [Google Scholar] [CrossRef]
- Forn, A.; Vaneetveld, G.; Pierret, J.C.; Menargues, S.; Baile, M.T.; Campillo, M.; Rassili, A. Thixoextrusion of A357 aluminium alloy. Trans. Nonferrous Met. Soc. China 2010, 20, s1005–s1009. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Omar, M.Z.; Sajuri, Z.; Salleh, M.S.; Alhawari, K.S. Trend and Development of Semisolid Metal Joining Processing. Adv. Mater. Sci. Eng. 2015, 2015, 846138. [Google Scholar] [CrossRef]
- Haga, T. Semi-solid roll casting of aluminum alloy strip by melt drag twin roll caster. J. Mater. Process. Technol. 2001, 111, 64–68. [Google Scholar] [CrossRef]
- Binesh, B.; Aghaie-Khafri, M. Microstructure and Properties of Semi-Solid Aluminum Alloys: A Literature Review. Metals 2018, 8, 181. [Google Scholar] [CrossRef]
- Modigell, M.; Pola, A.; Tocci, M. Rheological Characterization of Semi-Solid Metals: A Review. Metals 2018, 8, 245. [Google Scholar] [CrossRef]
- Kapranos, P. Current State of Semi-Solid Net-Shape Die Casting. Metals 2019, 9, 13. [Google Scholar] [CrossRef]
- Chang, Z.; Su, N.; Wu, Y.; Lan, Q.; Peng, L.; Ding, W. Semisolid rheoforming of magnesium alloys: A review. Mater. Des. 2020, 195, 108990. [Google Scholar] [CrossRef]
- Li, G.; Lu, H.; Hu, X.; Lin, F.; Li, X.; Zhu, Q. Current Progress in Rheoforming of Wrought Aluminum Alloys: A Review. Metals 2020, 10, 238. [Google Scholar] [CrossRef]
- Adachi, M.; Sasaki, H.; Harada, Y.; Sakamoto, T.; Sato, S.; Yoshida, A. Method and Apparatus for Shaping Semisolid Metals. Europe Patent EP0745694B1, 8 December 1996. [Google Scholar]
- Adachi, M. Characteristics of UBE rheocasting process. In Proceedings of the Japanese Die Casting Association, JD98-19, Yokohama, Japan, 11–13 November 1998; pp. 123–128. [Google Scholar]
- Muumbo, A.; Nomura, H.; Takita, M. Casting of semi-solid cast iron slurry using combination of cooling slope and pressurisation. Int. J. Cast Met. Res. 2004, 17, 39–46. [Google Scholar] [CrossRef]
- Kaufmann, H.; Uggowitzer, P.J. Fundamentals of the New Rheocasting Process for Magnesium Alloys. Adv. Eng. Mater. 2001, 3, 963–967. [Google Scholar] [CrossRef]
- Legoretta, E.C.; Atkinson, H.V.; Jones, H. Cooling slope casting to obtain thixotropic feedstock II: Observations with A356 alloy. J. Mater. Sci. 2008, 43, 5456–5469. [Google Scholar] [CrossRef]
- Kopp, R.; Neudenberger, D.; Winning, G. Different concepts of thixoforging and experiments for rheological data. J. Mater. Process. Technol. 2001, 111, 48–52. [Google Scholar] [CrossRef]
- Liu, T.Y.; Atkinson, H.V.; Kapranos, P.; Kirkwood, D.H.; Hogg, S.C. Rapid compression of aluminum alloys and its relationship to thixoformability. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2003, 34, 1545–1554. [Google Scholar] [CrossRef]
- Balan, T.; Becker, E.; Langlois, L.; Bigot, R. A new route for semi-solid steel forging. CIRP Ann. 2017, 66, 297–300. [Google Scholar] [CrossRef]
- Kang, C.G.; Seo, P.K.; Jung, H.K. Numerical analysis by new proposed coil design method in induction heating process for semi-solid forming and its experimental verification with globalization evaluation. Mater. Sci. Eng. A 2003, 341, 121–138. [Google Scholar] [CrossRef]
- Fan, Z.; Ji, S. Development of rheomoulding of light alloys. In Proceedings of the 6th International Conference on Semi-Solid Processing of Alloys and Composites, Turin, Italy, 27–29 September 2000. [Google Scholar]
- Peng, H.; Hsu, W.M. Development on rheomoulding of magnesium parts. In Proceedings of the 6th International Conference on Semi-Solid Processing of Alloys and Composites, Turin, Italy, 27–29 September 2000. [Google Scholar]
- Busk, R.S. Method for Making Thixotropic Materials. U.S. Patent 4694882, 22 September 1987. [Google Scholar]
- Bradley, N.L.; Wieland, R.D.; Schafer, W.J.; Niemi, A.N. Method and Apparatus for the Injection Molding of Metal Alloys. International Patent WO 90/09251, 23 August 1990. [Google Scholar]
- Pasternak, L.; Carnahan, R.; Decker, R.; Kilbert, R. Semi-solid production processing of magnesium alloys by thixomolding. In Proceedings of the Second International Conference on the Semi-Solid Processing of Alloys and Composites, Cambridge, MA, USA, 10–12 June 1992. [Google Scholar]
- Czerwinski, F. Magnesium alloy particulates for Thixomolding applications manufactured by rapid solidification. Mater. Sci. Eng. A 2004, 367, 261–271. [Google Scholar] [CrossRef]
- Tsukeda, T.; Takeya, K.; Saito, K.; Kubo, H. Mechanical and metallurgical properties of injection molded AZ91D magnesium alloy. J. Jpn. Inst. Light Met. 1999, 49, 287–290. [Google Scholar] [CrossRef]
- Patel, H.A.; Chen, D.L.; Bhole, S.D.; Sadayappan, K. Microstructure and tensile properties of thixomolded magnesium alloys. J. Alloys Compd. 2010, 496, 140–148. [Google Scholar] [CrossRef]
- Baadjou, R.; Shimahara, H.; Hirt, G. Automated Semi-Solid Forging of Steel Components by Means of Thixojoining. Solid State Phenom. 2006, 116–117, 383–386. [Google Scholar] [CrossRef]
- Obeidi, M.; McCarthy, É.; Brabazon, D. A review of semi-solid aluminium-steel joining processes. AIP Conf. Proc. 2016, 1769, 030005. [Google Scholar]
- Mendez, P.; Rice, C.S.; Brown, S. Joining using semisolid metals. Weld. J. 2002, 81, 181S–187S. [Google Scholar]
- Mohammed, M.N.; Omar, M.Z.; Salleh, M.S.; Zailani, M.A.; Alhawari, K.S. Joining two metals via partial remelting method. J. Asian Sci. Res. 2012, 2, 724–730. [Google Scholar]
- Baur, J. Thixoforging of a CuZn-alloy. In Proceedings of the 5th International Conference on Semi-Solid Processing of Alloys and Composites, Golden, CO, USA, 23–25 June 1998. [Google Scholar]
- Kiuchi, M.; Yanagimoto, J.; Sugiyama, S. Application of mushy/semi-solid joining—Part 3. J. Mater. Process. Technol. 2003, 140, 163–166. [Google Scholar] [CrossRef]
- Zhang, P.; Du, Y.; Liu, H.; Zeng, d.; Cui, J.; Ba, L. Semi-solid pressing bonding strength between steel and Cu-graphite composite. J. Mater. Sci. Technol. 2005, 21, 265–268. [Google Scholar]
- Xu, H.; Yang, H.; Luo, Q.; Zeng, Y.; Zhou, B.; Du, C. Strength and microstructure of semi-solid stirring brazing of SiCp/A356 composites and aluminum alloy in air. Adv. Mater. Lett. 2011, 2, 233–238. [Google Scholar] [CrossRef]
- Kiuchi, M.; Yanagimoto, J.; Sugiyama, S. Mushy-state joining, a new process for joining materials together. In Proceedings of the 5th International Conference on Semi-Solid Processing of Alloys and Composites, Golden, CO, USA, 23–25 June 1998. [Google Scholar]
- Mohammed, M.N.; Omar, M.Z.; Syarif, J.; Sajuri, Z.; Salleh, M.S.; Alhawari, K.S. Microstructural Evolution during DPRM Process of Semisolid Ledeburitic D2 Tool Steel. Sci. World J. 2013, 2013, 828926. [Google Scholar] [CrossRef]
- Siegert, K.; Wolf, A.; Baur, J. Thixoforging of Aluminium and Brass. Prod. Eng. 2000, 1, 21–24. [Google Scholar]
- Liu, H.W.; Guo, C.; Cheng, Y.; Liu, X.F.; Shao, G.J. Interfacial strength and structure of stainless steel–semi-solid aluminum alloy clad metal. Mater. Lett. 2006, 60, 180–184. [Google Scholar] [CrossRef]
- Quigley, B.F.; Abbaschian, G.J.; Wunderlin, R.; Mehrabian, R. A method for fabrication of aluminum-alumina composites. Metall. Trans. A 1982, 13, 93–100. [Google Scholar] [CrossRef]
- Kiuchi, M.; Yanagimoto, J.; Sugiyama, S. Application of mushy/semi-solid joining—part 2. In Proceedings of the 7th International Conference on Semi-Solid Processing of Alloys and Composites, Tsukuba, Japan, 25–27 September 2002. [Google Scholar]
- Kopp, R.; Kallweit, J.; Möller, T.; Seidl, I. Forming and joining of commercial steel grades in the semi-solid state. J. Mater. Process. Technol. 2002, 130, 562–568. [Google Scholar] [CrossRef]
- Hirt, G.; Baadjou, R.; Knauf, F. Investigations on Semisolid Joined Steel Components and their Bonding Quality. Steel Res. Int. 2010, 81, 589–596. [Google Scholar] [CrossRef]
- Shalchi Amirkhiz, B.; Aashuri, H.; Kokabi, A.H.; Abbasi Gharacheh, M.; Mola, J. Joining Metals by Combining Mechanical Stirring and Thermomechanical Treatment to Form a Globular Weld Structure. Solid State Phenom. 2006, 116–117, 397–401. [Google Scholar] [CrossRef]
- Narimannezhad, A.; Aashuri, H.; Kokabi, A.H.; Khosravani, A.; Kiani, M.; Foroughi, A. Semisolid Joining of Zinc AG40A Alloy by Partial Remelting and Mechanical Stirring. Solid State Phenom. 2008, 141–143, 225–230. [Google Scholar] [CrossRef]
- Alvani, S.M.J.; Aashuri, H.; Kokabi, A.; Beygi, R. Semisolid joining of aluminum A356 alloy by partial remelting and mechanical stirring. Trans. Nonferrous Met. Soc. China 2010, 20, 1792–1798. [Google Scholar] [CrossRef]
- Xu, H.B.; Luo, Q.X.; He, J.Y.; Zhou, B.F.; Zeng, Y.L.; Du, C.H. Study of Brazeability of SiCP/A356 Composites and Aluminum Alloy Using Semisolid Metal with High Solid Fraction by Stirring. Adv. Mater. Res. 2011, 239–242, 663–666. [Google Scholar]
- Xu, H.; Zhou, B.; Du, C.; Luo, Q.; Chen, H. Microstructure and Properties of Joint Interface of Semisolid Stirring Brazing of Composites. J. Mater. Sci. Technol. 2012, 28, 1163–1168. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Aashuri, H.; Kokabi, A.H. Characterization of newly developed semisolid stir welding method for AZ91 magnesium alloy by using Mg–25%Zn interlayer. Mater. Sci. Eng. A 2013, 565, 165–171. [Google Scholar] [CrossRef]
- Petkhwan, A.; Muangjunburee, P.; Wannasin, J. Investigation of Microstructure and Mechanical Properties of Semi-Solid State Joining of SSM Aluminum Alloys. Appl. Mech. Mater. 2014, 496–500, 371–375. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Omar, M.Z.; Al-Zubaidi, S.; Alhawari, K.S.; Abdelgnei, M.A. Microstructure and Mechanical Properties of Thixowelded AISI D2 Tool Steel. Metals 2018, 8, 316. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Omar, M.Z.; Salleh, M.S.; Alhawari, K.S. Study on Thixojoining Process Using Partial Remelting Method. Adv. Mater. Sci. Eng. 2013, 2013, 251472. [Google Scholar] [CrossRef]
- Kalaki, A.; Ketabchi, M.; Abbasi, M. Thixo-joining of D2 and M2 tool steels: Analysis of microstructure and mechanical properties. Int. J. Mater. Res. 2014, 105, 764–769. [Google Scholar] [CrossRef]
- Zhang, L.N.; Wang, S.Q.; Zhu, M.F.; Wang, N.; Wang, S.D. The extrusion behaviour of Zn-20% Al alloy in the semi-solid state. J. Mater. Process. Technol. 1994, 44, 91–98. [Google Scholar] [CrossRef]
- Zu, L.; Luo, S. Study on the powder mixing and semi-solid extrusion forming process of SiCp/2024Al composites. J. Mater. Process. Technol. 2001, 114, 189–193. [Google Scholar] [CrossRef]
- Uetani, Y.; Sueda, H.; Takagi, H.; Matsuda, K.; Ikeno, S. Effect of forced-air cooling on semi-solid extrusion of mechanically stirred Al-10%Mg alloy billet. In Proceedings of the 9th International Conference on Aluminium Alloys, Brisbane, Australia, 2–5 August 2004. [Google Scholar]
- Sugiyama, S.; Kuo, J.L.; Hsiang, S.H.; Yanagimoto, J. Semisolid Extrusion of Wrought Magnesium Alloy AZ61 and Its Mechanical Properties. In Proceedings of the 35th International MATADOR Conference, Taipei, Taiwan, 27 July 2007. [Google Scholar]
- Feng, J.; Zhang, D.; Hu, H.; Zhao, Y.; Chen, X.; Jiang, B.; Pan, F. Improved microstructures of AZ31 magnesium alloy by semi-solid extrusion. Mater. Sci. Eng. A 2021, 800, 140204. [Google Scholar] [CrossRef]
- Neag, A.; Favier, V.; Bigot, R.; Frunză, D. Study on Thixo-Extrusion of Semi-Solid Aluminium. Solid State Phenom. 2008, 141–143, 659–664. [Google Scholar] [CrossRef]
- Püttgen, W.; Bleck, W.; Seidl, I.; Kopp, R.; Bertrand, C. Investigation of Thixoforged Damper Brackets made of the Steel Grades HS6-5-3 and 100Cr6. Adv. Eng. Mater. 2005, 7, 726–735. [Google Scholar] [CrossRef]
- Omar, M.Z.; Palmiere, E.J.; Howe, A.A.; Atkinson, H.V.; Kapranos, P. Thixoforming of a high performance HP9/4/30 steel. Mater. Sci. Eng. A 2005, 395, 53–61. [Google Scholar] [CrossRef]
- Wang, Y.; Song, R.; Li, Y. Microstructural evolution and mechanical properties of 9Cr18 steel after thixoforging and heat treatment. Mater. Charact. 2017, 127, 64–72. [Google Scholar] [CrossRef]
- Knauf, F.; Baadjou, R.; Hirt, G. Analysis of semi-solid extrusion products made of steel alloy X210CRW12. Int. J. Mater. Form. 2009, 2, 733. [Google Scholar] [CrossRef]
- Muenstermann, S.; Uibel, K.; Tonnesen, T.; Telle, R. Semi-solid extrusion of steel grade X210CrW12 under isothermal conditions using ceramic dies. J. Mater. Process. Technol. 2009, 209, 3640–3649. [Google Scholar] [CrossRef]
- Guan, R.G.; Zhao, Z.Y.; Sun, X.P.; Huang, H.Q.; Dai, C.G.; Zhang, Q.S. Fabrication of AZ31 alloy wire by continuous semisolid extrusion process. Trans. Nonferrous Met. Soc. China 2010, 20, s729–s733. [Google Scholar] [CrossRef]
- Rattanochaikul, T.; Janudom, S.; Memongkol, N.; Wannasin, J. Development of aluminum rheo-extrusion process using semi-solid slurry at low solid fraction. Trans. Nonferrous Met. Soc. China 2010, 20, 1763–1768. [Google Scholar] [CrossRef]
- Rattanochaikul, T.; Janudom, S.; Memongkol, N.; Wannasin, J. Development of an aluminum semi-solid extrusion process. J. Met. Mater. Miner. 2010, 20, 17–21. [Google Scholar]
- Fan, Z.; Ji, S.; Bevis, M.J. Method and Apparatus for Making Metal Alloy Castings. Patent EP1307308B1, 8 December 2004. [Google Scholar]
- Roberts, K.; Fang, X.; Ji, S.; Fan, Z. Rheoextrusion of magnesium alloys. In Proceedings of the 7th International Conference on Semi-Solid Processing of Alloys and Composites, Tsukuba, Japan, 25–27 September 2002. [Google Scholar]
- Jabbari, A.; Abrinia, K. A metal additive manufacturing method: Semi-solid metal extrusion and deposition. Int. J. Adv. Manuf. Technol. 2018, 94, 3819–3828. [Google Scholar] [CrossRef]
- Alharbi, A.; Khan, A.; Todd, I.; Ramadan, M.; Mumtaz, K. Semisolid heat treatment processing window of Pb-40% Sn alloy for feedstock in the 3D printing thixo-forming process. Mater. Today Proc. 2022, 51, 403–410. [Google Scholar] [CrossRef]
- Lima, D.D.; Campo, K.N.; Button, S.T.; Caram, R. 3D thixo-printing: A novel approach for additive manufacturing of biodegradable Mg-Zn alloys. Mater. Des. 2020, 196, 109161. [Google Scholar] [CrossRef]
- Yun, M.; Lokyer, S.; Hunt, J.D. Twin roll casting of aluminium alloys. Mater. Sci. Eng. A 2000, 280, 116–123. [Google Scholar] [CrossRef]
- Liang, D.; Cowley, C. The twin-roll strip casting of magnesium. JOM 2004, 56, 26–28. [Google Scholar] [CrossRef]
- Ferry, M. Direct Strip Casting of Metals and Alloys: Processing, Microstructure and Properties; Woodhead Publishing; Maney Publishing: Cambridge, UK, 2006; pp. 101–150. [Google Scholar]
- Hunt, J.D.; Yun, M.; Lockyer, S.; Heywood, M.J. Advances in light metal casting technology: Twin roll casting. In Light Metals; Huglen, R., Ed.; Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1997; pp. 341–354. [Google Scholar]
- Haga, T. Semisolid strip casting using a twin roll caster equipped with a cooling slope. J. Mater. Process. Technol. 2002, 130–131, 558–561. [Google Scholar] [CrossRef]
- Kido, F.; Tetsuichi, M. Continuous casting of magnesium alloy sheet using semisolid slurry. Mater. Trans. 2012, 53, 495–499. [Google Scholar] [CrossRef]
- Haga, T.; Sakaguchi, H.; Inui, H.; Watari, H.; Kumai, S. Aluminium alloy semisolid strip casting using an unequal diameter twin roll caster. J. Achiev. Mater. Manuf. Eng. 2006, 14, 157–162. [Google Scholar]
- Wei, B.; Li, S.; Jiang, T.; Xu, G.; Li, Y.; Wang, Z. Effect of a novel semi-solid middle-layer structure formed during twin-roll casting on the mechanical properties of AA6022 alloy. Mater. Sci. Eng. A 2021, 812, 141083. [Google Scholar] [CrossRef]
- Lu, B.; Yu, W.; Li, Y.; Wang, Z.; Xu, G.; Li, J.; Qian, X. Formation of banded intergranular segregation and control via micro-alloying in twin-roll casted Al-Zn-Mg-Cu alloy with high solidification interval. Materialia 2022, 22, 101406. [Google Scholar] [CrossRef]
- Watari, H.; Davey, K.; Rasgado, M.T.A.; Haga, T.; Koga, N. Semi-solid Twin-roll Casting Process of Magnesium Alloy Sheets. AIP Conf. Proc. 2004, 712, 1314–1319. [Google Scholar]
- Watari, H.; Davey, K.; Rasgado, M.T.; Haga, T.; Izawa, S. Semi-solid manufacturing process of magnesium alloys by twin-roll casting. J. Mater. Process. Technol. 2004, 155–156, 1662–1667. [Google Scholar] [CrossRef]
- Maleki, A.; Taherizadeh, A.; Hoseini, N. Twin Roll Casting of Steels: An Overview. ISIJ Int. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Mao, W.M.; Zhao, A.; Yun, D.; Zhang, L.P.; Zhong, X.Y. Preparation study of semi-solid 60Si2Mn spring steel slurry. Acta Metall. Sin. (Engl. Lett.) 2003, 16, 483–488. [Google Scholar]
- Song, R.B.; Kang, Y.L.; Zhao, A. Fabrication of Semi-Solid Slurry for Steels and Their Rheo-Rolling Process. Solid State Phenom. 2008, 141–143, 457–461. [Google Scholar] [CrossRef]
- Mao, W.; Zhao, A.; Yun, D.; Zhang, L.; Zhong, X. Semi-solid slurry preparation and rolling of 1Cr18Ni9Ti stainless steel. J. Univ. Sci. Technol. Beijing 2003, 10, 35–39. [Google Scholar]
- Song, R.; Kang, Y.; Zhao, A. Semi-solid rolling process of steel strips. J. Mater. Process. Technol. 2008, 198, 291–299. [Google Scholar] [CrossRef]
- Li, J.; Kang, Y.; Zhao, A.; Sun, Y.; Cheng, M. Microstructural morphology of the semi-solid high carbon steel T12 before and after rheo-rolling. J. Univ. Sci. Technol. Beijing 2005, 12, 139–142. [Google Scholar]
- Kotadia, H.R.; Doernberg, E.; Patel, J.B.; Fan, Z.; Schmid-Fetzer, R. Solidification of Al-Sn-Cu Based Immiscible Alloys under Intense Shearing. Metall. Mater. Trans. A 2009, 40, 2202–2211. [Google Scholar] [CrossRef]
- Fang, X.; Fan, Z.; Ji, S.; Hu, Y. Processing of immiscible alloys by a twin-screw rheomixing process. In Proceedings of the 7th International Conference on Semi-Solid Processing of Alloys and Composites, Tsukuba, Japan, 25–27 September 2002. [Google Scholar]
- Fang, X.; Fan, Z. Microstructure of Zn–Pb immiscible alloys obtained by a rheomixing process. Mater. Sci. Technol. 2005, 21, 366–372. [Google Scholar] [CrossRef]
- Ji, S.; Zhen, Z.; Fan, Z. Effects of rheo-die casting process on the microstructure and mechanical properties of AM50 magnesium alloy. Mater. Sci. Technol. 2005, 21, 1019–1024. [Google Scholar] [CrossRef]
- Zhen, Z.; Qian, M.; Ji, S.; Fan, Z. The effects of rheo-diecasting on the integrity and mechanical properties of Mg–6Al–1Zn. Scr. Mater. 2006, 54, 207–211. [Google Scholar] [CrossRef]
- Fan, Z.; Fang, X.; Ji, S. Microstructure and mechanical properties of rheo-diecast (RDC) aluminium alloys. Mater. Sci. Eng. A 2005, 412, 298–306. [Google Scholar] [CrossRef]
- Ji, S.; Das, A.; Fan, Z. Solidification behavior of the remnant liquid in the sheared semisolid slurry of Sn-15 wt.%Pb alloy. Scr. Mater. 2002, 46, 205–210. [Google Scholar] [CrossRef]
- Spencer, D.B.; Mehrabian, R.; Flemings, M.C. Rheological behavior of Sn-15 pct Pb in the crystallization range. Metall. Mater. Trans. B 1972, 3, 1925–1932. [Google Scholar] [CrossRef]
- Kenney, M.P.; Courtois, J.A.; Evans, R.D.; Farrior, G.M.; Kyonka, C.P.; Koch, A.A.; Young, K.P. Metals Handbook, 2nd ed.; ASM International: Novelty, OH, USA, 1988; pp. 327–338. [Google Scholar]
- Niedick, I. Eignungsbewertung und Optimierung von Vormaterial für Thixoforming. Ph.D. Thesis, Aachen University, Aachen, Germany, 2000. [Google Scholar]
- Li, M.; Tamura, T.; Omura, N.; Murakami, Y.; Tada, S. Grain refinement of AZCa912 alloys solidified by an optimized electromagnetic stirring technique. J. Mater. Process. Technol. 2016, 235, 114–120. [Google Scholar] [CrossRef]
- Zoqui, E.J.; Paes, M.; Es-Sadiqi, E. Macro- and microstructure analysis of SSM A356 produced by electromagnetic stirring. J. Mater. Process. Technol. 2002, 120, 365–373. [Google Scholar] [CrossRef]
- Vivès, C. Crystallization of aluminum alloys in the presence of vertical electromagnetic force fields. J. Cryst. Growth 1997, 173, 541–549. [Google Scholar] [CrossRef]
- Vivès, C. Crystallization of semi-solid magnesium alloys and composites in the presence of magnetohydrodynamic shear flows. J. Cryst. Growth 1994, 137, 653–662. [Google Scholar] [CrossRef]
- Kopper, A. Microstructure evolution during re-heating of 357 aluminium alloy and its effect on the flow properties in a semi-solid metal casting operation. In Proceedings of the 6th International Conference on Semi-Solid Processing of Alloys and Composites, Turin, Italy, 27–29 September 2000. [Google Scholar]
- Haga, T.; Suzuki, S. Casting of aluminum alloy ingots for thixoforming using a cooling slope. J. Mater. Process. Technol. 2001, 118, 169–172. [Google Scholar] [CrossRef]
- Birol, Y.; Akdi, S. Cooling slope casting to produce EN AW 6082 forging stock for manufacture of suspension components. Trans. Nonferrous Met. Soc. China 2014, 24, 1674–1682. [Google Scholar] [CrossRef]
- Yang, H.; Xie, S.; Li, L. Numerical simulation of the preparation of semi-solid metal slurry with damper cooling tube method. Int. J. Miner. Metall. Mater. 2007, 14, 443–448. [Google Scholar] [CrossRef]
- Khosravi, H.; Akhlaghi, F. Comparison of microstructure and wear resistance of A356–SiCp composites processed via compocasting and vibrating cooling slope. Trans. Nonferrous Met. Soc. China 2015, 25, 2490–2498. [Google Scholar] [CrossRef]
- Haga, T.; Suzuki, S. A downward melt drag single roll caster for casting semisolid slurry. J. Mater. Process. Technol. 2004, 157–158, 695–700. [Google Scholar] [CrossRef]
- Budiman, H.; Omar, M.Z.; Jalar, A. Effect of Water Cooling on the Production of Al-Si Thixotropic Feedstock by Cooling Slope Casting. Eur. J. Sci. Res. 2009, 32, 158–166. [Google Scholar]
- Guan, R.G.; Zhao, Z.Y.; Li, Y.D.; Chen, T.J.; Xu, S.X.; Qi, P.X. Microstructure and properties of squeeze cast A356 alloy processed with a vibrating slope. J. Mater. Processing Technol. 2016, 229, 514–519. [Google Scholar] [CrossRef]
- El Mahallawi, I.; Mahmoud, T.; Gaafer, A.; Mahmoud, F. Effect of Pouring Temperature and Water Cooling on the Thixotropic Semi-solid Microstructure of A319 Aluminium Cast Alloy. Mater. Res. 2015, 18, 170–176. [Google Scholar] [CrossRef]
- Pan, Q.Y.; Findon, M.; Apelian, D. The continuous rheoconversion process (CRP): A novel SSM approach. In Proceedings of the 8th International Conference on Semi-Solid Processing of Alloys and Composites, Limassol, Cyprus, 21–23 September 2004. [Google Scholar]
- Bernard, W.J. The Continuous Rheoconversion Process: Scale-Up and Optimization. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, May 2005. [Google Scholar]
- Pan, Q.; Apelian, D.; Hogan, P. The continuous rheoconversion process (CRP™): Optimization & industrial applications. Mater. Sci. Technol. 2006, 24. Available online: https://www.fracturae.com/index.php/MST/article/view/1120 (accessed on 22 May 2022).
- Flemings, M.C.; Martinez-Ayers, R.A.; Figueredo, A.M.D.; Yurko, J.A. Metal Alloy Compositions and Process. U.S. Patent US6645323B2, 11 November 2003. [Google Scholar]
- Canyook, R.; Wannasin, J.; Wisuthmethangkul, S.; Flemings, M.C. Characterization of the microstructure evolution of a semi-solid metal slurry during the early stages. Acta Mater. 2012, 60, 3501–3510. [Google Scholar] [CrossRef]
- Wannasin, J.; Martinez, R.A.; Flemings, M.C. Grain refinement of an aluminum alloy by introducing gas bubbles during solidification. Scr. Mater. 2006, 55, 115–118. [Google Scholar] [CrossRef]
- Wannasin, J.; Thanabumrungkul, S. Development of a semi-solid metal processing technique for aluminium casting applications. Songklanakarin J. Sci. Technol. 2008, 30, 215–220. [Google Scholar]
- Wannasin, J.; Martinez, R.; Flemings, M. A Novel Technique to Produce Metal Slurries for SemiSolid Metal Processing. Solid State Phenom. 2006, 116–117, 366–369. [Google Scholar] [CrossRef]
- Wannasin, J.; Janudom, S.; Rattanochaikul, T.; Canyook, R.; Burapa, R.; Chucheep, T.; Thanabumrungkul, S. Research and development of gas induced semi-solid process for industrial applications. Trans. Nonferrous Met. Soc. China 2010, 20, s1010–s1015. [Google Scholar] [CrossRef]
- Abdi, M.; Shabestari, S.G. Semi-Solid Slurry Casting Using Gas Induced Semi-Solid Technique to Enhance the Microstructural Characteristics of Al-4.3Cu Alloy. Solid State Phenom. 2019, 285, 253–258. [Google Scholar] [CrossRef]
- Wannasin, J.; Flemings, M.C. Process for Preparing Molten Metals for Casting at a Low to Zero Superheat Temperature. WO 2015/174937 A1, 16 May 2014. [Google Scholar]
- Langlais, J.; Lemieux, A.; Bouchard, D.; Sheehy, C. Development of a Versatile Rheocasting Technology; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2006. [Google Scholar] [CrossRef]
- Hussey, M.J.; Browne, D.J.; Brabazon, D.; Car, A.J. A direct thermal method of attaining globular morphology in the primary phase of alloys. In Proceedings of the 7th International Conference on Semi-solid Processing of Alloys and Composites, Tsukuba, Japan, 25–27 September 2002. [Google Scholar]
- Jarfors, A.E.W. Semisolid Casting of Metallic Parts and Structures. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Oxford, UK, 2022; pp. 100–116. [Google Scholar]
- Doutre, D.; Hay, G.; Wales, P.; Gabathuler, J.P. Seed: A New Process for Semi-Solid Forming. Can. Metall. Q. 2004, 43, 265–272. [Google Scholar] [CrossRef]
- Ferrante, M.; de Freitas, E. Rheology and microstructural development of a Al–4wt%Cu alloy in the semi-solid state. Mater. Sci. Eng. A 1999, 271, 172–180. [Google Scholar] [CrossRef]
- Qian, M. Creation of semisolid slurries containing fine and spherical particles by grain refinement based on the Mullins–Sekerka stability criterion. Acta Mater. 2006, 54, 2241–2252. [Google Scholar] [CrossRef]
- Le, Q.C.; Cui, J.; Xia, K. Thixotropic Mg Alloys through Liquidus and Sub-Liquidus Casting. Mater. Sci. Forum 2005, 488/489, 303–306. [Google Scholar] [CrossRef]
- Liu, D.; Atkinson, H.V.; Kapranos, P.; Jirattiticharoean, W.; Jones, H. Microstructural evolution and tensile mechanical properties of thixoformed high performance aluminium alloys. Mater. Sci. Eng. A 2003, 361, 213–224. [Google Scholar] [CrossRef]
- Xia, K.; Tausig, G. Liquidus casting of a wrought aluminum alloy 2618 for thixoforming. Mater. Sci. Eng. A 1998, 246, 1–10. [Google Scholar] [CrossRef]
- Lü, S.; Wu, S.; Lin, C.; Hu, Z.; An, P. Preparation and rheocasting of semisolid slurry of 5083 Al alloy with indirect ultrasonic vibration process. Mater. Sci. Eng. A 2011, 528, 8635–8640. [Google Scholar] [CrossRef]
- Abramov, O.V. Ultrasound in Liquid and Solid Metals; CRC Press: Boca Raton, FL, USA, 1993; pp. 138–159. [Google Scholar]
- Wu, S.; Xie, L.; Zhao, J.; Nakae, H. Formation of non-dendritic microstructure of semi-solid aluminum alloy under vibration. Scr. Mater. 2008, 58, 556–559. [Google Scholar] [CrossRef]
- Jian, X.; Xu, H.; Meek, T.T.; Han, Q. Effect of power ultrasound on solidification of aluminum A356 alloy. Mater. Lett. 2005, 59, 190–193. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Le, Q.C.; Cui, J.Z. Microstructures and mechanical properties of AZ80 alloy treated by pulsed ultrasonic vibration. Trans. Nonferrous Met. Soc. China 2008, 18, s113–s116. [Google Scholar] [CrossRef]
- Luo, S.J.; Keung, W.C.; Kang, Y.L. Theory and application research development of semi-solid forming in China. Trans. Nonferrous Met. Soc. China 2010, 20, 1805–1814. [Google Scholar] [CrossRef]
- Qi, M.; Xu, Y.; Li, J.; Kang, Y.; Wulabieke, Z. Microstructure refinement and corrosion resistance improvement mechanisms of a novel Al-Si-Fe-Mg-Cu-Zn alloy prepared by ultrasonic vibration-assisted rheological die-casting process. Corros. Sci. 2021, 180, 109180. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Guan, R.; Wen, J. Formation and Evolution of Non-dendritic Microstructures of Semi-solid Alloys Prepared by Shearing/Cooling Roll Process. J. Mater. Sci. Technol. 2009, 22, 195–199. [Google Scholar]
- Kiuchi, M.; Sugiyama, S. A New Process to Manufacture Semi-solid Alloys. ISIJ Int. 1995, 35, 790–797. [Google Scholar] [CrossRef]
- Young, K.P.; Kyonka, C.P.; Courtois, J.A. Fine Grained Metal Composition. U.S. Patent US4415374A, 15 November 1983. [Google Scholar]
- Kirkwood, D.H.; Kapranos, P. Semi-solid processing of alloys. Met. Mater. 1989, 5, 16–19. [Google Scholar]
- Kirkwood, D.H.; Sellars, C.M.; Elias-Boyed, L.G. Thixotropic Materials. International Patent WO 87/06957, 19 November 1987. [Google Scholar]
- Binesh, B.; Aghaie-Khafri, M. RUE-based semi-solid processing: Microstructure evolution and effective parameters. Mater. Des. 2016, 95, 268–286. [Google Scholar] [CrossRef]
- Grant, P.S. Spray forming. Prog. Mater. Sci. 1995, 39, 497–545. [Google Scholar] [CrossRef]
- Ward, P.J.; Atkinson, H.V.; Anderson, P.R.G.; Elias, L.G.; Garcia, B.; Kahlen, L.; Rodriguez-ibabe, J.M. Semi-solid processing of novel MMCs based on hypereutectic aluminium-silicon alloys. Acta Mater. 1996, 44, 1717–1727. [Google Scholar] [CrossRef]
- Tzimas, E.; Zavaliangos, A. Evolution of near-equiaxed microstructure in the semisolid state. Mater. Sci. Eng. A 2000, 289, 228–240. [Google Scholar] [CrossRef]
- Manson-Whitton, E.D.; Stone, I.C.; Jones, J.R.; Grant, P.S.; Cantor, B. Isothermal grain coarsening of spray formed alloys in the semi-solid state. Acta Mater. 2002, 50, 2517–2535. [Google Scholar] [CrossRef]
- Ahmad, A.H.; Naher, S.; Brabazon, D. The Effect of Direct Thermal Method, Temperature and Time on Microstructure of a Cast Aluminum Alloy. Mater. Manuf. Process. 2014, 29, 134–139. [Google Scholar] [CrossRef]
- Ahmad, A.; Naher, S.; Brabazon, D. Direct Thermal Method of Aluminium 7075. Adv. Mater. Res. 2014, 939, 400–408. [Google Scholar] [CrossRef]
- Omar, M.Z.; Alfan, A.; Syarif, J.; Atkinson, H.V. Microstructural investigations of XW-42 and M2 tool steels in semi-solid zones via direct partial remelting route. J. Mater. Sci. 2011, 46, 7696–7705. [Google Scholar] [CrossRef]
- Omar, M.Z.; Atkinson, H.; Palmiere, E.; Howe, A.; Kapranos, P. Microstructural Development of HP9/4/30 Steel During Partial Remelting. Steel Res. Int. 2004, 75, 552–560. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.M.; Zou, M.H.; Han, Z.T. Microstructure evolution of components of ZAlSi8Cu3Fe alloy in processing of thixo-diecasting. Trans. Nonferrous Met. Soc. China 2008, 18, 109–115. [Google Scholar] [CrossRef]
- Omar, M.Z.; Atkinson, H.V.; Kapranos, P. Thixotropy in Semisolid Steel Slurries under Rapid Compression. Metall. Mater. Trans. A 2011, 42, 2807–2819. [Google Scholar] [CrossRef]
- Wessen, M.; Cao, H. A Method of and a Device for Producing a Liquid-Solid Metal Composition. Europe Patent EP1838885B1, 7 August 2013. [Google Scholar]
- Fragner, W.; Peterlechner, C.; Potzinger, R. Scale-up of magnesium new rheocasting from a laboratory level to an industrial process. In Proceedings of the 2005 TMS Annual Meeting, San Francisco, CA, USA, 13–17 February 2005. [Google Scholar]
- Granath, O.; Wessén, M.; Cao, H. Porosity reduction possibilities in commercial Aluminium A380 and Magnesium AM60 alloy components using the RheoMetalTM process. Mater. Sci. Technol. 2010, 28, 1–10. [Google Scholar]
- Gupta, R.; Sharma, A.; Pandel, U.; Ratke, L. Coarsening kinetics in Al alloy cast through rapid slurry formation (RSF) Process. Acta Metall. Slovaca 2017, 23, 12–21. [Google Scholar] [CrossRef]
- Rogal, Ł. Critical assessment: Opportunities in developing semi-solid processing: Aluminium, magnesium, and high-temperature alloys. Mater. Sci. Technol. 2017, 33, 759–764. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, X.; Wu, Y.; Peng, L.; Ding, W. Review on criteria for assessing the processability of semisolid alloys. Mater. Lett. 2021, 282, 128835. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, H.; Atkinson, H. What is the Process Window for Semi-solid Processing? Metall. Mater. Trans. A 2016, 47, 1–5. [Google Scholar] [CrossRef]
- Vogel, A.; Doherty, R.; Cantor, B. Stir-cast microstructure and slow crack growth. In Solidification and Casting of Metals, Proceedings of the an International Conference on Solidification, London, UK, 18–21 July 1979; Metals Society: London, UK, 1979. [Google Scholar]
- Doherty, R.D.; Lee, H.I.; Feest, E.A. Microstructure of stir-cast metals. Mater. Sci. Eng. 1984, 65, 181–189. [Google Scholar] [CrossRef]
- Ji, S. The fragmentation of primary dendrites during shearing in semisolid processing. J. Mater. Sci. 2003, 38, 1559–1564. [Google Scholar] [CrossRef]
- Niroumand, B.; Xia, K. 3D study of the structure of primary crystals in a rheocast Al–Cu alloy. Mater. Sci. Eng. 2000, 283, 70–75. [Google Scholar] [CrossRef]
- Mullis, A.M. Growth induced dendritic bending and rosette formation during solidification in a shearing flow. Acta Mater. 1999, 47, 1783–1789. [Google Scholar] [CrossRef]
- Dragnevski, K.; Mullis, A.M.; Walker, D.J.; Cochrane, R.F. Mechanical deformation of dendrites by fluid flow during the solidification of undercooled melts. Acta Mater. 2002, 50, 3743–3755. [Google Scholar] [CrossRef]
- Flemings, M.C.; Yurko, J.A.; Martinez, R.A. Solidification processes and microstructures. In Proceedings of the 2004 TMS Annual Meeting, Charlotte, NC, USA, 14–18 March 2004. [Google Scholar]
- Flemings, M.C.; Yurko, J.A.; Martinez, R.A. Semi-solid forming: Our understanding today and its implications for improved process. In Proceedings of the 8th International Conference on Semi-Solid Processing of Alloys and Composites, Limassol, Cyprus, 21–23 September 2004. [Google Scholar]
- Hellawell, A. Grain evaluation in conventional rheocasting. In Proceedings of the Fourth International Conference on Semi-solid Processing of Alloys and Composites, Sheffield, UK, 19–21 June 1996. [Google Scholar]
- Jackson, K.; Hunt, J.; Uhlmann, D.; Seward, T. On origin of equiaxed zone in castings. Trans. Metall. Soc. AIME 1966, 236, 149–157. [Google Scholar]
- Ruvalcaba, D.; Mathiesen, R.H.; Eskin, D.G.; Arnberg, L.; Katgerman, L. In situ observations of dendritic fragmentation due to local solute-enrichment during directional solidification of an aluminum alloy. Acta Mater. 2007, 55, 4287–4292. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z. Solidification behavior of Sn-15 wt pct Pb alloy under a high shear rate and high intensity of turbulence during semisolid processing. Metall. Mater. Trans. A 2002, 33, 3511–3520. [Google Scholar] [CrossRef]
- Ji, S.; Qian, M.; Fan, Z. Semisolid processing characteristics of AM series Mg alloys by rheo-diecasting. Metall. Mater. Trans. A 2006, 37, 779–787. [Google Scholar] [CrossRef]
- Das, A.; Ji, S.; Fan, Z. Morphological development of solidification structures under forced fluid flow: A Monte-Carlo simulation. Acta Mater. 2002, 50, 4571–4585. [Google Scholar] [CrossRef]
- Li, T.; Lin, X.; Huang, W. Morphological evolution during solidification under stirring. Acta Mater. 2006, 54, 4815–4824. [Google Scholar] [CrossRef]
- Sannes, S.; Gjestland, H.; Arnberg, L.; Solberg, J.K. Microstructure coarsening of semi-solid Mg alloys. In Proceedings of the 3rd International Conference on Semisolid Processing of Alloys and Composites, University of Tokyo, Tokyo, Japan, 13–15 June 1994. [Google Scholar]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids. 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wagner, C. Theory of precipitate change by redissolution. Z. Elektrochem. 1961, 65, 581–591. [Google Scholar]
- Ji, S.; Roberts, K.; Fan, Z. Isothermal coarsening of fine and spherical particles in semisolid slurry of Mg–9Al–1Zn alloy under low shear. Scr. Mater. 2006, 55, 971–974. [Google Scholar] [CrossRef]
- Peeratatsuwan, C.; Pandee, P.; Patakham, U.; Limmaneevichitr, C. Microstructure and rheological properties of a semisolid A356 alloy with erbium addition. J. Rare Earths 2021, 40, 1148–1155. [Google Scholar] [CrossRef]
- Mao, W.M.; Chen, Z.Z.; Liu, H.W.; Li, Y.G. Preparation and Rheo-Die Casting of Semi-Solid A356 Aluminum Alloy Slurry through a Serpentine Pouring Channel. Solid State Phenom. 2013, 192–193, 404–409. [Google Scholar] [CrossRef]
- Qi, M.; Kang, Y.; Li, J.; Wulabieke, Z.; Xu, Y.; Li, Y.; Liu, A.; Chen, J. Microstructures refinement and mechanical properties enhancement of aluminum and magnesium alloys by combining distributary-confluence channel process for semisolid slurry preparation with high pressure die-casting. J. Mater. Process. Technol. 2020, 285, 116800. [Google Scholar] [CrossRef]
- Li, N.Y.; Mao, W.M.; Geng, X.X. Preparation of semi-solid 6061 aluminum alloy slurry by serpentine channel pouring. Trans. Nonferrous Met. Soc. China. 2022, 32, 739–749. [Google Scholar] [CrossRef]
- Binesh, B.; Aghaie-Khafri, M. Phase Evolution and Mechanical Behavior of the Semi-Solid SIMA Processed 7075 Aluminum Alloy. Metals 2016, 6, 42. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Wang, Y.; Xiao, G.; Liu, Y.; Huang, M. Recrystallization process of hot-extruded 6A02 aluminum alloy in solid and semi-solid temperature ranges. J. Alloys Compd. 2022, 893, 162311. [Google Scholar] [CrossRef]
- Lin, B.; Fan, T.; Li, H.-y.; Zhao, Y.-l.; Zhang, W.-w.; Liu, K. Microstructure and high temperature tensile properties of Al–Si–Cu–Mn–Fe alloys prepared by semi-solid thixoforming. Trans. Nonferrous Met. Soc. China 2021, 31, 2232–2249. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.B.; Cao, Z.Y.; Zhang, Y.F.; Zhang, Q.Q. Effects of isothermal process parameters on the microstructure of semisolid AZ91D alloy produced by SIMA. J. Mater. Process. Technol. 2009, 209, 792–797. [Google Scholar] [CrossRef]
- Yan, G.; Zhao, S.; Ma, S.; Shou, H. Microstructural evolution of A356.2 alloy prepared by the SIMA process. Mater. Charact. 2012, 69, 45–51. [Google Scholar] [CrossRef]
- Loué, W.R.; Suéry, M. Microstructural evolution during partial remelting of AlSi7Mg alloys. Mater. Sci. Eng. A 1995, 203, 1–13. [Google Scholar] [CrossRef]
- Blais, S.; Loue, W.; Pluchon, C. Structure control by electromagnetic stirring and reheating at semi-solid state. In Proceedings of the 4th International Conference on Semi-Solid Processing of Alloys and Composites, Sheffield, UK, 19–21 June 1996. [Google Scholar]
- Annavarapu, S.; Doherty, R.D. Inhibited coarsening of solid-liquid microstructures in spray casting at high volume fractions of solid. Acta Metall. Mater. 1995, 43, 3207–3230. [Google Scholar] [CrossRef]
- Wang, T.; Yin, Z.M.; Sun, Q. Effect of homogenization treatment on microstructure and hot workability of high strength 7B04 aluminium alloy. Trans. Nonferrous Met. Soc. China 2007, 17, 335–339. [Google Scholar] [CrossRef]
- Fan, X.G.; Jiang, D.M.; Meng, Q.C.; Zhang, B.Y.; Wang, T. Evolution of eutectic structures in Al-Zn-Mg-Cu alloys during heat treatment. Trans. Nonferrous Met. Soc. China 2006, 16, 577–581. [Google Scholar] [CrossRef]
- Campo, K.N.; de Freitas, C.C.; da Fonseca, E.B.; Caram, R. CrCuFeMnNi high-entropy alloys for semisolid processing: The effect of copper on phase formation, melting behavior, and semisolid microstructure. Mater. Charact. 2021, 178, 111260. [Google Scholar] [CrossRef]
- Zhang, L.J.; Fan, J.T.; Liu, D.J.; Zhang, M.D.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; Liu, R.P. The microstructural evolution and hardness of the equiatomic CoCrCuFeNi high-entropy alloy in the semi-solid state. J. Alloys Compd. 2018, 745, 75–83. [Google Scholar] [CrossRef]
- Campo, K.N.; de Freitas, C.C.; Fanton, L.; Caram, R. Melting behavior and globular microstructure formation in semi-solid CoCrCuxFeNi high-entropy alloys. J. Mater. Sci. Technol. 2020, 52, 207–217. [Google Scholar] [CrossRef]
- Rogal, Ł. Semi-solid processing of the CoCrCuFeNi high entropy alloy. Mater. Des. 2017, 119, 406–416. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, M.; Wang, Y.; Liu, Y.; Zhang, Y. Microstructure evolution and formation mechanism of CoCrCu1.2FeNi high entropy alloy during the whole process of semi-solid billet preparation. J. Mater. Sci. Technol. 2022, 120, 172–185. [Google Scholar] [CrossRef]
- Haga, T.; Kapranos, P. Simple rheocasting processes. J. Mater. Process. Technol. 2002, 130–131, 594–598. [Google Scholar] [CrossRef]
- Birol, Y. Cooling slope casting and thixoforming of hypereutectic A390 alloy. J. Mater. Process. Technol. 2008, 207, 200–203. [Google Scholar] [CrossRef]
- Motegi, T.; Tanabe, F. New semi-solid casting of copper alloys using AN inclined cooling plate. In Proceedings of the Eighth International Conference on Semi-Solid Processing of Alloys and Composites, Limassol, Cyprus, 21–23 September 2004. [Google Scholar]
- Robert, M.H.; Zoqui, E.J.; Tanabe, F.; Motegi, T. Producing thixotropic semi-solid A356 alloy: Microstructure formation x forming behaviour. J. Achiev. Mater. Manuf. Eng. 2006, 20, 19–26. [Google Scholar]
- Nili-Ahmadabadi, M.; Pahlevani, F.; Babaghorbani, P. Effect of slope plate variable and reheating on the semi-solid structure of ductile cast iron. Tsinghua Sci. Technol. 2008, 13, 147–151. [Google Scholar] [CrossRef]
- Taghavi, F.; Ghassemi, A. Study on the effects of the length and angle of inclined plate on the thixotropic microstructure of A356 aluminum alloy. Mater. Des. 2009, 30, 1762–1767. [Google Scholar] [CrossRef]
- Satya, S.J.; Kumar, V.; Barekar, N.S.; Biswas, K.; Dhindaw, B.K. Microstructural Evolution Under Low Shear Rates During Rheo Processing of LM25 Alloy. J. Mater. Eng. Perform. 2012, 21, 2283–2289. [Google Scholar] [CrossRef][Green Version]
- Curle, U.; Möller, H.; Wilkins, J. Shape rheocasting of high purity aluminium. Scr. Mater. 2011, 64, 479–482. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z. Solidification Behavior and Microstructural Evolution of Near-Eutectic Zn-Al Alloys under Intensive Shear. Metall. Mater. Trans. A 2009, 40, 185–195. [Google Scholar] [CrossRef][Green Version]
- Curle, U.A.; Wilkins, J.D. Semi-Solid Casting of Pure Magnesium. Solid State Phenom. 2019, 285, 464–469. [Google Scholar] [CrossRef]
- Curle, U.A.; Möller, H.; Wilkins, J.D. Shape rheocasting of unmodified Al–Si binary eutectic. Mater. Lett. 2011, 65, 1469–1472. [Google Scholar] [CrossRef]
- Hu, Z.H.; Wu, G.H.; Zhang, P.; Ding, W.J. Rheo-Processing of Near-Eutectic ADC12 Alloy. Solid State Phenom. 2013, 192–193, 116–122. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D. Fundamentals of Solidification; Trans Tech Publications Limited: Aedermannsdorf, Switzerland, 1989; pp. 293–305. [Google Scholar]
- Elliott, R. Eutectic Solidification Processing: Crystalline and Glassy Alloys; Butterworth-Heinemann: London, UK, 1983; pp. 105–183. [Google Scholar]
- Murakami, Y.; Omura, N. Control of Amount of α-Al Phase Particles in near Eutectic Al-Si Alloy by Electromagnetic Stirring. Solid State Phenom. 2022, 327, 250–254. [Google Scholar] [CrossRef]







| Substrate A | Substrate B | Interlayer Combination | Ref. |
|---|---|---|---|
| CuZn40Al2 | Tool steel | [47] | |
| Stainless steel | Aluminium alloy | [48] | |
| Aluminium alloy, stainless steel or cast iron | Particles, balls, short fibres of materials including ceramic, aluminium alloys, copper alloys, or stainless steels | [40,42,49,50] | |
| Stainless steel | M2 tool steel | [51] | |
| X210CrW12, 100Cr6, X5CrNil18-10 | 9SMn28, CuCo2Be, CuSn12, and GBZ12 | [37,52] | |
| Sn-15 wt%Pb | Sn-15 wt%Pb | Sn-5wt%Pb | [39] |
| Pb-15 wt%Sn | Pb-15 wt%Sn | [53] | |
| Zinc AG40A die cast alloy | Zinc AG40A die cast alloy | [54] | |
| A356 | A356 | [55] | |
| SiCP/A356 composites | 2024 aluminium alloy | Zn-Al | [56] |
| SiCP/A356 composites | SiCP/A356 composites | Zn-Al | [57] |
| AZ91 alloy | AZ91 alloy | Mg-25wt%Zn | [58] |
| A356 aluminium alloy | A356 aluminium alloy | [59] | |
| D2 tool steel | D2 tool steel | [46,60] | |
| D2 tool steel | 304 stainless steel | [61] | |
| D2 tool steel | M2 tool steel | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Wang, K.; Dong, X. An Overview on the Process Development and the Formation of Non-Dendritic Microstructure in Semi-Solid Processing of Metallic Materials. Crystals 2022, 12, 1044. https://doi.org/10.3390/cryst12081044
Ji S, Wang K, Dong X. An Overview on the Process Development and the Formation of Non-Dendritic Microstructure in Semi-Solid Processing of Metallic Materials. Crystals. 2022; 12(8):1044. https://doi.org/10.3390/cryst12081044
Chicago/Turabian StyleJi, Shouxun, Kai Wang, and Xixi Dong. 2022. "An Overview on the Process Development and the Formation of Non-Dendritic Microstructure in Semi-Solid Processing of Metallic Materials" Crystals 12, no. 8: 1044. https://doi.org/10.3390/cryst12081044
APA StyleJi, S., Wang, K., & Dong, X. (2022). An Overview on the Process Development and the Formation of Non-Dendritic Microstructure in Semi-Solid Processing of Metallic Materials. Crystals, 12(8), 1044. https://doi.org/10.3390/cryst12081044








