Recent Trends in Elpasolite Single Crystal Scintillators for Radiation Detection
Abstract
:1. Introduction
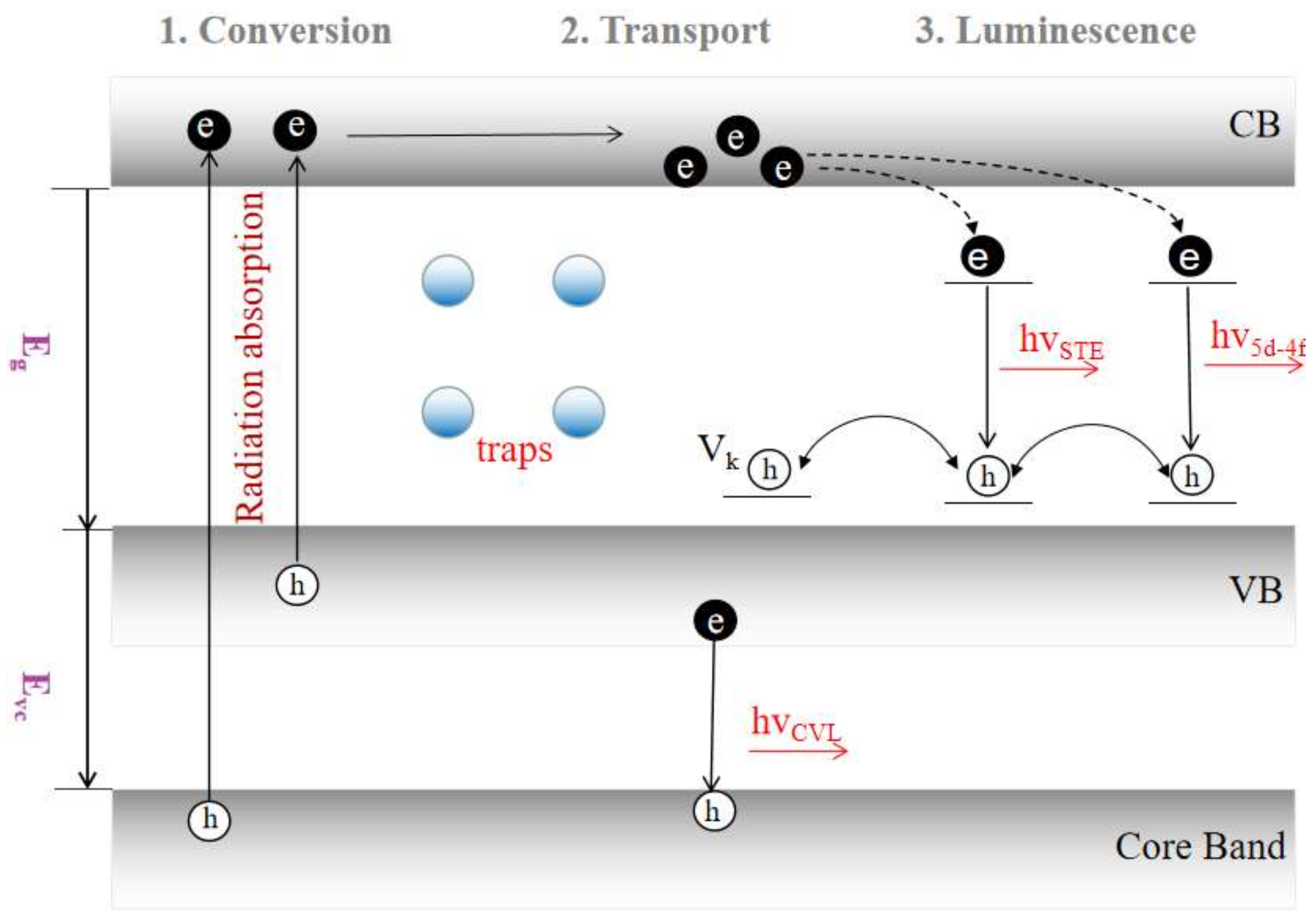
2. Scintillator Properties
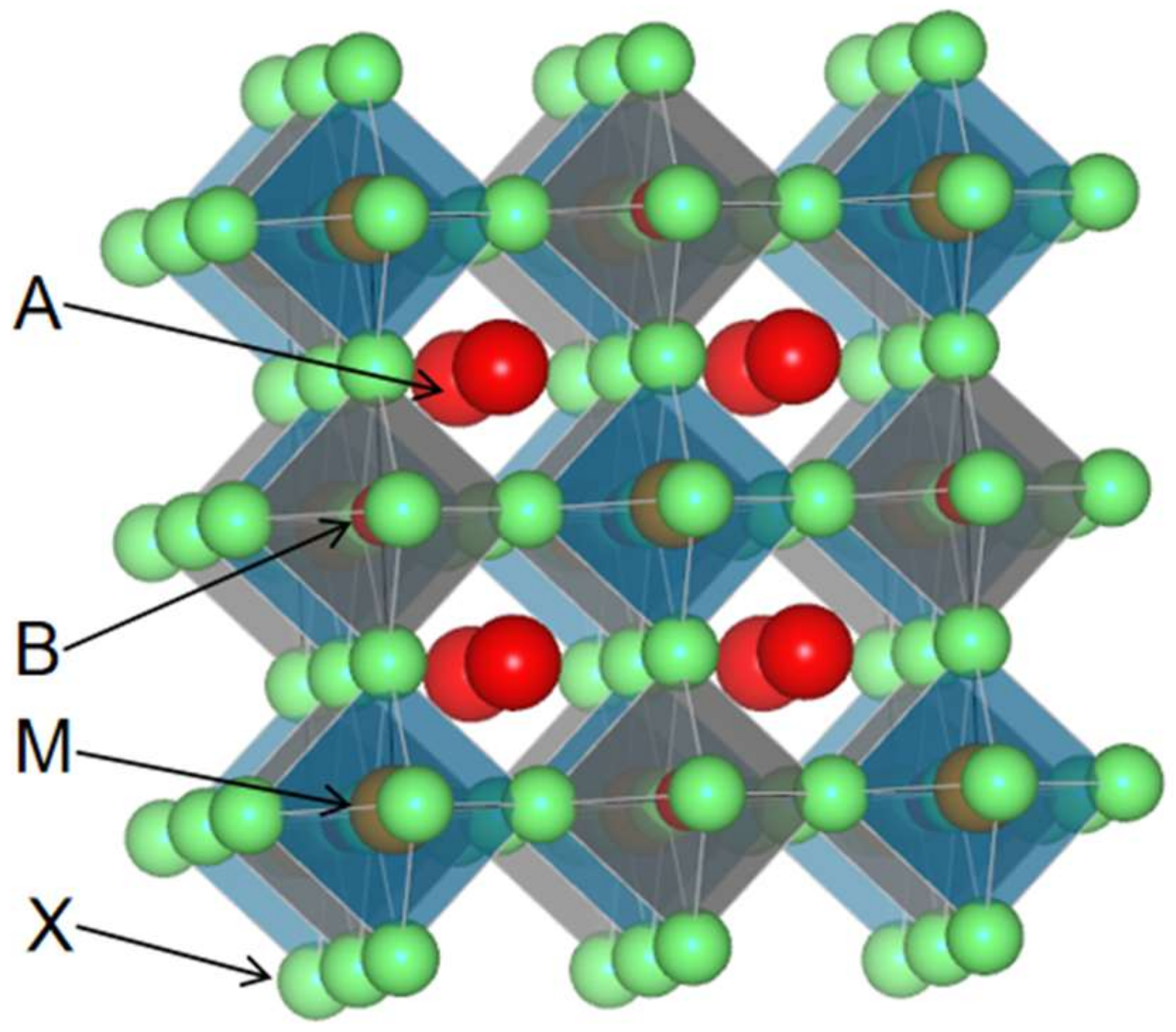
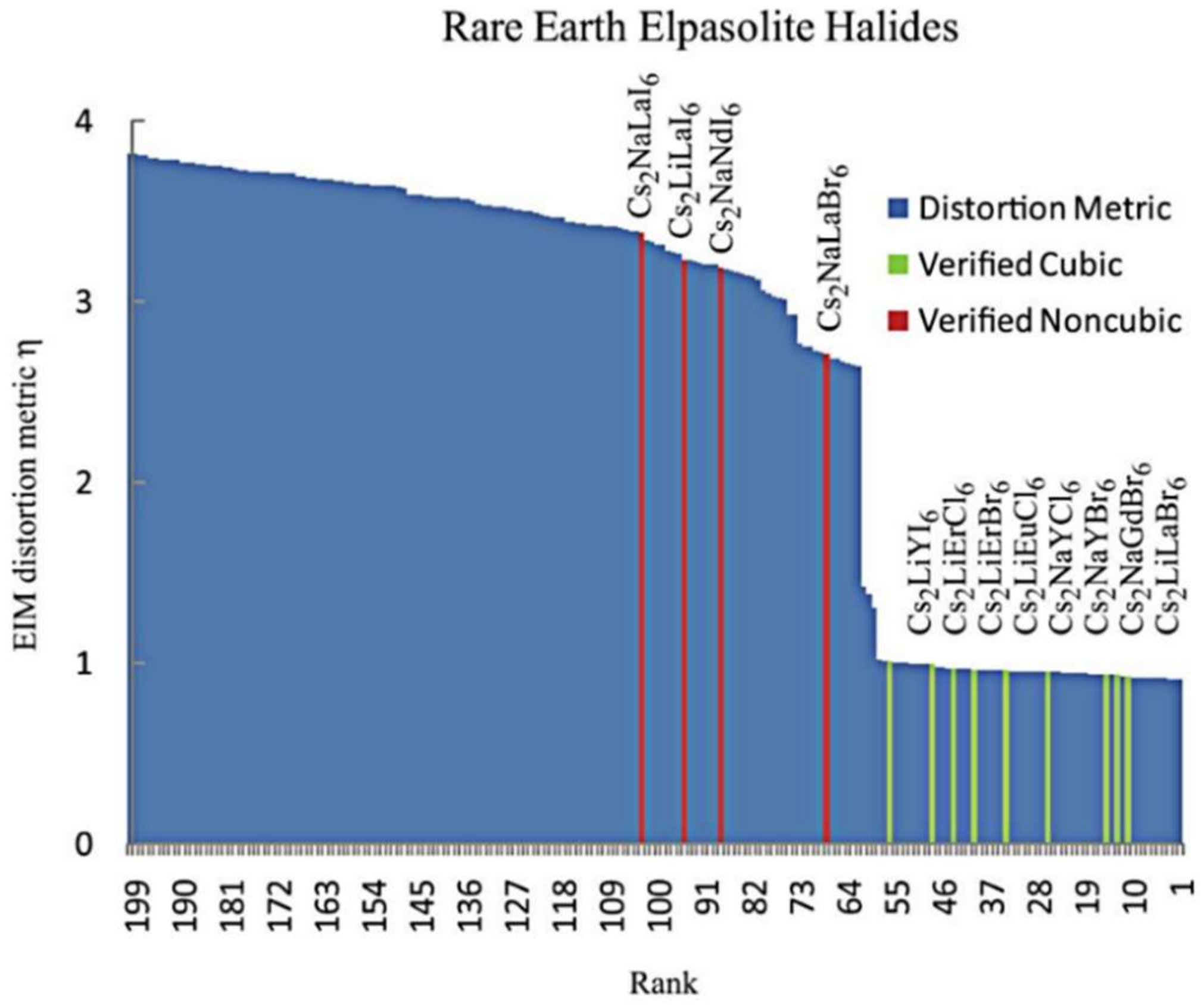
3. Structure of Elpasolite Scintillators
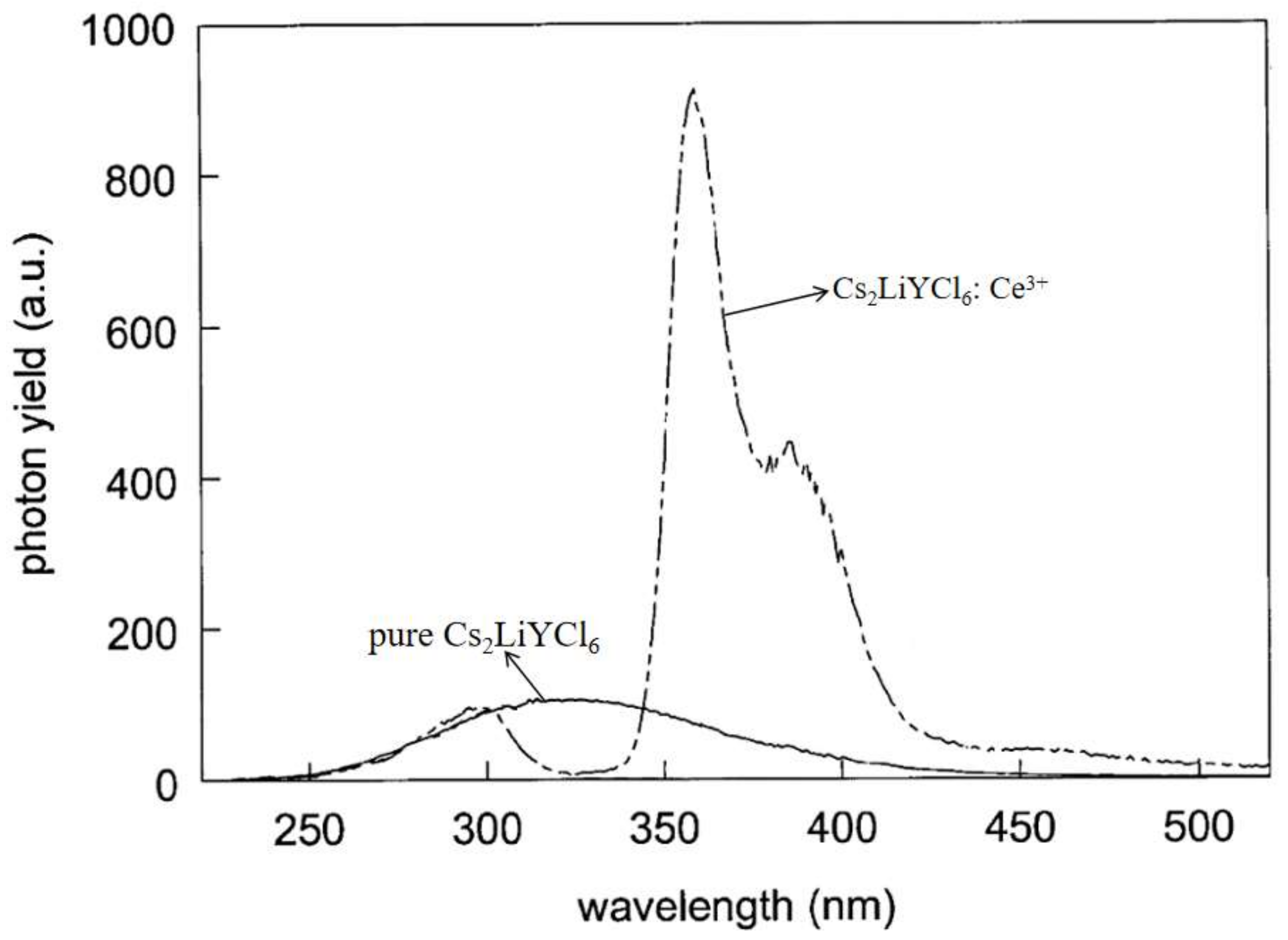
4. Cs2LiYCl6 (CLYC)
5. Cs2LiLaCl6 (CLLC)
6. Cs2LiLaBr6 (CLLB)
7. The Elpasolite Scintillators for Neutron Detection
8. Other Elpasolites
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.J.; Yang, P.Z.; Liao, J.Y. Progress on LnX(3) (Ce) scintillator crystals. J. Inorg. Mater. 2005, 20, 522–528. [Google Scholar]
- Nikl, M.; Yoshikawa, A.; Kamada, K.; Nejezchleb, K.; Stanek, C.R.; Mares, J.A.; Blazek, K. Development of LuAG-based scintillator crystals-A review. Prog. Cryst. Growth Charact. Mater. 2013, 59, 47–72. [Google Scholar] [CrossRef]
- Maddalena, F.; Tjahjana, L.; Xie, A.; Zeng, S.; Wang, H.; Coquet, P.; Drozdowski, W.; Dujardin, C.; Dang, C.; Birowosuto, M.D. Inorganic, Organic, and Perovskite Halides with Nanotechnology for High-Light Yield X- and gamma-ray Scintillators. Crystals 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- McGregor, D.S. Materials for Gamma-Ray Spectrometers: Inorganic Scintillators. Annu. Rev. Mater. Sci. 2018, 48, 245–277. [Google Scholar] [CrossRef]
- Rontgen, W.C. On a New Kind of Rays. Science 1896, 3, 227–231. [Google Scholar] [CrossRef]
- Sakai, E. Recent Measurements on Scintillator-photodetector Systems. IEEE Trans. Nucl. Sci. 1987, 34, 418–422. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Imrie, D.C. Thermal annealing and optical darkening effects in CsI (Tl) crystals. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 1999, 432, 138–146. [Google Scholar] [CrossRef]
- Boukeffoussa, K.; Bouakaz, K. Analytical method for calculating the efficiency and solid angle of an NaI (Tl) detector. Appl. Radiat. Isot. 2021, 173, 138–146. [Google Scholar] [CrossRef]
- Mao, R.H.; Wu, C.; Dai, L.E.; Lu, S. Crystal growth and scintillation properties of LSO and LYSO crystals. J. Cryst. Growth 2013, 368, 97–100. [Google Scholar] [CrossRef]
- Yamamoto, S.; Imaizumi, M.; Shimosegawa, E.; Hatazawa, J. Development of a compact and high spatial resolution gamma camera system using LaBr3 (Ce). Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2010, 622, 261–269. [Google Scholar] [CrossRef]
- Yanagida, T. Study of rare-earth-doped scintillators. Opt. Mater. 2013, 35, 1987–1992. [Google Scholar] [CrossRef]
- Hawrami, R.; Hines, C.; Abselem, I.; Biteman, V.; Vaghini, J.; Glodo, J.; O’Dougherty, P.; Shah, K.S.; Cherepy, N.; Payne, S.; et al. Latest Advances in Large Diameter SrI:Eu & CLYC: Ce Scintillators for Isotope Identification. SPIE Conf. Hard X-Ray Gamma-Ray Neutron Detect. Phys. XIV 2012, 8507, 155–162. [Google Scholar]
- Shirwadkar, U.; Glodo, J.; van Loef, E.V.; Hawrami, R.; Mukhopadhyay, S.; Churilov, A.; Higgins, W.M.; Shah, K.S. Scintillation properties of Cs2LiLaBr6 (CLLB) crystals with varying Ce3+ concentration. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2011, 652, 268–270. [Google Scholar] [CrossRef]
- Tremsin, A.S.; Perrodin, D.; Losko, A.S.; Vogel, S.C.; Bourke, M.A.; Bizarri, G.A.; Bourret, E.D. Real-time Crystal Growth Visualization and Quantification by Energy-Resolved Neutron Imaging. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.L.; Du, M.H. Discrete Electronic Bands in Semiconductors and Insulators: Potential High-Light-Yield Scintillators. Phys. Rev. Appl. 2015, 3, 054005. [Google Scholar] [CrossRef]
- Gundiah, G.; Brennan, K.; Yan, Z.; Samulon, E.C.; Wu, G.; Bizarri, G.A.; Derenzo, S.E.; Bourret-Courchesne, E.D. Structure and scintillation properties of Ce3+-activated Cs2NaLaCl6, Cs3LaCl6, Cs2NaLaBr6, Cs3LaBr6, Cs2NaLaI6 and Cs3LaI6. J. Lumin. 2014, 149, 374–384. [Google Scholar] [CrossRef]
- Dong, L.W.; Liu, Y.P.; Wen, K.C.; Chen, D.J.; Rao, D.W.; Liu, J.P.; Yuan, B.T.; Dong, Y.F.; Wu, Z.; Liang, Y.F.; et al. High-Polarity Fluoroalkyl Ether Electrolyte Enables Solvation-Free Li+ Transfer for High-Rate Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104699. [Google Scholar] [CrossRef]
- Van Eijk, C.W.E. Neutron detection and neutron dosimetry. Radiat. Prot. Dosim. 2004, 110, 5–13. [Google Scholar] [CrossRef]
- Thomas, D.J. Neutron spectrometry for radiation protection. Radiat. Prot. Dosim. 2004, 110, 141–149. [Google Scholar] [CrossRef]
- Runkle, R.C.; Bernstein, A.; Vanier, P.E. Securing special nuclear material: Recent advances in neutron detection and their role in nonproliferation. J. Appl. Phys. 2010, 108, 111101. [Google Scholar] [CrossRef]
- Dong, L.W.; Liu, Y.P.; Chen, D.J.; Han, Y.P.; Ji, Y.P.; Liu, J.P.; Yuan, B.T.; Dong, Y.F.; Li, Q.; Zhou, S.Y.; et al. Stabilization of high-voltage lithium metal batteries using a sulfone-based electrolyte with bi-electrode affinity and LiSO2F-rich interphases. Energy Storage Mater. 2022, 44, 527–536. [Google Scholar] [CrossRef]
- Shirwadkar, U.; Hawrami, R.; Glodo, J.; van Loef, E.V.D.; Shah, K.S. Novel Scintillation Material CS(2)LiLaBr(6-x)Clx: Ce for Gamma-ray and Neutron Spectroscopy. In Proceedings of the IEEE Nuclear Science Symposium/Medical Imaging Conference Record (NSS/MIC)/19th Room-Temperature Semiconductor X-ray and Gamma-ray Detector Workshop, Anaheim, CA, USA, 29 October–3 November 2012; pp. 1963–1967. [Google Scholar]
- Dong, L.W.; Liu, J.P.; Chen, D.J.; Han, Y.P.; Liang, Y.F.; Yang, M.Q.; Yang, C.H.; He, W.D. Suppression of Polysulfide Dissolution and Shuttling with Glutamate Electrolyte for Lithium Sulfur Batteries. Acs Nano. 2019, 13, 14172–14181. [Google Scholar] [CrossRef] [PubMed]
- Glodo, J.; Higgins, W.M.; van Loef, E.V.D.; Shah, K.S. Cs2LiYCl6: Ce Scintillator for Nuclear Monitoring Applications. IEEE Trans. Nucl. Sci. 2009, 56, 1257–1261. [Google Scholar] [CrossRef]
- Lecoq, P. Development of new scintillators for medical applications. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2016, 809, 130–139. [Google Scholar] [CrossRef]
- Dujardin, C.; Auffray, E.; Bourret-Courchesne, E.; Dorenbos, P.; Lecoq, P.; Nikl, M.; Vasil’ev, A.N.; Yoshikawa, A.; Zhu, R.Y. Needs, Trends, and Advances in Inorganic Scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 1977–1997. [Google Scholar] [CrossRef] [Green Version]
- Bizarri, G.; Cherepy, N.J.; Choong, W.S.; Hull, G.; Moses, W.W.; Payne, S.A.; Singh, J.; Valentine, J.D.; Vasilev, A.N.; Williams, R.T. Progress in Studying Scintillator Proportionality: Phenomenological Model. IEEE Trans. Nucl. Sci. 2009, 56, 2313–2320. [Google Scholar] [CrossRef] [Green Version]
- Khodyuk, I.V.; Dorenbos, P. Trends and Patterns of Scintillator Nonproportionality. IEEE Trans. Nucl. Sci. 2012, 59, 3320–3331. [Google Scholar] [CrossRef]
- Onken, D.R.; Perrodin, D.; Bourret, E.D.; Vogel, S.C. The crystal structure and temperature dependence of the elpasolite Tl2LiYCl6. J. Appl. Crystallogr. 2021, 54, 604–611. [Google Scholar] [CrossRef]
- Flerov, I.N.; Gorev, M.V.; Aleksandrov, K.S.; Tressaud, A.; Grannec, J.; Couzi, M. Phase transitions in elpasolites (ordered perovskites). Mater. Sci. Eng. R Rep. 1998, 24, 81–151. [Google Scholar] [CrossRef]
- Doty, F.P.; Zhou, X.W.; Yang, P.; Rodriguez, M.A. Elpasolite scintillators. In Office of Scientific & Technical Information Technical Reports; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2012. [Google Scholar]
- Combes, C.M.; Dorenbos, P.; van Eijk, C.W.E.; Kramer, K.W.; Gudel, H.U. Optical and scintillation properties of pure and Ce3+-doped Cs2LiYCl6 and Li3YCl6: Ce3+ crystals. J. Lumin. 1999, 82, 299–305. [Google Scholar] [CrossRef]
- Higgins, W.M.; Glodo, J.; Shirwadkar, U.; Churilov, A.; van Loef, E.; Hawrami, R.; Ciampi, G.; Hines, C.; Shah, K.S. Bridgman growth of Cs2LiYCl6: Ce and Li-6-enriched (Cs2LiYCl6)-Li-6: Ce crystals for high resolution gamma ray and neutron spectrometers. J. Cryst. Growth 2010, 312, 1216–1220. [Google Scholar] [CrossRef]
- Glodo, J.; Hawrami, R.; Shah, K.S. Development of Cs2LiYCl6 scintillator. J. Cryst. Growth 2013, 379, 73–78. [Google Scholar] [CrossRef]
- Li, K.N.; Zhang, X.P.; Gui, Q.; Jin, P.; Tian, G. Characterization of the new scintillator Cs2LiYCl6: Ce3+. Nucl. Sci. Technol. 2018, 29, 11. [Google Scholar] [CrossRef]
- Bessiere, A.; Dorenbos, P.; van Eijk, C.W.E.; Kramer, K.W.; Gudel, H.U. Luminescence and scintillation properties Of Cs2LiYCl6: Ce3+ for gamma and neutron detection. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2005, 537, 242–246. [Google Scholar] [CrossRef]
- Lee, D.W.; Stonehill, L.C.; Klimenko, A.; Terry, J.R.; Tornga, S.R. Pulse-shape analysis of Cs2LiYCl6: Ce scintillator for neutron and gamma-ray discrimination. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2012, 664, 1–5. [Google Scholar] [CrossRef]
- Ruta, F.L.; Swider, S.; Lam, S.; Feigelson, R.S. Understanding phase equilibria and segregation in Bridgman growth of Cs2LiYCl6 scintillator. J. Mater. Res. 2017, 32, 2373–2380. [Google Scholar] [CrossRef]
- Whitney, C.; Stapels, C.; Johnson, E.; Chapman, E.; Alberghini, G.; Glodo, J.; Shah, K.; Christian, J. 6-Li Enriched Cs(2)LiYCl(6): Ce Based Thermal Neutron Detector Coupled with CMOS Solid-State Photomultipliers for a Portable Detector Unit. In Proceedings of the Conference on Medical Imaging 2011-Physics of Medical Imaging, Valencia, Spain, 23–29 October 2011; Volume 7961. [Google Scholar]
- Lejay, J.; Blahuta, S.; Ouspenski, V.; Menge, P.; Richaud, D. Large CLYC: Ce and CLLB: Ce Crystals for Gamma-Neutron Detection Systems; Saint-Gobain Crystals: Hiram, OH, USA, 2013; p. 1. [Google Scholar]
- Rodnyi, P.A.; Mikhailik, V.B.; Stryganyuk, G.B.; Voloshinovskii, A.S.; van Eijk, C.W.E.; Zimmerer, G.F. Luminescence properties of Ce-doped Cs2LiLaCl6 crystals. J. Lumin. 2000, 86, 161–166. [Google Scholar] [CrossRef]
- Glodo, J.; Hawrami, R.; Loef, E.V.; Higgins, W.; Shah, K.S. Dual gamma neutron detection with Cs2LiLaCl6. In Proceedings of the SPIE-The International Society for Optical Engineering, San Diego, CA, USA, 1 January 2009. [Google Scholar]
- Zhu, H.B.; Zhang, P.; Pan, S.K.; Li, H.Y.; Jiang, Y.; Zhang, J.Y.; Zhang, Z.; Ren, G.H.; Pan, J.G.; Chen, H.B. Growth and characterization of Cs2LiLaCl6: Ce single crystals. J. Cryst. Growth 2019, 507, 332–337. [Google Scholar] [CrossRef]
- Yang, K.; Menge, P.R.; Lejay, J.L.; Ouspenski, V. Scintillation Properties and Temperature Responses of Cs2LiLaBr6: Ce3+. In Proceedings of the 60th IEEE Nuclear Science Symposium (NSS)/Medical Imaging Conference (MIC)/20th International Workshop on Room-Temperature Semiconductor X-ray and Gamma-ray Detectors, Seoul, South Korea, 27 October–2 November 2013. [Google Scholar]
- Glodo, J.; van Loef, E.; Hawrami, R.; Higgins, W.M.; Churilov, A.; Shirwadkar, U.; Shah, K.S. Selected Properties of Cs2LiYCl6, Cs2LiLaCl6, and Cs2LiLaYBr6 Scintillators. IEEE Trans. Nucl. Sci. 2011, 58, 333–338. [Google Scholar] [CrossRef]
- Woolf, R.S.; Wulf, E.A.; Phlips, B.F.; Chowdhury, P.; Jackson, E.G. Identification of internal radioactive contaminants in elpasolites (CLYC, CLLB, CLLBC) and other inorganic scintillators. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2020, 954, 161228. [Google Scholar] [CrossRef]
- Lin, J.; Wei, Q.H.; Zhang, D.; Tang, G.; Qin, L.S.; Shi, H.S. Crystal Growth and Scintillation Properties of Non-Stoichiometric Cs2LiLaBr6: Ce. Cryst. Res. Technol. 2019, 54, 1900047. [Google Scholar] [CrossRef]
- Menge, P.R.; Lejay, J.; Ouspenski, V. Design and Performance of a Compact Cs2LiLaBr6 (Ce) Neutron/Gamma Detector Using Silicon Photomultipliers. In Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), San Diego, CA, USA, 31 October–7 November 2015. [Google Scholar]
- Kouzes, R.T.; Ely, J.H.; Erikson, L.E.; Kernan, W.J.; Lintereur, A.T.; Siciliano, E.R.; Stephens, D.L.; Stromswold, D.C.; van Ginhoven, R.M.; Woodring, M.L. Neutron detection alternatives to He-3 for national security applications. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2010, 623, 1035–1045. [Google Scholar] [CrossRef]
- Cieslak, M.J.; Gamage, K.A.A.; Glover, R. Critical Review of Scintillating Crystals for Neutron Detection. Crystals 2019, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Hirai, M.; Niimura, N.; Ishida, A. Epithermal and thermal-neutron beamline monitor using LI-6 glass scintillator. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 1987, 259, 497–500. [Google Scholar] [CrossRef]
- Cowles, C.; Behling, S.; Baldez, P.; Folsom, M.; Kouzes, R.; Kukharev, V.; Lintereur, A.; Robinson, S.; Siciliano, E.; Stave, S.; et al. Development of a lithium fluoride zinc sulfide based neutron multiplicity counter. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2018, 887, 59–63. [Google Scholar] [CrossRef]
- Bessiere, A.; Dorenbos, P.; van Eijk, C.W.E.; Kramer, K.W.; Gudel, H.U. New thermal neutron scintillators: Cs2LiYCl6: Ce3+ and Cs2LiYBr6: Ce3+. IEEE Trans. Nucl. Sci. 2004, 51, 2970–2972. [Google Scholar] [CrossRef] [Green Version]
- Glodo, J.; Hawrami, R.; van Loef, E.; Shirwadkar, U.; Shah, K.S. Pulse Shape Discrimination with Selected Elpasolite Crystals. IEEE Trans. Nucl. Sci. 2012, 59, 2328–2333. [Google Scholar] [CrossRef]
- Mesick, K.E.; Coupland, D.D.S.; Stonehill, L.C. Pulse-shape discrimination and energy quenching of alpha particles in Cs2LiLaBr6: Ce3+. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2017, 841, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Rooh, G.; Kim, H.J.; Park, H.; Kim, S. Investigation of scintillation and luminescence properties of cerium doped Rb2LiGdCl6 single crystals. J. Cryst. Growth 2013, 377, 28–31. [Google Scholar] [CrossRef]
- Ferrulli, F.; Labalme, M.; Silari, M. Investigation of CLYC-6 for thermal neutron detection and CLYC-7 for fast neutron spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2022, 1029, 166460. [Google Scholar] [CrossRef]
- Mishin, A.N.; Rodnyi, P.A.; Sidorenko, A.V.; Voloshinovskii, A.S.; Dorenbos, P. Search for new scintillators for x- and gamma-ray detectors. In Proceedings of the 4th International Workshop on Nondestructive Testing and Computer Simulations in Science and Engineering, St. Petersburg, Russia, 12–17 June 2000; Volume 4348, pp. 47–51. [Google Scholar]
- Rooh, G.; Kang, H.; Kim, H.J.; Park, H.; Kim, S. The growth and scintillation properties of Cs2NaCeCl6 single crystal. J. Cryst. Growth 2009, 311, 2470–2473. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, H.J.; Khan, A.; Park, J.M. Crystal growth and characterization of K2LiCeCl6, a novel elpasolite scintillator. Radiat. Meas. 2021, 141, 106524. [Google Scholar] [CrossRef]
- Kim, H.J.; Rooh, G.; Park, H.; Kim, S. Luminescence and scintillation properties of the new Ce-doped Tl2LiGdCl6 single crystals. J. Lumin. 2015, 164, 86–89. [Google Scholar] [CrossRef]
- Zhang, X.G.; Cai, Z.C.; Kang, Z.; Zhai, H.W.; Yin, Z.; Jie, W.Q.; Wang, T. Crystal Growth and Optimization of Cs2LiLaBr6 Scintillator via the Cs2LaBr5-LiBr Phase Diagram Construction. IEEE Trans. Nucl. Sci. 2022, 69, 56–60. [Google Scholar] [CrossRef]
- Shirwadkar, U.; Glodo, J.; van Loef, E.; Hawrami, R.; Mukhopadhyay, S.; Shah, K.S. Investigating Scintillation Properties of Ce Doped Cs2LiYBr6. In Proceedings of the IEEE Nuclear Science Symposium (NSS)/Medical Imaging Conference (MIC)/17th International Workshop on Room-Temperature Semiconductor X-ray and Gamma-ray Detectors, Knoxville, TN, USA, 30 October–6 November 2010; pp. 1585–1588. [Google Scholar]
- Jang, J.; Kim, H.J.; Rooh, G.; Kim, S. Development and measurement of luminescence properties of Ce-doped Cs2LiGdBr6 crystals irradiated with X-ray, gamma-ray and proton beam. J. Korean Phys. Soc. 2017, 71, 796–801. [Google Scholar] [CrossRef]
- Kim, S.; Rooh, G.; Kim, H.J.; Kim, D.S.; Kang, S.J. Crystal growth and scintillation properties of Cs2LiLuBr6:Ce3+. J. Cryst. Growth 2011, 317, 84–86. [Google Scholar] [CrossRef]
- Kim, S.; Rooh, G.; Kim, H.J.; Kim, W.; Hong, U. Crystal Growth and Scintillation Properties of Cs2NaCeBr6. IEEE Trans. Nucl. Sci. 2010, 57, 1251–1254. [Google Scholar] [CrossRef]
- Rooh, G.; Kim, H.; Park, H.; Kim, S. Luminescence and Scintillation Characteristics of Rb2LiCeBr6 Single Crystal. IEEE Trans. Nucl. Sci. 2010, 57, 3836–3840. [Google Scholar]
- Glodo, J.; van Loef, E.V.D.; Higgins, W.M.; Shah, K.S. Scintillation Properties of Cs2NaLaI6: Ce. In Proceedings of the 15th International Workshop on Room-Temperature Semiconductor X- and Gamma-Ray Detectors/2006 IEEE Nuclear Science Symposium, San Diego, CA, USA, 29 October–4 November 2006; pp. 1208–1211. [Google Scholar]

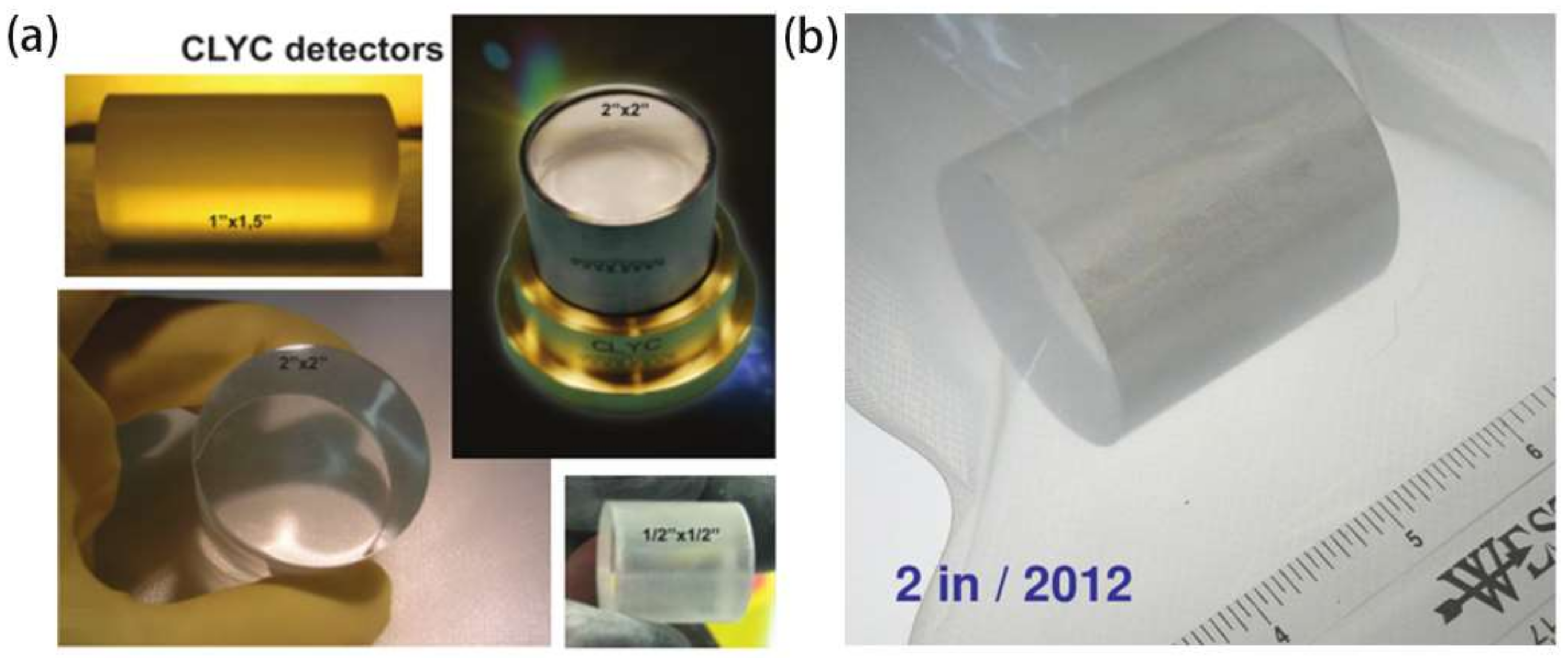



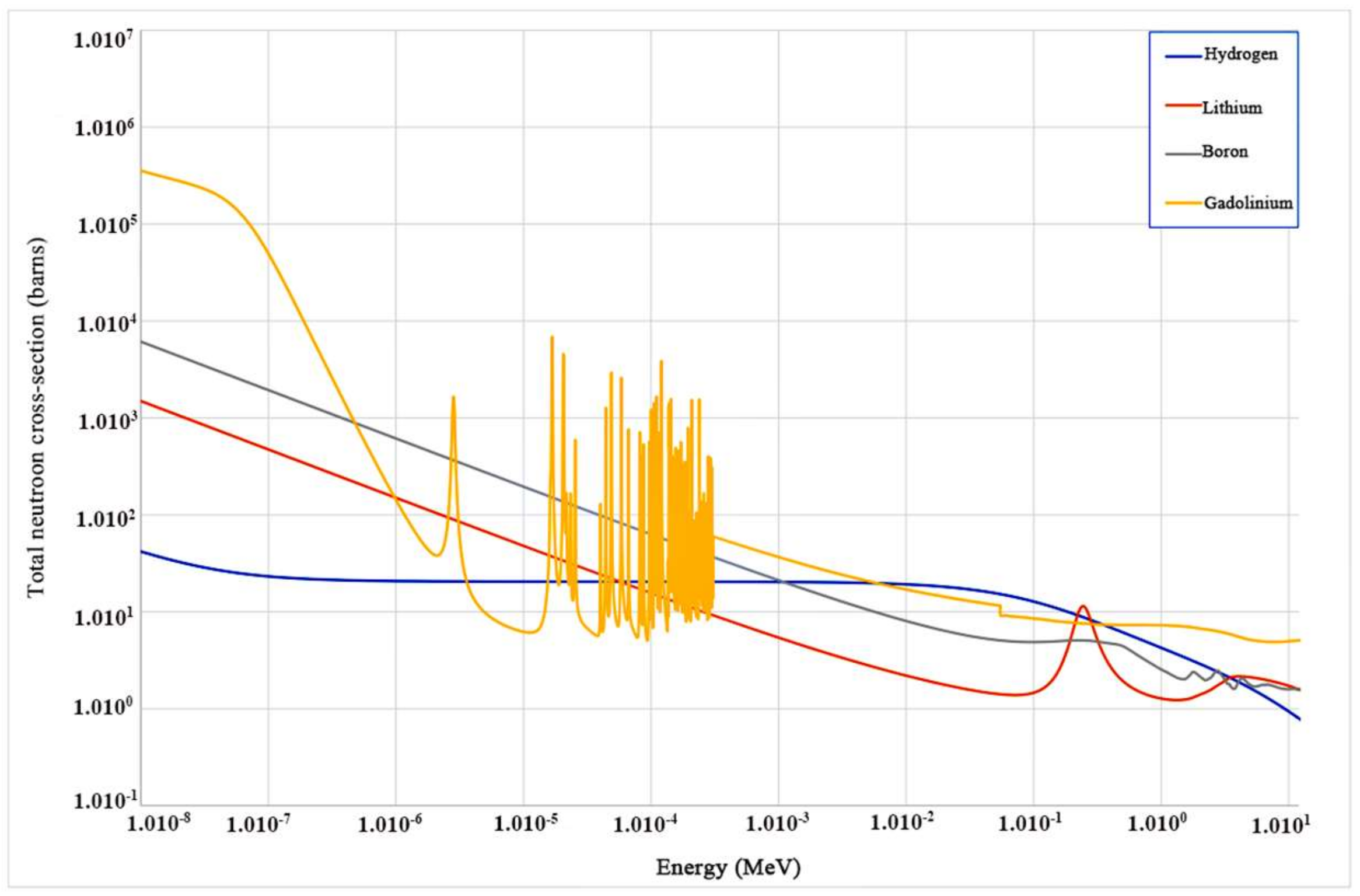
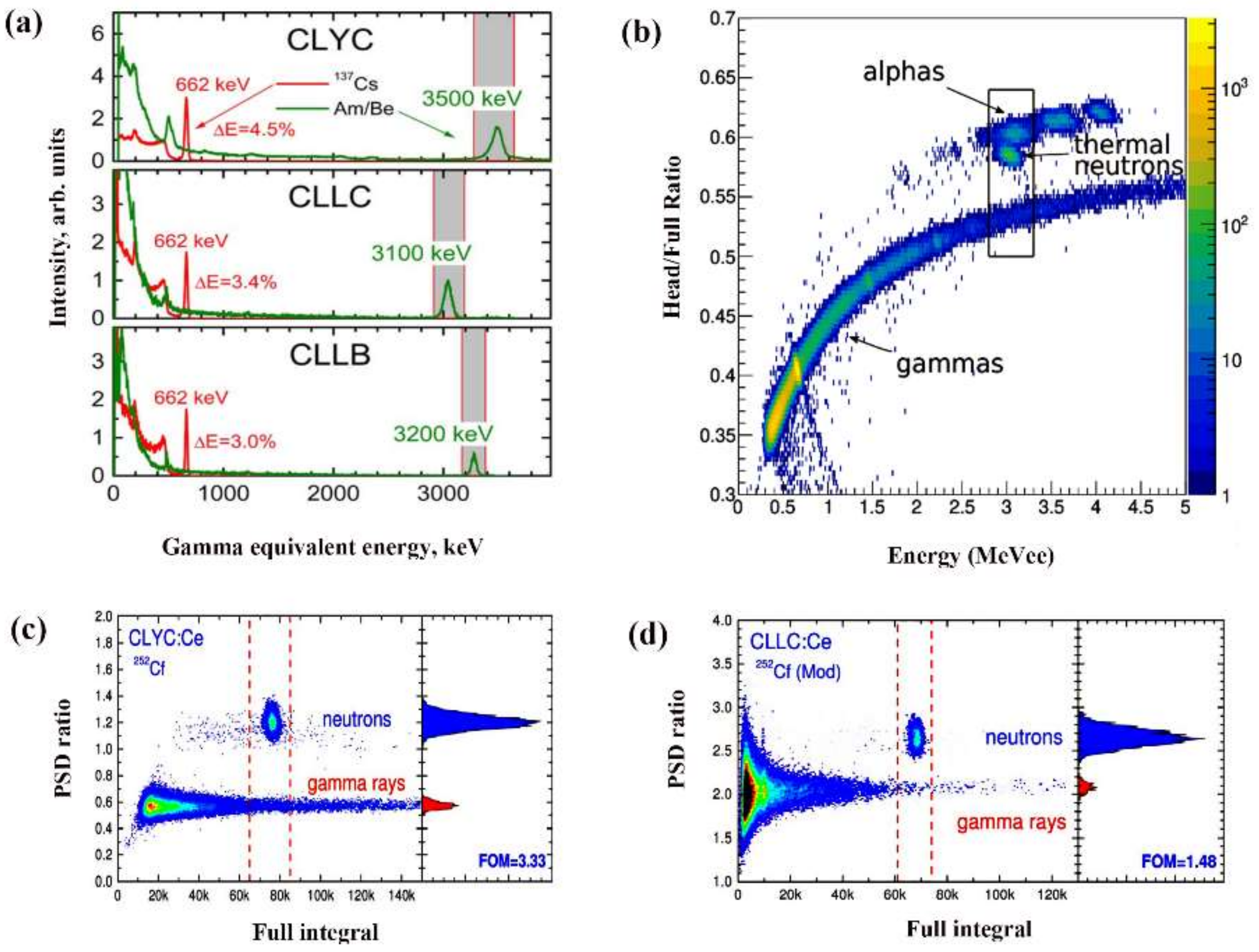
| Chemical Component | Density (g/cm3) | M. P. (K) | Gamma-ray LY (ph/MeV) | E. R. At 662 keV (%) | Decay Times (ns) | Neutron Detection | HY | Price |
|---|---|---|---|---|---|---|---|---|
| NaI (Tl) | 3.67 | 924 | 40,000 | 6.5 | 230 | Yes | Yes | Low |
| CsI (Tl) | 4.51 | 894 | 18,000 | 4.3 | 1250 | No | Slight | Low |
| SrI2 (Eu) | 4.55 | 675 | 80,000 | 3 | 1.2103 | No | Yes | Med |
| LaCl3 (Ce) | 3.9 | 1148 | 50,000 | 3.1 | 24 | No | Yes | High |
| LaBr3 (Ce) | 5.63 | 1056 | 70,000 | 2.4 | 16 | No | Yes | High |
| LSO (Ce) | 7.41 | 2320 | 30,000 | 9.0 | 40 | No | No | High |
| YSO (Ce) | 4.45 | 2200 | 18,500 | 9.3 | 42 | No | No | High |
| BGO | 7.13 | 1323 | 3600 | 20 | 300 | No | No | Low |
| Cs2LiYCl6 | 3.31 | 913 | 20,000 | 3.6 | 3, 50 | Yes | Yes | High |
| NaI (Tl) | 3.67 | 924 | 40,000 | 6.5 | 230 | Yes | Yes | Low |
| Chemical Component | Ce Con. (mol %) | Gamma Ray LY (ph/MeV) | E. R. At 662 keV (%) | Decay Times (ns) | Thermal Neutron Detection | Fast Neutron Detection | FOM | References |
|---|---|---|---|---|---|---|---|---|
| Cs2LiYCl6 | 0.5 | 20,000 | 3.6 | 3, 50, 1.0103 | ▲ | ▲ | 3.33 | [57] |
| Cs2LiLaCl6 | 1 | 35,000 | 3.4 | 85, 450, 1.3103 | ▲ | ▲ | 1.48 | [58] |
| Cs2NaCeCl6 | 0 | 20,000 | 8.3 | 91, 3.2103 | ▲ | [59] | ||
| K2LiCeCl6 | 0 | 21,000 | 16 | 70, 360, 2.0103 | ▲ | ▲ | 0.81 | [60] |
| Tl2LiYCl6 | 1.5 | 27,000 | 4.0 | 37, 341, 947 | ▲ | ▲ | [29] | |
| Tl2LiGdCl6 | 10 | 58,000 | 4.6 | 34, 191, 1.2103 | ▲ | ▲ | [61] | |
| Cs2LiLaBr6 | 2 | 60,000 | 2.9 | 122, 661 | ▲ | 1.9 | [62] | |
| Cs2LiYBr6 | 1 | 24,000 | 4.1 | 50, 270 | ▲ | 1.23 | [63] | |
| Cs2LiGdBr6 | 15 | 37,800 | 6.2 | 62, 224, 1.0103 | ▲ | [64] | ||
| Cs2LiLuBr6 | 1 | 25,000 | 19 | 37, 337, >103 | ▲ | [65] | ||
| Cs2NaCeBr6 | 0 | 25,000 | 6.7 | 140, 880 | [66] | |||
| Rb2LiCeBr6 | 0 | 33,000 | 6.3 | 55, 284 | ▲ | [67] | ||
| Cs2NaLaI6 | 5 | 46,000 | 5.0 | 45, 170 | [68] | |||
| NaI | 4 (Tl) | 40,000 | 6.5 | 230 | ▲ | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.; Hao, S.; Shang, Y.; Lei, Z.; Yang, C. Recent Trends in Elpasolite Single Crystal Scintillators for Radiation Detection. Crystals 2022, 12, 887. https://doi.org/10.3390/cryst12070887
Jin T, Hao S, Shang Y, Lei Z, Yang C. Recent Trends in Elpasolite Single Crystal Scintillators for Radiation Detection. Crystals. 2022; 12(7):887. https://doi.org/10.3390/cryst12070887
Chicago/Turabian StyleJin, Taiguang, Shuwei Hao, Yunfei Shang, Zuotao Lei, and Chunhui Yang. 2022. "Recent Trends in Elpasolite Single Crystal Scintillators for Radiation Detection" Crystals 12, no. 7: 887. https://doi.org/10.3390/cryst12070887
APA StyleJin, T., Hao, S., Shang, Y., Lei, Z., & Yang, C. (2022). Recent Trends in Elpasolite Single Crystal Scintillators for Radiation Detection. Crystals, 12(7), 887. https://doi.org/10.3390/cryst12070887








