High-Temperature Oxidation Behaviour of Duplex Fe-Mn-Al-Ni-C Lightweight Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
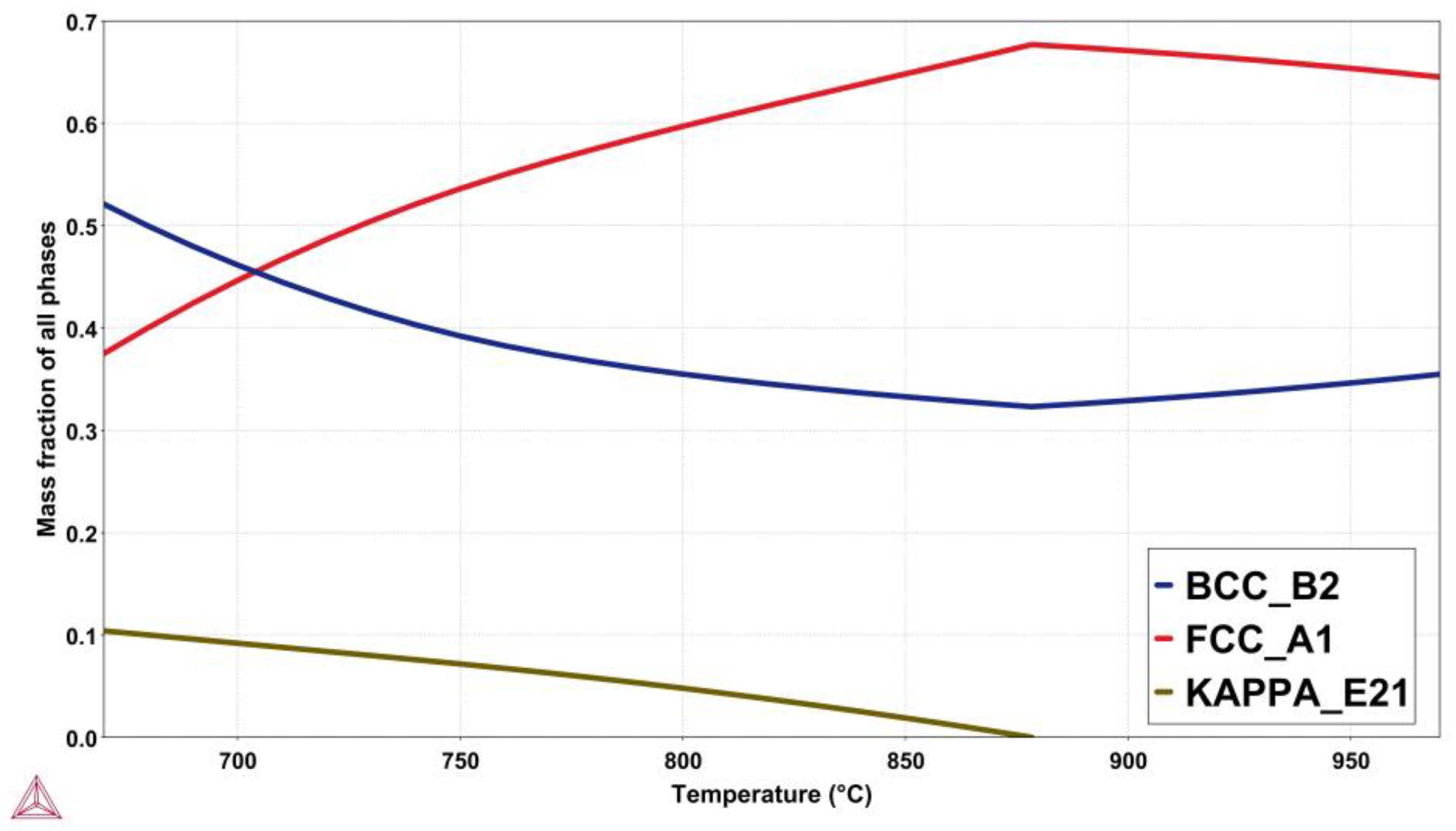
3.1. CALPHAD Calculations
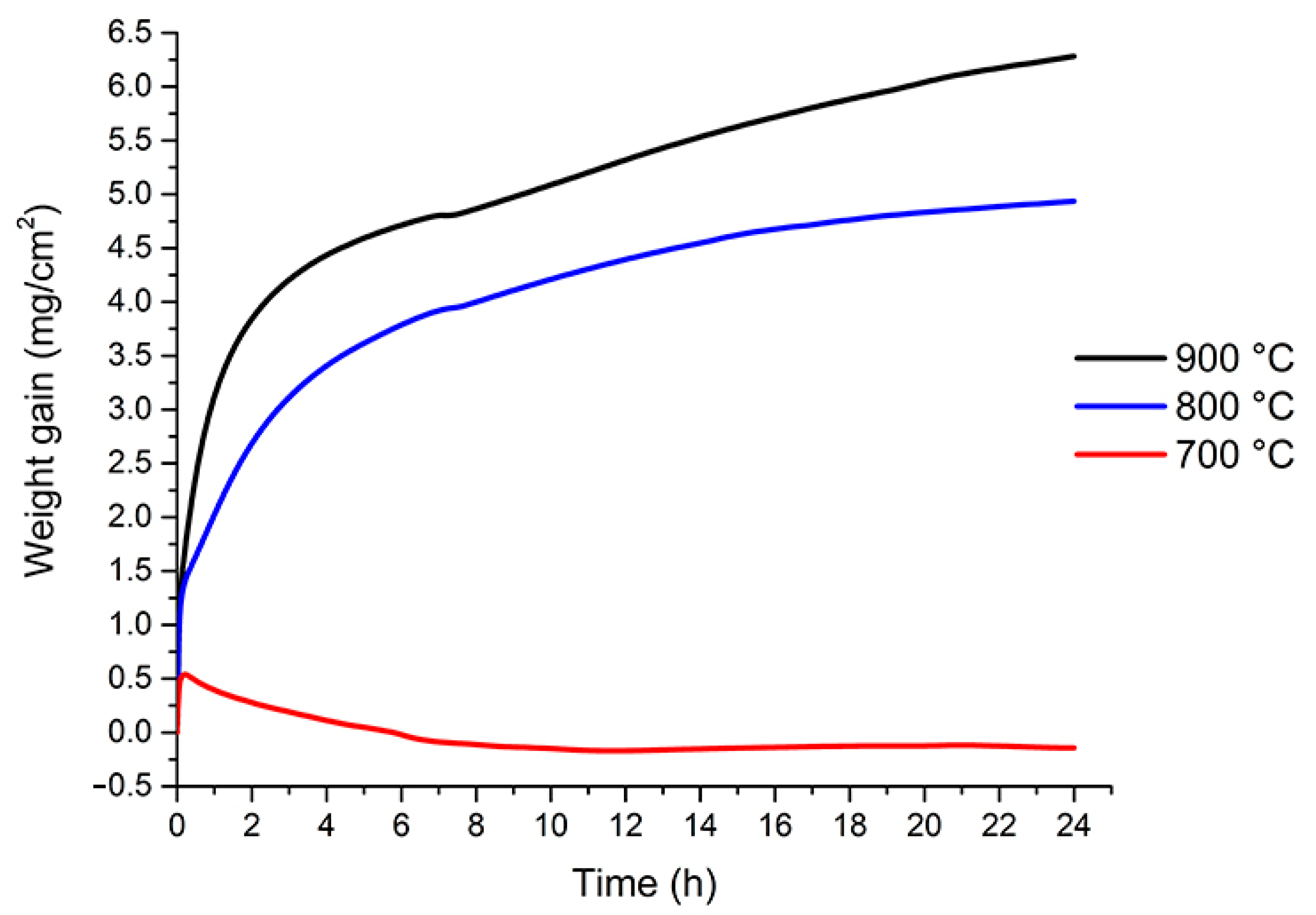
3.2. Thermogravimetric Analysis
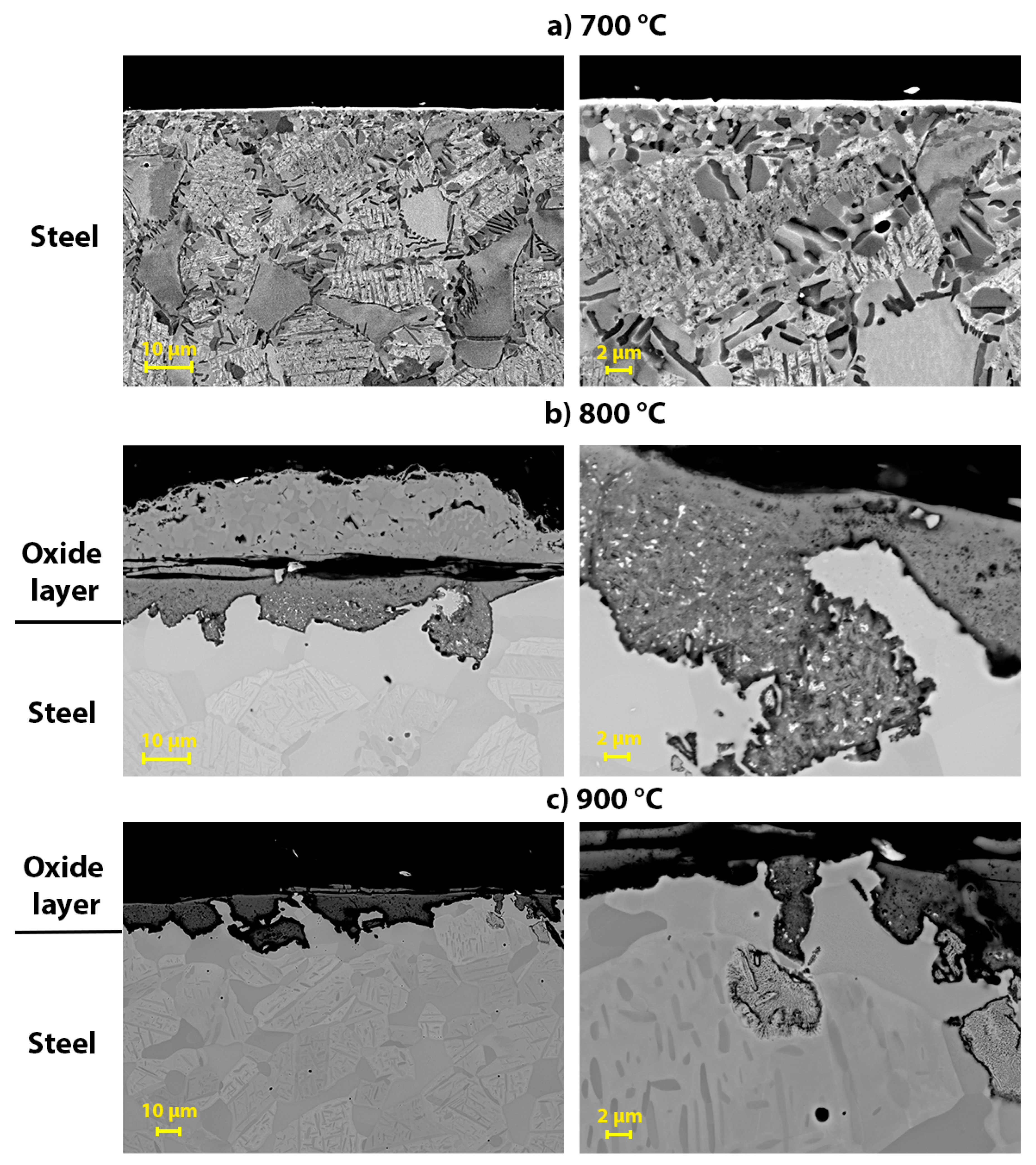
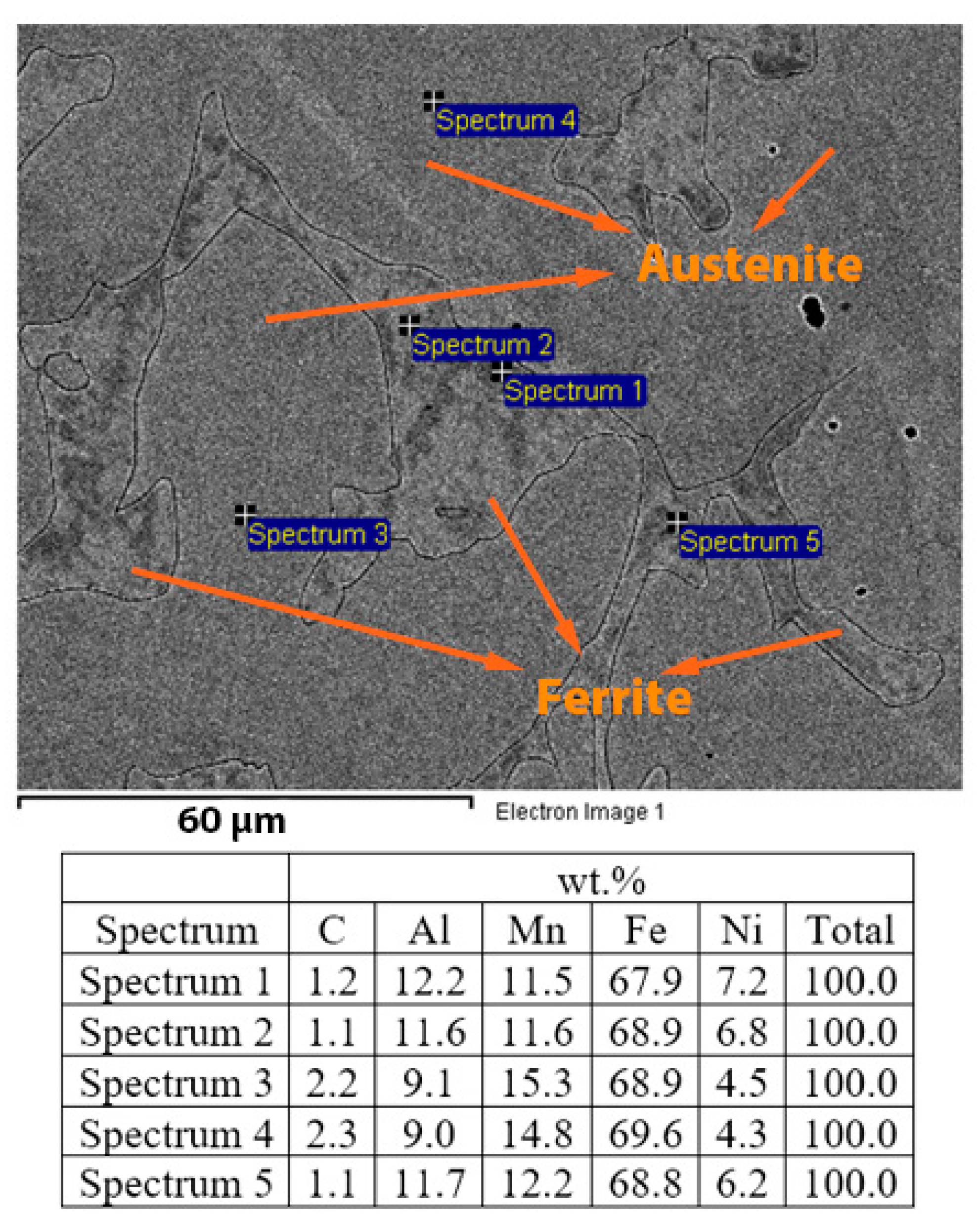
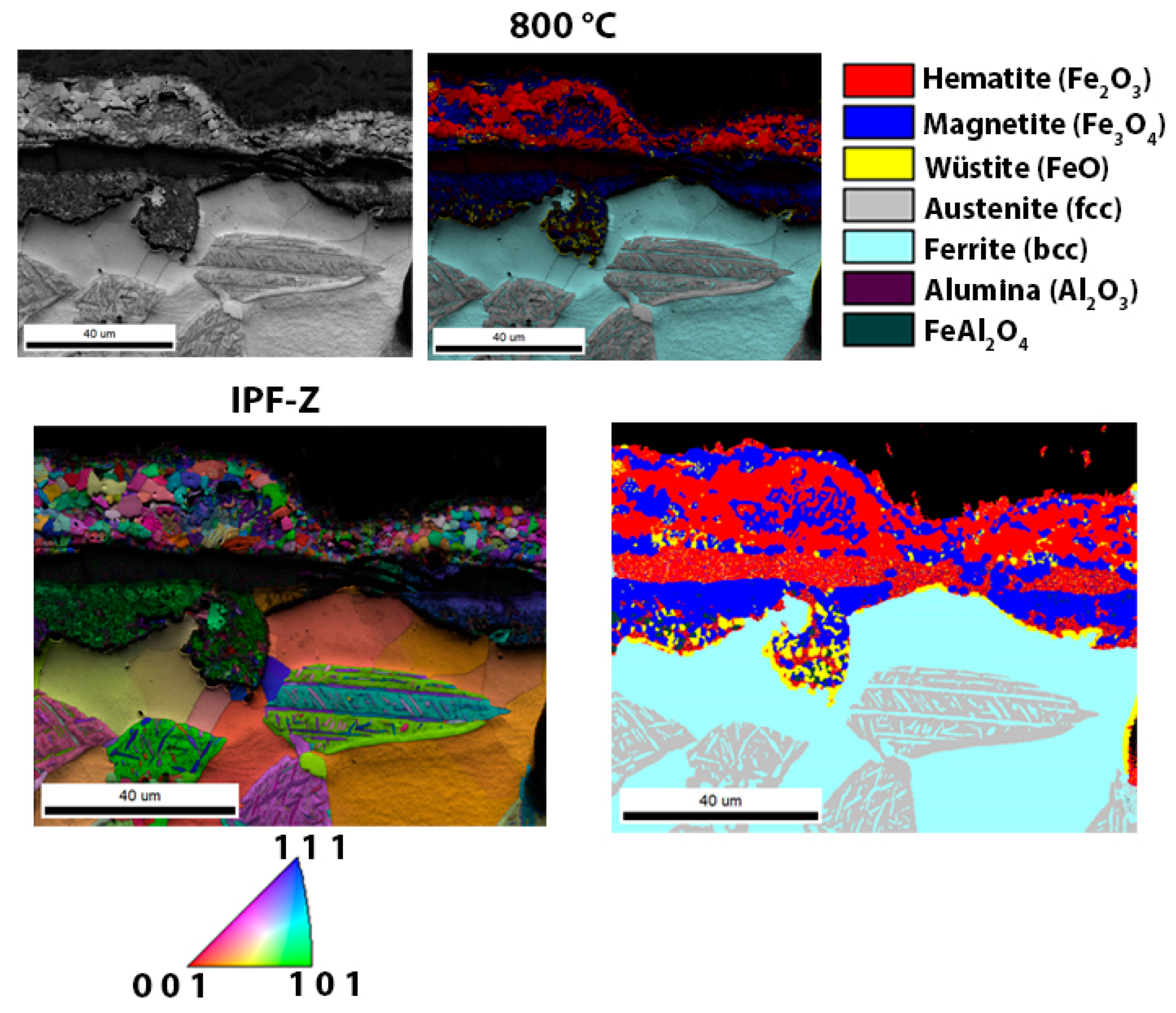
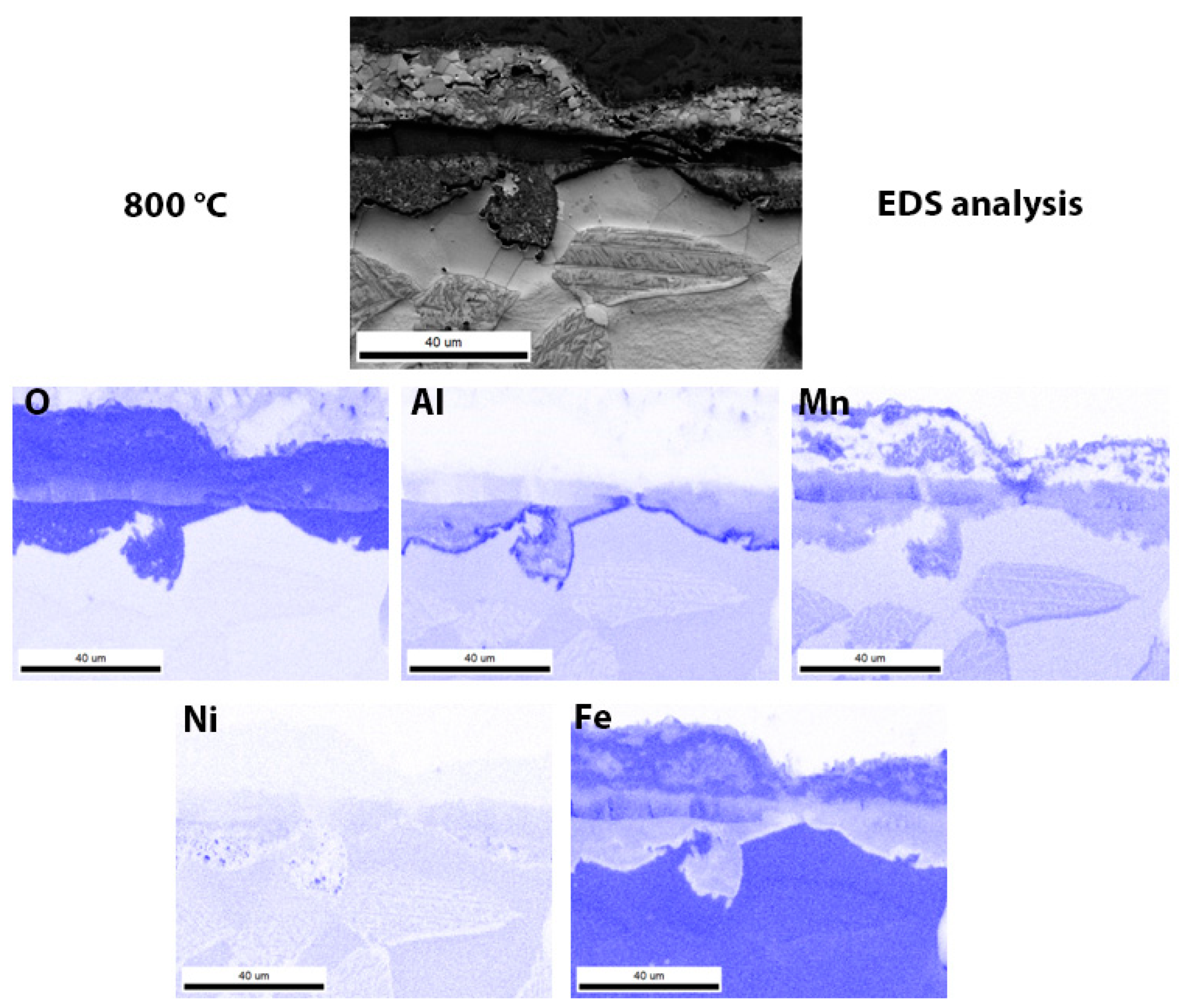
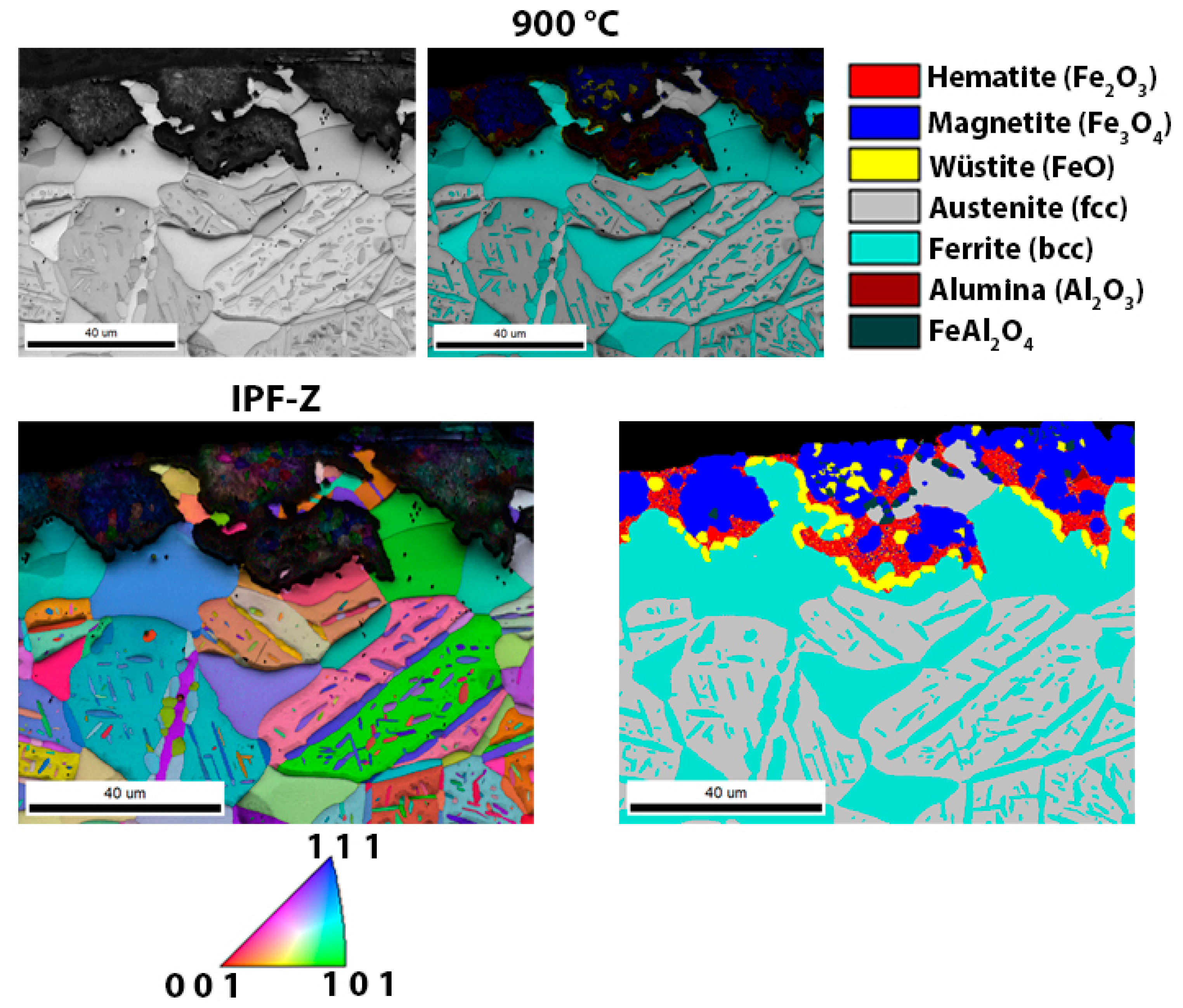
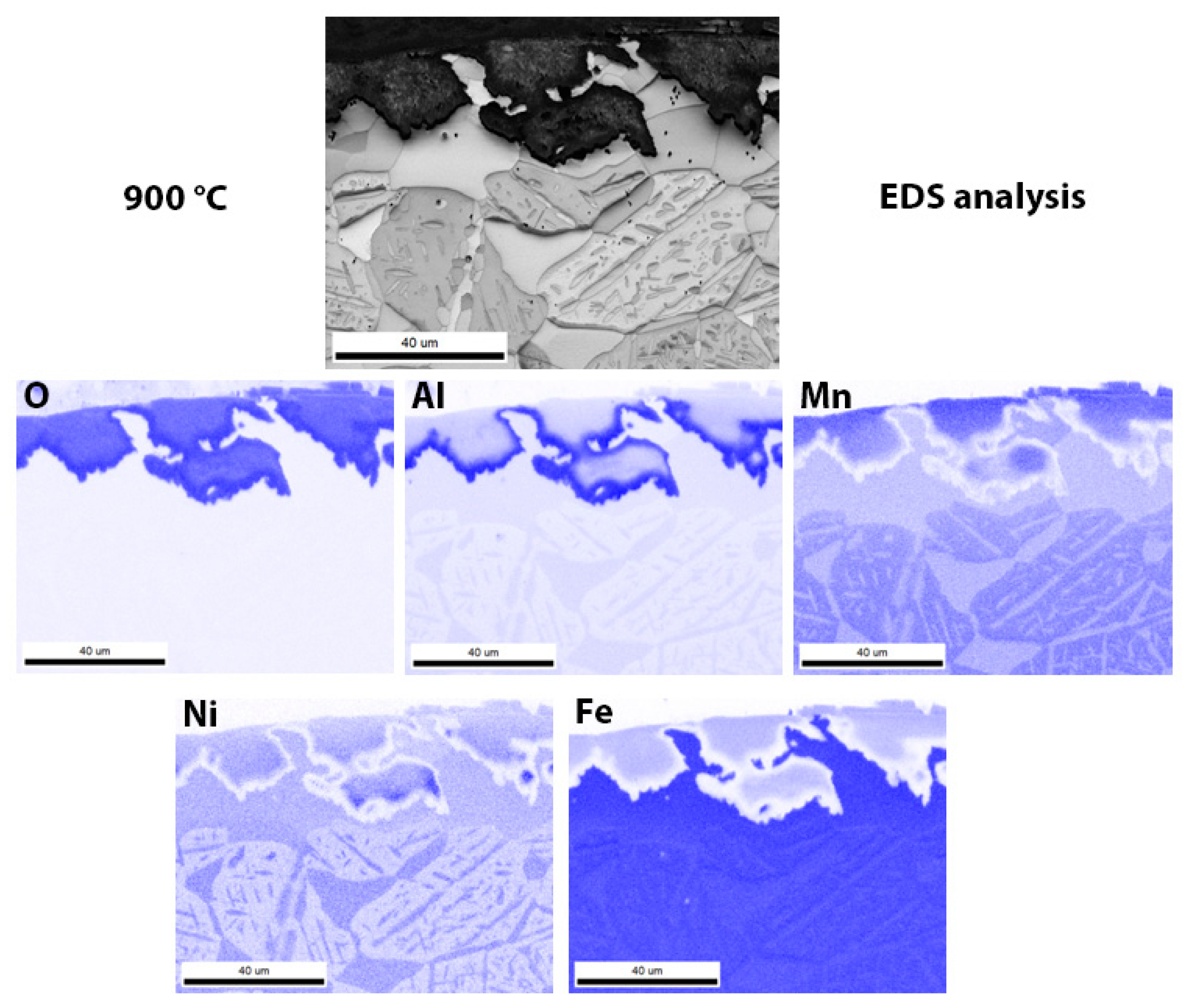
3.3. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Howell, R.A.; Van Aken, D.C. A literature review of age hardening Fe-Mn-Al-C alloys. Iron Steel Technol. 2009, 6, 193–212. [Google Scholar]
- Rana, R.; Lahaye, C.; Ray, R.K. Overview of Lightweight Ferrous Materials: Strategies and Promises. JOM 2014, 66, 1734–1746. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, L.; Van Aken, D. High Manganese and Aluminum Steels for the Military and Transportation Industry. JOM 2014, 66, 1770–1784. [Google Scholar] [CrossRef]
- Rana, R. Low-Density Steels. JOM 2014, 66, 1730–1733. [Google Scholar] [CrossRef] [Green Version]
- Kalashnikov, I.; Acselrad, O.; Shalkevich, A.; Pereira, L.C. Chemical composition optimization for austenitic steels of the Fe-Mn-Al-C system. J. Mater. Eng. Perform. 2000, 9, 597–602. [Google Scholar] [CrossRef]
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, L.; Ishida, K. High-strength Fe-20Mn-Al-C-based alloys with low density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Raabe, D.; Springer, H.; Gutierrez-Urrutia, I.; Roters, F.; Bausch, M.; Seol, J.B.; Koyama, M.; Choi, P.P.; Tsuzaki, K. Alloy Design, Combinatorial Synthesis, and Microstructure–Property Relations for Low-Density Fe-Mn-Al-C Austenitic Steels. JOM 2014, 66, 1845–1856. [Google Scholar] [CrossRef]
- Zuazo, I.; Hallstedt, B.; Lindahl, B.; Selleby, M.; Soler, M.; Etienne, A.; Perlade, A.; Hasenpouth, D.; Cazottes, S.; Kleber, X.; et al. Low-Density Steels: Complex Metallurgy for Automotive Applications. JOM 2014, 66, 1747–1758. [Google Scholar] [CrossRef]
- Jahn, M.T.; Chang, S.C.; Hsiao, Y.H. Transverse tensile and fatigue properties of Fe-Mn-Al-C alloys. J. Mater. Sci. Lett. 1989, 8, 723–724. [Google Scholar] [CrossRef]
- Yoo, J.D.; Park, K.T. Microband-induced plasticity in a high Mn-Al-C light steel. Mater. Sci. Eng. A 2008, 496, 417–424. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brox, U. Microstructures and Mechanical Properties of High-Strength Fe-Mn-AI-C Light-Weight TRIPLEX Steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Z.; Xu, Y.; Ran, Q.; Xu, W.; Li, J.; Xiao, X. Internal oxidation behaviour of Fe-Mn-Al-C duplex light-weight steels with good combination of strength and ductility. Corros. Sci. 2017, 120, 148–157. [Google Scholar] [CrossRef]
- Wang, R.; Straszheim, M.J.; Rapp, R.A. A high-temperature oxidation-resistant Fe-Mn-Al-Si alloy. Oxid. Met. 1984, 21, 71–79. [Google Scholar] [CrossRef]
- Jackson, P.R.S.; Wallwork, G.R. High temperature oxidation of iron-manganese-aluminum based alloys. Oxid. Met. 1984, 21, 135–170. [Google Scholar] [CrossRef]
- Jeong, T.K.; Jung, G.; Lee, K.; Kang, Y.B.; Bhadeshia, H.K.D.H.; Suh, D.W. Selective oxidation of Al rich Fe–Mn–Al–C low density steels. Mater. Sci. Technol. 2014, 30, 1805–1814. [Google Scholar] [CrossRef]
- Peng, W.; Wang, J.; Zhang, H.; Hong, X.; Wu, Z.; Xu, Y.; Li, J.; Xiao, X. Insights into the role of grain refinement on high-temperature initial oxidation phase transformation and oxides evolution in high aluminium Fe-Mn-Al-C duplex lightweight steel. Corros. Sci. 2017, 126, 197–207. [Google Scholar] [CrossRef]
- Zambrano, O.A. A general perspective of Fe–Mn–Al–C steels. J. Mater. Sci. 2018, 53, 14003–14062. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.H.; Wan, C.M. Effect of manganese on the oxidation of Fe-Mn-Al-C alloys. J. Mater. Sci. 1988, 23, 744–752. [Google Scholar] [CrossRef]
- Wang, C.J.; Chang, Y.C. Formation and growth morphology of nodules in the high-temperature oxidation of Fe-Mn-Al-C alloy. Mater. Chem. Phys. 2003, 77, 738–743. [Google Scholar] [CrossRef]
- Wang, C.J.; Chang, Y.C. TEM study of the internal oxidation of an Fe-Mn-Al-C alloy after hot corrosion. Oxid. Met. 2002, 57, 363–378. [Google Scholar] [CrossRef]
- Gleeson, B.; Hadavi, S.M.M.; Young, D.J. Isothermal transformation behavior of thermally-grown wustite. Mater. High Temp. 2000, 17, 311–318. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Thomas, H.; Lars, H.; Pingfang, S.; Bo, S. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Balaško, T.; Burja, J.; Medved, J. Effect of Ni on solidification of duplex low-density steels. J. Therm. Anal. Calorim. 2020, 142, 1605–1611. [Google Scholar] [CrossRef]
- Burja, J.; Batič, B.Š.; Balaško, T. Influence of the addition of ni on as-cast microstructure of duplex fe-mn-al-c lightweight steel. Crystals 2021, 11, 1551. [Google Scholar] [CrossRef]
- Balaško, T.; Vončina, M.; Burja, J.; Batič, B.Š. High-Temperature Oxidation Behavior of Tool Steel with Increased Thermal Conductivity. Oxid. Met. 2022, 98, 135–161. [Google Scholar] [CrossRef]

| Sample | C | Si | Cu | Ni | Mn | Al | Fe |
|---|---|---|---|---|---|---|---|
| FeMnAl5.6NiC | 0.82 | 0.20 | 0.17 | 5.6 | 14.8 | 10.1 | balance |
| Temperature (°C) | TGA (%) | TGA (mg) | TGA (mg cm−2) |
|---|---|---|---|
| 700 | −0.030 | −0.124 | −0.144 |
| 800 | 1.031 | 4.222 | 4.935 |
| 900 | 1.310 | 5.324 | 6.282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaško, T.; Šetina Batič, B.; Medved, J.; Burja, J. High-Temperature Oxidation Behaviour of Duplex Fe-Mn-Al-Ni-C Lightweight Steel. Crystals 2022, 12, 957. https://doi.org/10.3390/cryst12070957
Balaško T, Šetina Batič B, Medved J, Burja J. High-Temperature Oxidation Behaviour of Duplex Fe-Mn-Al-Ni-C Lightweight Steel. Crystals. 2022; 12(7):957. https://doi.org/10.3390/cryst12070957
Chicago/Turabian StyleBalaško, Tilen, Barbara Šetina Batič, Jožef Medved, and Jaka Burja. 2022. "High-Temperature Oxidation Behaviour of Duplex Fe-Mn-Al-Ni-C Lightweight Steel" Crystals 12, no. 7: 957. https://doi.org/10.3390/cryst12070957
APA StyleBalaško, T., Šetina Batič, B., Medved, J., & Burja, J. (2022). High-Temperature Oxidation Behaviour of Duplex Fe-Mn-Al-Ni-C Lightweight Steel. Crystals, 12(7), 957. https://doi.org/10.3390/cryst12070957









