Functionalized Microbial Consortia with Silver-Doped Hydroxyapatite (Ag@HAp) Nanostructures for Removal of RO84 from Industrial Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silver Nanoparticles (Ag NPs)
2.2. Silver-Doped Hydroxyapatite (Ag@HAp) NPs
2.3. Growth Experiment of Consortia
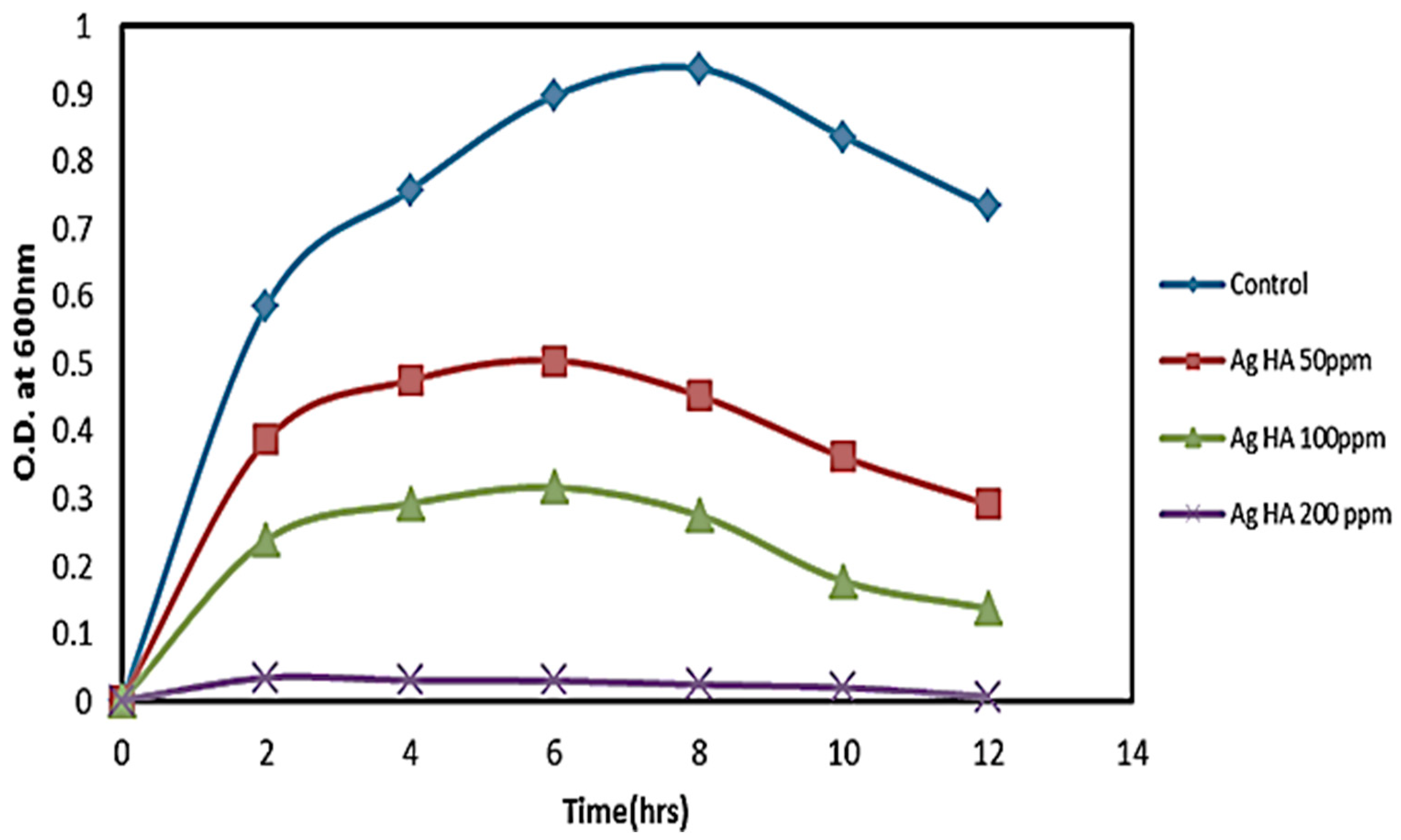
2.4. Preparation of Consortia and Their Growth Test with Ag and without Ag@HAp
2.5. Standardization of Dye Solution
2.5.1. Remediation Experiment
2.5.2. Effect of pH
2.6. Characterization Techniques
3. Results and Discussions
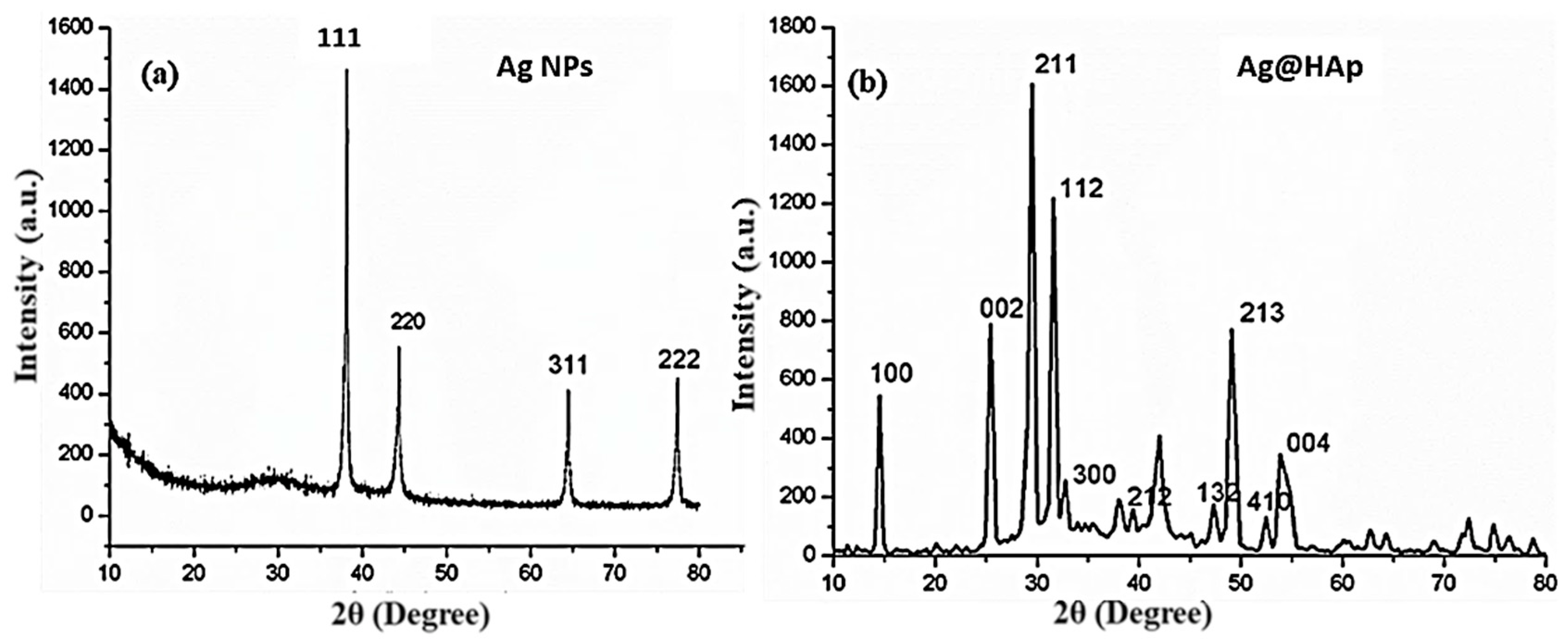
3.1. Structural Confirmations: XRD Analysis
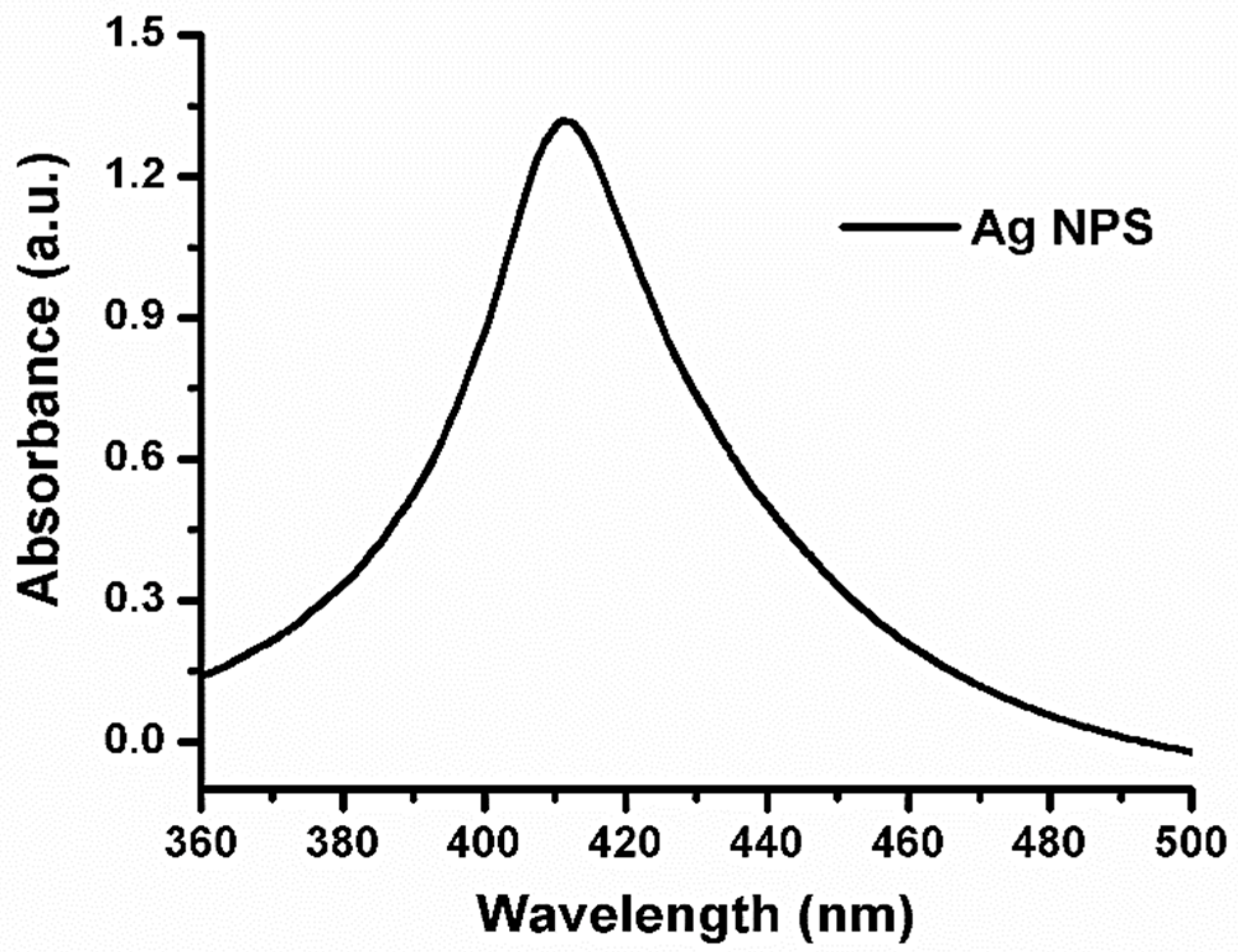
3.2. UV-Vis Absorbance Spectra
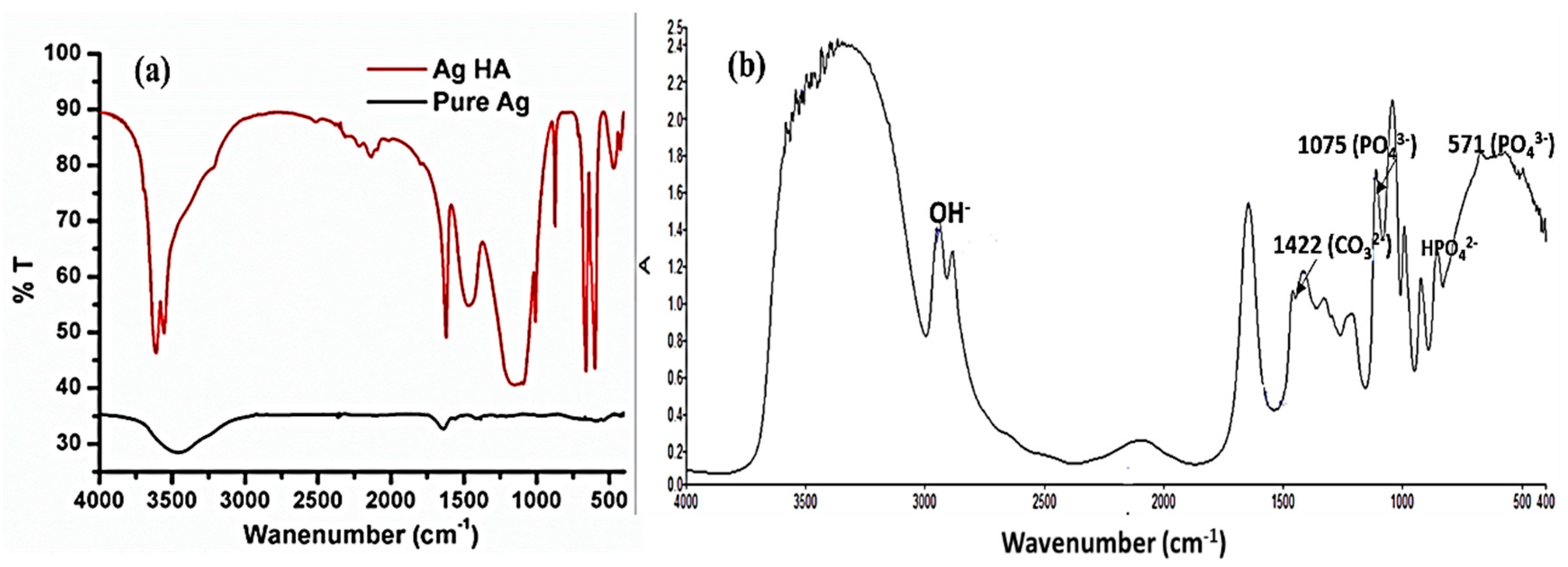
3.3. Functional Group Confirmations: FTIR Analysis
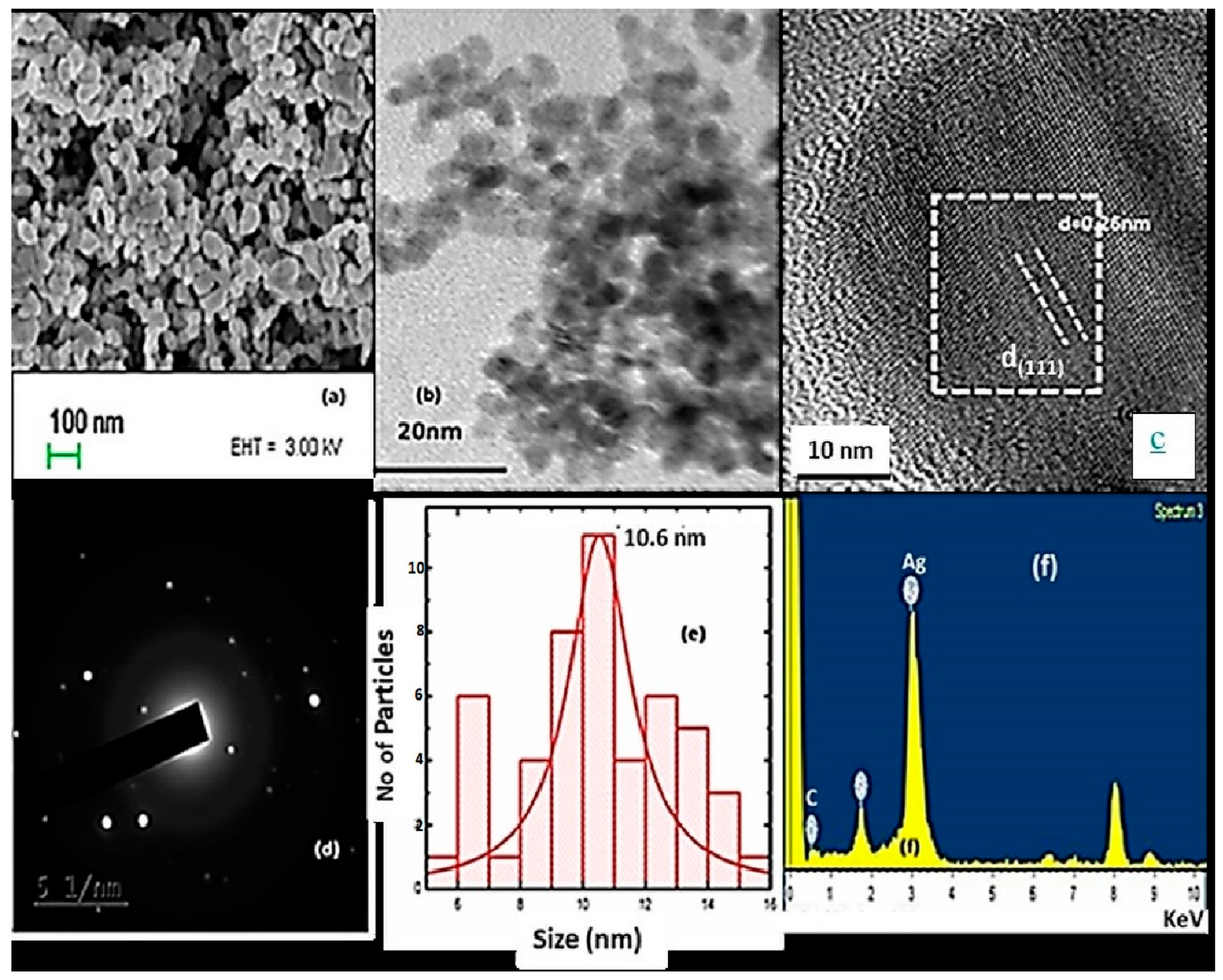
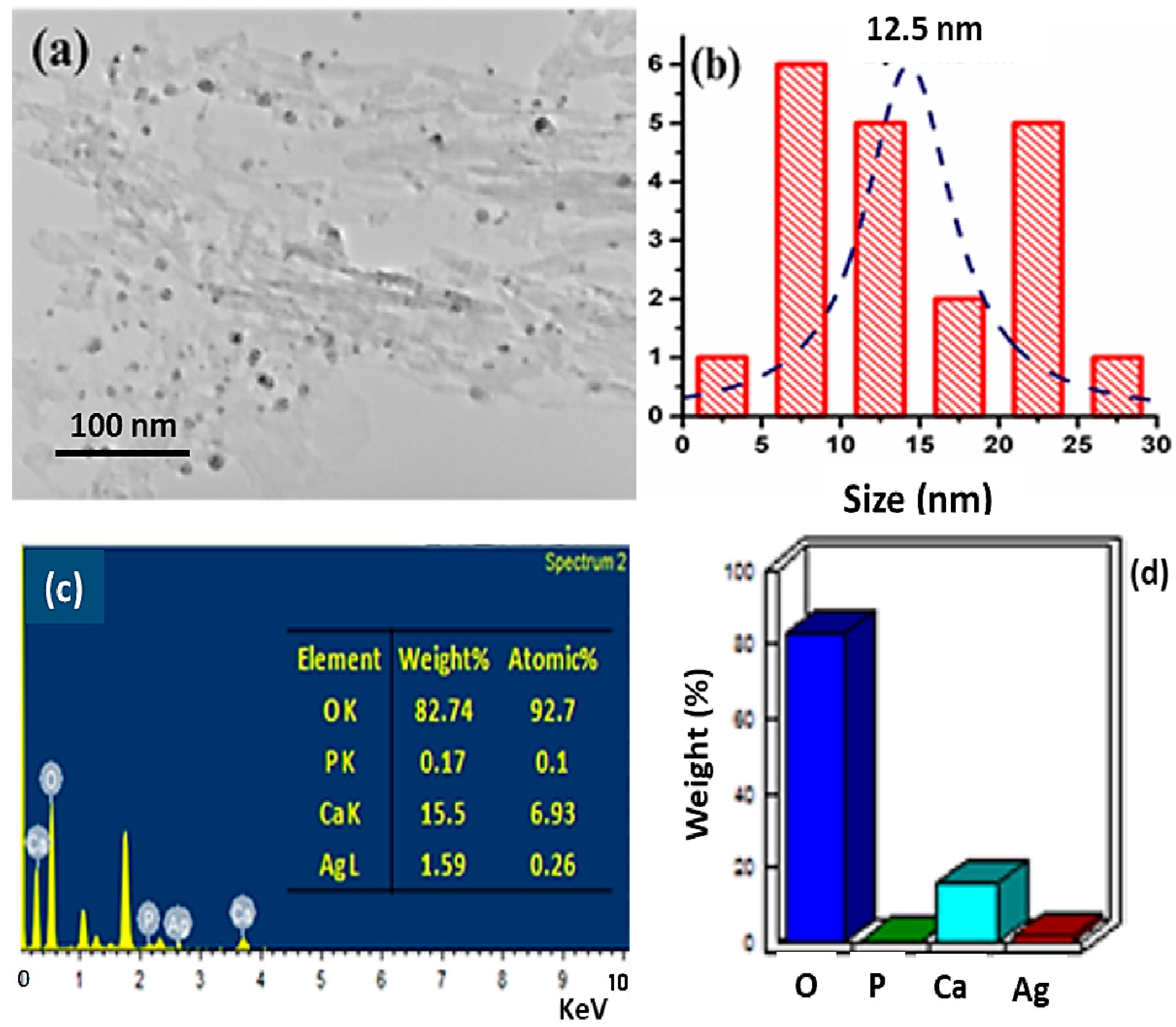
3.4. Morphological Studies: HRTEM Analysis
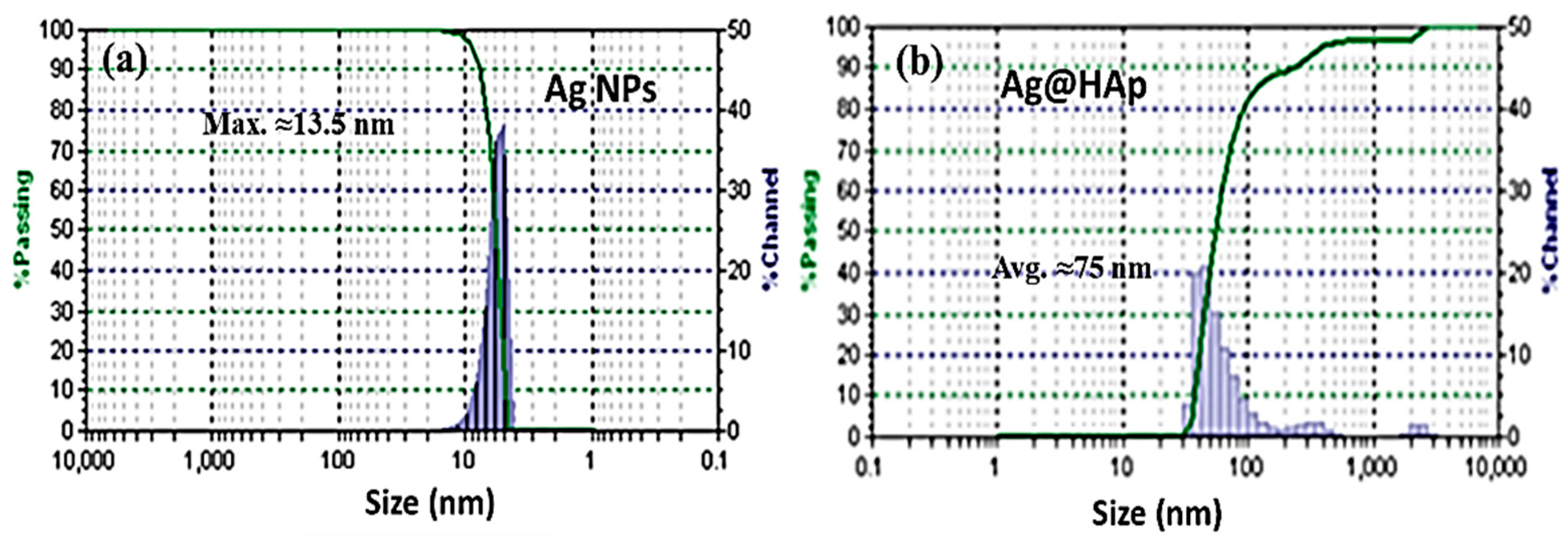
3.5. DLS Study
4. Remediation of Industrial Effluents
4.1. Removal of Microbial Pathogens
4.2. Adsorption of Consortia by Nanoparticles
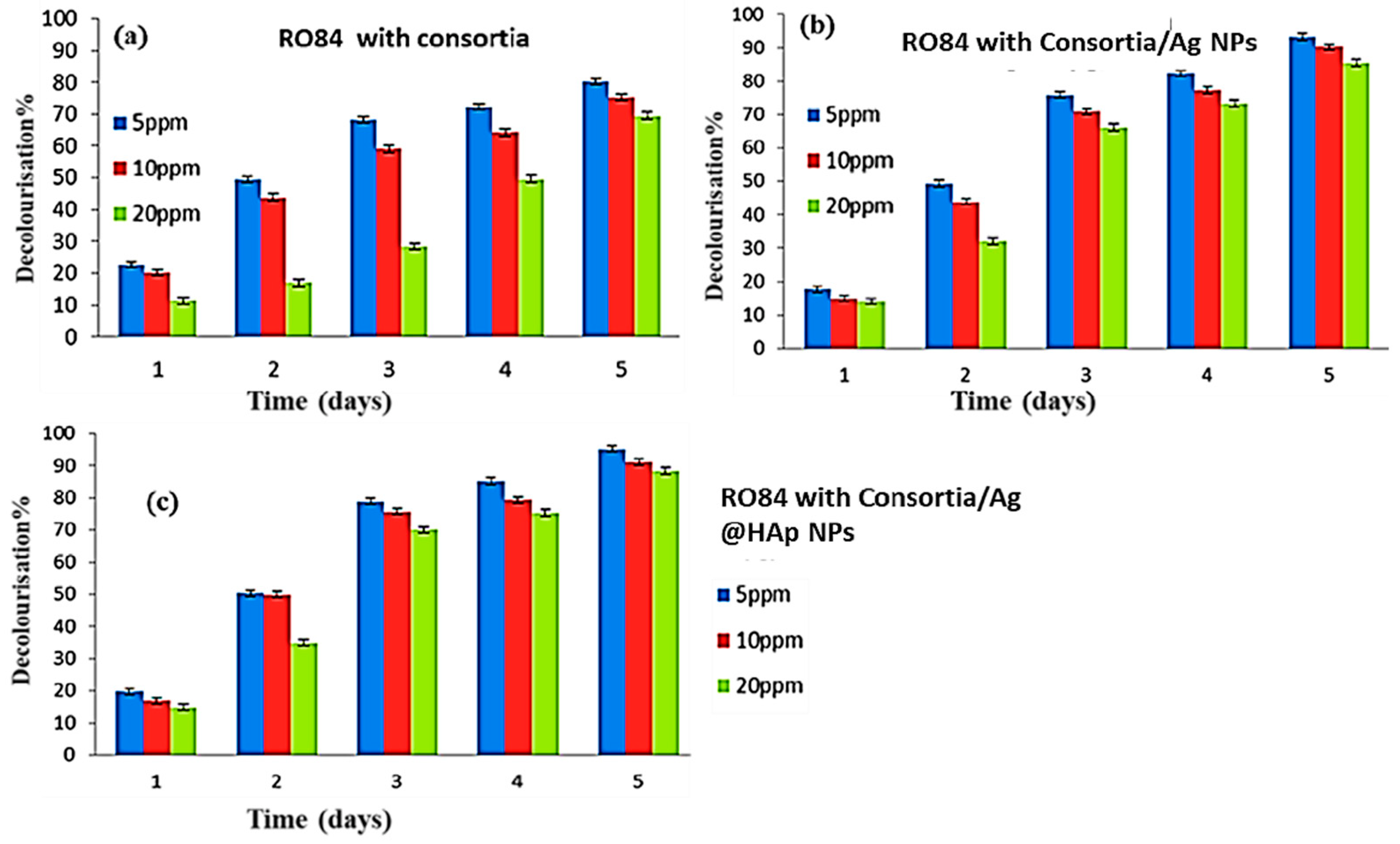
5. Remediation of Reactive Orange 84 (RO 84)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algethami, J.S.; Hassan, M.S.; Alorabi, A.Q.; Alhemiary, N.A.; Fallatah, A.M.; Alnaam, Y.; Almusabi, S.; Amna, T. Manganese Ferrite–Hydroxyapatite Nanocomposite Synthesis: Biogenic Waste Remodeling for Water Decontamination. Nanomaterials 2022, 12, 1631. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Inwati, G.K.; Yadav, V.K.; Ali, I.H.; Vuggili, S.B.; Kakodiya, S.D.; Solanki, M.K.; Yadav, K.K.; Ahn, Y.; Yadav, S.; Islam, S.; et al. 2D Personality of Multifunctional Carbon Nitrides towards Enhanced Catalytic Performance in Energy Storage and Remediation. Appl. Sci. 2022, 12, 3753. [Google Scholar] [CrossRef]
- Rajendran, S.; Inwati, G.K.; Yadav, V.K.; Choudhary, N.; Solanki, M.B.; Abdellattif, M.H.; Yadav, K.K.; Gupta, N.; Islam, S.; Jeon, B.-H. Enriched Catalytic Activity of TiO2 Nanoparticles Supported by Activated Carbon for Noxious Pollutant Elimination. Nanomaterials 2021, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Suriyaprabha, R.; Inwati, G.K.; Gupta, N.; Singh, B.; Lal, C.; Kumar, P.; Godha, M.; Kalasariya, H. A Noble and Economical Method for the Synthesis of Low Cost Zeolites From Coal Fly Ash Waste. Adv. Mater. Process. Technol. 2021, 1–19. [Google Scholar] [CrossRef]
- Inwati, G.K.; Kumar, P.; Roos, W.; Swart, H. Thermally induced structural metamorphosis of ZnO:Rb nanostructures for antibacterial impacts. Colloids Surf. B Biointerfaces 2020, 188, 110821. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Inwati, G.; Ghosh, S.; Kumar, V.; Roos, W.; Swart, H. Optical and surface properties of Zn doped CdO nanorods and antimicrobial applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125369. [Google Scholar] [CrossRef]
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Maze, J.; Swart, H.C. Recent advances on ferrites nanomaterial’s as photocatalyst for environment. In Advances in Nanostructured Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 381–409. [Google Scholar]
- Inwati, G.; Kumar, P.; Roos, W.; Swart, H.; Singh, M. UV-irradiation effects on tuning LSPR of Cu/Ag nanoclusters in ion exchanged glass matrix and its thermodynamic behaviour. J. Alloy. Compd. 2020, 823, 153820. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The role of calix[n]arenes and pillar[n]arenes in the design of silver nanopar-ticles: Self-assembly and application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [Green Version]
- Batool, M.; Daoush, W.M.; Hussain, M.K. Dye Sequestration Using Biosynthesized Silver Nanoparticles Adsorbent in Aqueous Solutions. Crystals 2022, 12, 662. [Google Scholar] [CrossRef]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Lim, P.; Shi, Z.; Tay, B.; Neoh, K. Silver-Doped Apatite as a Bioactive and an Antimicrobial Bone Material. Key Eng. Mater. 2012, 493–494, 27–30. [Google Scholar] [CrossRef]
- Modi, S.; Inwati, G.K.; Gacem, A.; Abullais, S.S.; Prajapati, R.; Yadav, V.K.; Syed, R.; Alqahtani, M.S.; Yadav, K.K.; Islam, S.; et al. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics 2022, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.A.C.; Vercik, L.C.D.O.; Monteiro, F.J.; Rigo, E.C.D.S. Preparation, characterization and antibacterial properties of silver nanoparticles–hydroxyapatite composites by a simple and eco-friendly method. Ceram. Int. 2016, 42, 2271–2280. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.M.; Selvaraj, R.; Pugazhendhi, A. A review on the synthesis of hydroxyapatite, its composites and adsorptive removal of pollutants from wastewater. J. Water Process Eng. 2020, 38, 101574. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Rathi, M.; Almaary, K.S.; Alkhattaf, F.S.; Elbadawi, Y.B.; Chang, S.W.; Ravindran, B. Preparation and antibacterial application of hydroxyapatite doped Silver nanoparticles derived from chicken bone. J. King Saud Univ. Sci. 2022, 34, 101749. [Google Scholar] [CrossRef]
- Inwati, G.; Rao, Y.; Singh, M. Thermodynamically induced in Situ and Tunable Cu Plasmonic Behaviour. Sci. Rep. 2018, 8, 3006. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Kumar Yadav, V.; Ali, D.; Ramar, K.; Gokhlesh Kumar Narayanan, V. New designing (NH4)2SiP4O13 nanowires and effective photocatalytic degradation of Malachite green and antimicrobial properties. Chem. Phys. Lett. 2022, 803, 139817. [Google Scholar] [CrossRef]
- Malik, P.; Inwati, G.K.; Mukherjee, T.K.; Singh, S.; Singh, M. Green silver nanoparticle and Tween-20 modulated pro-oxidant to antioxidant curcumin transformation in aqueous CTAB stabilized peanut oil emulsions. J. Mol. Liq. 2019, 291, 111252. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganoderma lucidum Extract and Evaluation of Their Theranostic Applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Jadalannagari, S.; Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Antimicrobial activity of hemocompatible silver doped hydroxyapatite nanoparticles synthesized by modified sol–gel technique. Appl. Nanosci. 2014, 4, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inwati, G.; Rao, Y.; Singh, M. In Situ Growth of Low-Dimensional Silver Nanoclusters with Their Tunable Plasmonic and Thermodynamic Behavior. ACS Omega 2017, 2, 5748–5758. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and Antimicrobial Activity of Silver-Doped Hydroxyapatite Nanoparticles. BioMed Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [Green Version]
- Inwati, G.K.; Kumar, P.; Singh, M.; Yadav, V.K.; Kumar, A.; Soma, V.R.; Swart, H. Study of photoluminescence and nonlinear optical behaviour of AgCu nanoparticles for nanophotonics. Nano-Struct. Nano-Objects 2021, 28, 100807. [Google Scholar] [CrossRef]
- Wei, W.; Yang, L.; Zhong, W.H.; Li, S.Y.; Cui, J.; Wei, Z.G. Fast removal of methylene blue from aqueous solution by adsorption onto poorly crystalline hydroxyapatite nanoparticles. Dig. J. Nanomater. Biostructures 2015, 10, 1343–1363. [Google Scholar]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Hoque, N.A.; Thakur, P.; Das, S. A facile vacuum assisted synthesis of nanoparticle impregnated hydroxyapatite composites having excellent antimicrobial properties and biocompatibility. Ceram. Int. 2018, 44, 1066–1077. [Google Scholar] [CrossRef]
- Piccirillo, C.; Castro, P. Calcium hydroxyapatite-based photocatalysts for environment remediation: Characteristics, performances and future perspectives. J. Environ. Manag. 2017, 193, 79–91. [Google Scholar] [CrossRef]
- Narendran, P.; Rajendran, A.; Garhnayak, M.; Garhnayak, L.; Nivedhitha, J.; Devi, C.; Pattanayak, D.K. Influence of pH on wet-synthesis of silver decorated hydroxyapatite nanopowder. Colloids Surf. B Biointerfaces 2018, 169, 143–150. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Ahmed, E.; Akhtar, M.S.; Khalid, N. Facile and inexpensive synthesis of Ag doped ZnO/CNTs composite: Study on the efficient photocatalytic activity and photocatalytic mechanism. J. Mol. Liq. 2020, 311, 113326. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Inwati, G.K.; Swart, H.C.; Roos, W. Graphene oxide based semiconducting nanomaterial’s composites for environmental applications. In Nanoscale Compound Semiconductors and Their Optoelectronics Applications; Woodhead Publishing: Cambridge, UK, 2022; pp. 407–431. [Google Scholar]
- Inwati, G.K.; Kumar, P.; Swart, H.C. Multifunctional properties of hybrid semiconducting nanomaterials and their applications. In Nanoscale Compound Semiconductors and their Optoelectronics Applications; Woodhead Publishing: Cambridge, UK, 2022; pp. 315–350. [Google Scholar]
- Modi, S.; Prajapati, R.; Inwati, G.K.; Deepa, N.; Tirth, V.; Yadav, V.K.; Yadav, K.K.; Islam, S.; Gupta, P.; Kim, D.-H.; et al. Recent Trends in Fascinating Applications of Nanotechnology in Allied Health Sciences. Crystals 2021, 12, 39. [Google Scholar] [CrossRef]
- Yadav, V.K.; Choudhary, N.; Khan, S.H.; Malik, P.; Inwati, G.K.; Suriyaprabha, R.; Ravi, R.K. Synthesis and characterisation of nano-biosorbents and their applications for waste water treatment. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; Gheorghe, D., Ashok, V., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 252–290. [Google Scholar]
- Kumar, P.; Mathpal, M.C.; Ghosh, S.; Inwati, G.K.; Maze, J.R.; Duvenhage, M.-M.; Roos, W.; Swart, H. Plasmonic Au nanoparticles embedded in glass: Study of TOF-SIMS, XPS and its enhanced antimicrobial activities. J. Alloys Compd. 2022, 909, 164789. [Google Scholar] [CrossRef]
- Kumar, N.; Inwati, G.K.; Ahmed, E.M.; Lal, C.; Makwana, B.; Yadav, V.K.; Islam, S.; Ahn, H.-J.; Yadav, K.K.; Jeon, B.-H. Modified 7-Chloro-11H-indeno[1,2-b]quinoxaline Heterocyclic System for Biological Activities. Catalysts 2022, 12, 213. [Google Scholar] [CrossRef]


| S. No. | Notation | Identified Organism | Accession Number NCBI |
|---|---|---|---|
| 1 | SP1 | Serratia liquefaciens | Applied for accession |
| 2 | SP2 | Bacillus velezensis | MH730069 |
| 3 | SP3 | Achromobacter pulmonis | Applied for accession |
| 4 | SP4 | Pseudomonas Stutzeri | MH730067 |
| 5 | SP5 | Pseudomonas Stutzeri | MH730068 |
| 6 | SP6 | Pseudomonas Stutzeri | MH730066 |
| Samples | 5 ppm | 10 ppm | 20 ppm |
|---|---|---|---|
| Consortia | 80.32 | 75.45 | 69.54 |
| Consortia/Ag | 93.34 | 90.19 | 85.43 |
| Consortia/Ag@HAp | 95.34 | 91.20 | 88.43 |
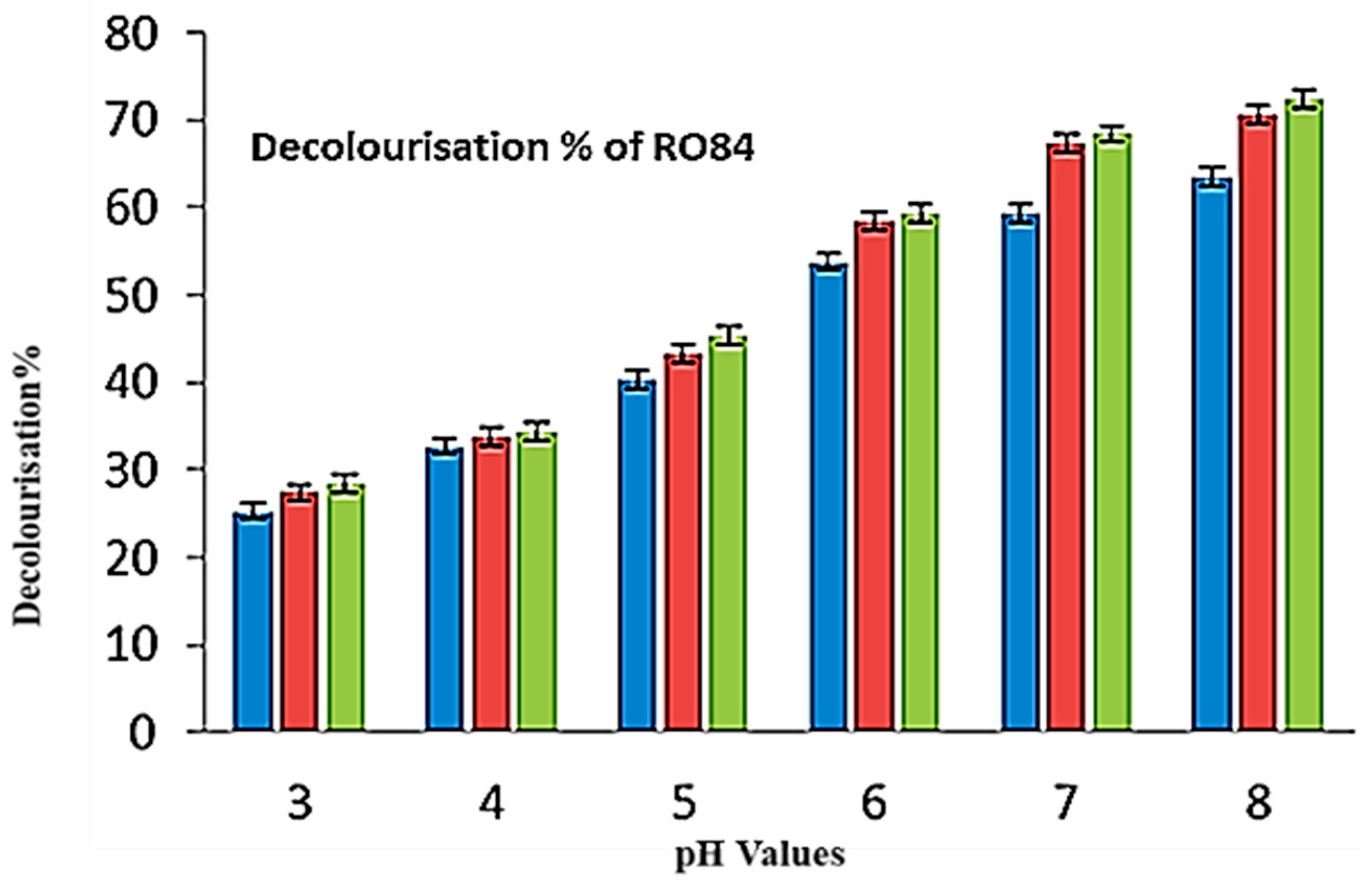
| pH | Consortia | Consortia/(Ag) | Consortia/ (Ag: HA) |
|---|---|---|---|
| 3 | 25.33 | 27.43 | 28.54 |
| 4 | 32.73 | 33.76 | 34.34 |
| 5 | 40.35 | 43.34 | 45.35 |
| 6 | 53.85 | 58.54 | 59.43 |
| 7 | 59.34 | 67.46 | 68.43 |
| 8 | 63.47 | 70.67 | 72.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, S.; Yadav, V.K.; Gacem, A.; Algethami, J.S.; Alqahtani, M.S.; Aldakheel, F.M.; Binshaya, A.S.; Alharthi, N.S.; Khan, I.A.; Islam, S.; et al. Functionalized Microbial Consortia with Silver-Doped Hydroxyapatite (Ag@HAp) Nanostructures for Removal of RO84 from Industrial Effluent. Crystals 2022, 12, 970. https://doi.org/10.3390/cryst12070970
Rajendran S, Yadav VK, Gacem A, Algethami JS, Alqahtani MS, Aldakheel FM, Binshaya AS, Alharthi NS, Khan IA, Islam S, et al. Functionalized Microbial Consortia with Silver-Doped Hydroxyapatite (Ag@HAp) Nanostructures for Removal of RO84 from Industrial Effluent. Crystals. 2022; 12(7):970. https://doi.org/10.3390/cryst12070970
Chicago/Turabian StyleRajendran, Suriyaprabha, Virendra Kumar Yadav, Amel Gacem, Jari S. Algethami, Mohammed S. Alqahtani, Fahad M. Aldakheel, Abdulkarim S. Binshaya, Nahed S. Alharthi, Imtiaz A. Khan, Saiful Islam, and et al. 2022. "Functionalized Microbial Consortia with Silver-Doped Hydroxyapatite (Ag@HAp) Nanostructures for Removal of RO84 from Industrial Effluent" Crystals 12, no. 7: 970. https://doi.org/10.3390/cryst12070970
APA StyleRajendran, S., Yadav, V. K., Gacem, A., Algethami, J. S., Alqahtani, M. S., Aldakheel, F. M., Binshaya, A. S., Alharthi, N. S., Khan, I. A., Islam, S., Ahn, Y., & Jeon, B.-H. (2022). Functionalized Microbial Consortia with Silver-Doped Hydroxyapatite (Ag@HAp) Nanostructures for Removal of RO84 from Industrial Effluent. Crystals, 12(7), 970. https://doi.org/10.3390/cryst12070970









