Abstract
Perovskite rare-earth ferrites (REFeO3) have attracted great attention for their high ferroelectric and magnetic transition temperatures, strong magnetoelectric coupling, and electric polarization. We report on the flux method growth of rare-earth iron oxide Lu1−xScxFeO3 single crystals through a K2CO3-B2O3-Bi2O3 mixture as a flux solution, and give a detailed characterization of the microstructure, magnetism, and ferroelectric properties. X-ray diffraction (XRD) and energy dispersive X-ray spectroscopy (EDX) measurements revealed that the obtained single crystals can be designated to three different crystal structures of different chemical compositions, that is, Lu0.96Sc0.04FeO3 (perovskite phase), Lu0.67Sc0.33FeO3 (hexagonal phase), and Lu0.2Sc0.8FeO3 (bixbyite phase), respectively. Magnetic measurements indicate that the perovskite Lu0.96Sc0.04FeO3 is an anisotropic hard ferromagnetic material with a high Curie transition temperature, the bixbyite Lu0.2Sc0.8FeO3 is a low temperature soft ferromagnetic material, and the hexagonal Lu0.67Sc0.33FeO3 exhibits multiferroic properties. Lu0.67Sc0.33FeO3 possesses a weak ferromagnetic transition at about 162 K. We further investigate the ferroelectric domain structures in hexagonal sample by scanning electron microscope and the characteristic atomic structures in ferroelectric domain walls by atomically resolved scanning transmission electron microscope. Our successful growth of perovskite Lu1−xScxFeO3 single crystals with distinct crystal structures and stochiometric Lu-Sc substitutions is anticipated to provide a useful ferrites system for furthering exploitation of their multiferroic properties and functionalities.
1. Introduction
Multiferroicity is an important topic of condensed matter physics and has attracted much attention in recent years [1]. The couplings of ferroelectric and magnetic orders in multiferroics not only give rise to many novel physical properties, but also enable potential applications in spintronics-based devices with enhanced functionalities, such as data storage [2], magnetic sensor [3], and energy harvesting [4]. To achieve practical applications, realization of multiferroicity above room temperature is one of the most prominent goals [5]. However, discovery of such room-temperature multiferroic materials remains a great challenge, in part due to the lack of suitable material systems that exhibit room-temperature multiferroicity. Presently, BiFeO3 appears to be the only room-temperature multiferroic compound, though the magnetoelectric coupling in BiFeO3 has been demonstrated to be rather small [6]. In recent years, REMnO3 (RE = rare-earth elements) materials have become one of the most extensively studied multiferroic systems [7]. Depending on the choice of substituted rare-earth elements, REMnO3 compounds are found to crystalize in either orthorhombic or hexagonal phases. Previous investigation [8] has concluded that substituted RE ions with large RE ionic radius (RE = La–Dy) are better accommodated in orthorhombic structure (o-REMnO3) with Pbnm space group, while the ones with small RE ionic radius (RE = Ho–Lu, Y, or Sc) are better accommodated in hexagonal structure (h-REMnO3) with P63cm space group. Unfortunately, most of these materials have very weak polarization, which is induced by their magnetic structures, and the observation of M-E coupling was hindered [7]. Nevertheless, hexagonal REMnO3 compounds show high polarization and high ferroelectric transition temperature near 900 K due to their P63cm non-centrosymmetric structure [9]. However, the magnetic order in these compounds appears below 100 K [10], and its antiferromagnetism can hinder the coupling between spins and ferroelectric orders.
Isostructural with the h-REMnO3, the hexagonal rare-earth ferrite h-REFeO3 is anticipated to exhibit multiferroic properties because the Fe3+ ions are of high magnetic moments and possibly possess strong indirect exchange coupling. Recently, the occurrence of multiferroicity at room temperature was found in h-LuFeO3 and LuFe2O4 heterojunction [11]. The coexistence of ferrimagnetic and ferroelectric orders was also reported in the orthorhombic LuFe1-xMnxO3 [12]. Moreover, the magnetic transition temperature of h-(Lu,Sc)FeO3 is above 160 K, which is the highest value when compared the ones in the REMO3 (M = Mn or Fe) series compounds [13]. Structurally, unlike REMnO3 compounds, REFeO3 compounds crystallize in orthorhombic structures for most of the rare-earth elements [13] with the exception of scandium [14,15,16,17,18]. It is noted that bixbyite ScFeO3 crystallizes in cubic structures with Ia-3 (No. 206) space group [19]. Therefore, it remains a challenge to obtain single-phase hexagonal REFeO3 that are anticipated to exhibit multiferroicity. In the literature, a successful strategy has been demonstrated to prepare hexagonal REFeO3 polycrystalline samples by a judicious mixture of rare-earth ions for suitable mean radius of RE elements [18]. This strategy of mixture of RE element has been widely explored in a range of sample preparation methods, such as conventional solid phase reaction, low-pressure metal-organic chemical vapor deposition or pulsed laser deposition. For synthesis of nanosized REFeO3 powder, the spray-ICP method [20] or the solution-based precursor method [21] can also be used. However, growth of single crystal hexagonal REFeO3 for studying its relevant multiferroic properties remains a challenging task.
In this work, we explored a conventional flux method to prepare single-phase h-REFeO3 single crystals with a particular focus on the judicious choice of flux solution. Through different mixture of flux solutions, we have successfully prepared three types of single crystals Lu1−xScxFeO3 with distinct orthorhombic, hexagonal, and cubic crystal structures. We then carried out systematic structural and multiferroic property characterizations of these compounds via X-ray diffraction, electron microscopy, and magnetization measurements.
2. Experimental Section
The first step is to prepare Lu0.5Sc0.5FeO3 polycrystalline precursors by conventional solid-state reaction of stochiometric mixture of high-purity Lu2O3 (99.99%, Alfa), Sc2O3 (99.99%, Alfa), and Fe2O3 (99.99%, Alfa) powders. All these raw powders were purchased from the Alfa Aesar Thermo Fisher Scientific via its distributor in Shanghai, China. The powder mixtures were thoroughly pulverized and pressed into pellets, which were sintered at 1100 °C for 12 h and then naturally cooled to room temperature; these processes of precursor preparation were repeated twice. Subsequently, the obtained Lu0.5Sc0.5FeO3 polycrystalline precursors were pulverized and divided into three parts, marking them as sample A, B, and C, together with the different choice of flux solution as following.
Sample A: per 10 g Lu0.5Sc0.5FeO3 powder was mixed with 18.9 g B2O3 (99.99%, Alfa) and 63.24 g Bi2O3 (99.99%, Alfa).
Sample B: per 10 g Lu0.5Sc0.5FeO3 powder was mixed with 63.24 g Bi2O3 (99.99%, Alfa).
Sample C: per 10 g Lu0.5Sc0.5FeO3 powder was mixed with 37.52 g K2CO3 (99.99%, Alfa), 18.9 g B2O3 (99.99%, Alfa), and 63.24 g Bi2O3 (99.99%, Alfa).
These samples were loaded into a 70 mL platinum crucible covered with a platinum lid, and placed in a high-temperature resistance furnace at 1300 °C. The reaction temperature was maintained for about 16 h. Subsequently, the samples were cooled at a rate about 2.5 °C/h from 1300 down to 950 °C. Then the crucibles were removed from the furnace and allowed to cool naturally to room temperature. The single crystals were extracted from the solidified mixture by immersing the crucible in hot dilute HNO3:H2O with a volume ratio of approximately 1:6, while keeping the temperature at 80~90 °C. Finally, the extracted single crystals were washed three times with deionized water.
Powder X-ray diffraction (XRD) measurements were performed on a Bruker D8 Discover diffractometer equipped with Cu Kα radiation. Single crystal X-ray diffraction (XRD) measurements were performed on a Bruker D8 Venture diffractometer equipped with Mo Kα radiation. Morphologies and chemical compositions of samples were analyzed on a Hitachi model S-4800 scanning electron microscope (SEM) with an Energy Dispersive X-ray (EDX) spectrometer. Microstructures and atomic analysis were performed on an aberration-corrected transmission electron microscope (JEM-ARM200F, JEOL Inc., Akishima, Tokyo). Magnetic properties measurement was performed in a Vibrating Sample Magnetometer (VSM, Quantum Design, San Diego, CA, USA) from 2 to 300 K under various applied magnetic fields in field-cooling (FC) and zero-field cooling (ZFC) modes.
3. Results and Discussion
Figure 1a–c shows the optical images of Lu1−xScxFeO3 single crystals and Figure 1d–f are their corresponding X-ray Laue diffraction patterns. Analyses of X-ray Laue diffraction patterns reveal that the red-brown rod crystals picked from sample A are single crystals of orthorhombic phase Lu0.96Sc0.04FeO3, the yellow-brown cube crystals picked from sample B are single crystals of cubic phase Lu0.2Sc0.8FeO3, and the black flake crystals extracted from sample C are single crystals of hexagonal phase Lu0.67Sc0.33FeO3. It should be noted that the Laue diffraction spots of the hexagonal single crystal (Figure 1f) samples are rather diffuse, which indicates that there is large number of lattice defects in the hexagonal single crystal Lu0.67Sc0.33FeO3. The crystallographic structures of the three single-crystal samples are refined by powder X-ray diffraction data to yield the average atomic structure and the chemical compositions. In addition, we performed SEM-EDX measurements to confirm that the chemical formulae of the three samples are orthorhombicLu0.96Sc0.04FeO3, hexagonal Lu0.67Sc0.33FeO3 and cubic Lu0.20Sc0.80FeO3. Table 1, Table 2 and Table 3 tabulate the corresponding crystallographic atomic sites and the chemical compositions. We further carried out powder X-ray diffraction measurements on the pulverized sample of Lu0.67Sc0.33FeO3 (Figure 2a) and the single crystal (Figure 2b). The insets to Figure 2a,b are the corresponding atomic structure and the SEM morphology.
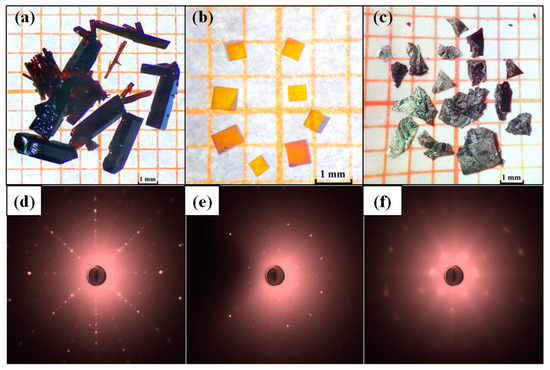
Figure 1.
Morphologies of the as-synthesized single crystals and the corresponding X-ray Laue diffraction patterns. (a–c) show the photographs of the single crystals of Lu0.96Sc0.04FeO3, Lu0.2Sc0.8FeO3 and Lu0.67Sc0.33FeO3, respectively. (d–f) are the [100]-oriented X-ray Laue diffraction patterns for the single crystals of Lu0.96Sc0.04FeO3, Lu0.2Sc0.8FeO3 and Lu0.67Sc0.33FeO3, respectively.

Table 1.
Crystal data and atomic coordinates of Lu0.96Sc0.04FeO3 at 300 K.

Table 2.
Crystal data and atomic coordinates of Lu0.2Sc0.8FeO3 at 300 K.

Table 3.
Crystal data and atomic coordinates of Lu0.67Sc0.33FeO3 at 300 K.
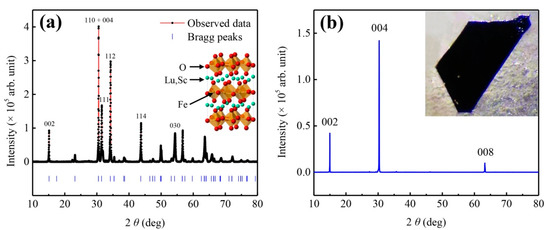
Figure 2.
X-ray diffraction data of Lu0.67Sc0.33FeO3 single crystal. (a) Powder X-ray diffraction profile. Inset shows the atomic representation of the hexagonal crystal structure. (b) [100]-oriented X-ray diffraction of single crystal Lu0.67Sc0.33FeO3. Inset shows a typical crystalline plate.
Based on the systematic growth experiments with varying flux solution, we found that the composition ratios of K2CO3-B2O3-Bi2O3 flux play a key role in determining the crystal structure and the compositions of the final Lu0.67Sc0.33FeO3 products. Figure 3 summarizes the relationship between flux compositions and single crystal products, in which the weight ratios of three compounds as flux are represented in the triangle relationship. An empirical rule is obtained: with a high content of Bi2O3 up to 100%, the product is dominated by cubic Lu0.2Sc0.8FeO3 bixbyite phase; with a low content of Bi2O3 down to 0%, the product is dominated by the normal ferrite phase with orthorhombic structure. Our results of crystal growth experiments indicate that a high yield of hexagonal single crystal samples can be obtained using a flux solution K2CO3-B2O3-Bi2O3 with molar ratio of 2:2:1. We note that in most cases the three phases are mixed and sometimes contain with a small amount of Lu3Fe5O12 crystalline grains. It is worth mentioning that we also used PbO-PbF2 as flux and obtained large size of Lu3Fe5O12 single crystals. However, we did not obtain any single crystals only by using K2CO3 flux, implying that the used potassium carbonate acts as a template in the reaction system, which induces the formation of hexagonal phase.
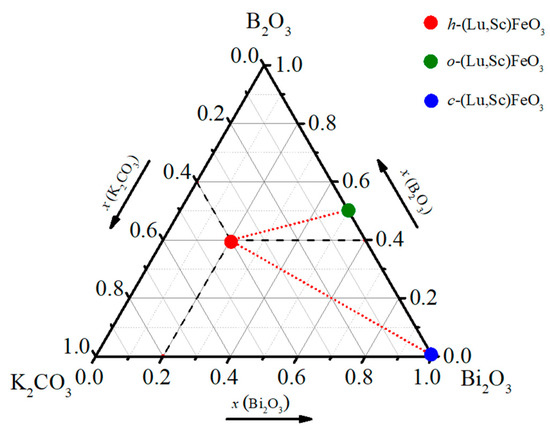
Figure 3.
A triangle relationship is used to represent the weight ratios of the three compounds used as a flux. Depending on the mixture of flux composition used in the solid-state reaction, the final products of Lu1−xScx FeO3 single crystals with distinct compositions and crystal structures are indicated.
Next, we investigated the magnetic properties of the three types of Lu1−xScxFeO3 single crystals using Vibrating Sample Magnetometer (VSM). Figure 4a shows the temperature-dependent magnetization M-T curve of the orthorhombic phase, displaying a ferromagnetic ordering with strong magnetic anisotropy. In the direction parallel to b axis, the magnetic moment increases with the decrease in temperature, but the growth rate decreases gradually and becomes almost flat near 2 K. In the direction perpendicular to b, the magnetic moment first increases and then decreases with the decrease in temperature. The field-dependent magnetization M-H curves clearly exhibit the magnetic hysteresis loops with the temperature-dependent coercive fields close to 1 T. In a fixed temperature, the coercivity in the direction parallel to b is about 20% of that in the direction perpendicular to b. The saturated magnetic field of this material is far beyond the measurable range of our VSM. In the cubic phase Lu0.20Sc0.80FeO3, the M-T curve in Figure 4b reveals that the Lu0.20Sc0.80FeO3 exhibits a paramagnetic behavior down to very low temperature of around 20 K. The M-H curve of Lu0.20Sc0.80FeO3 reveals a very small coercivity and small remnant magnetization below 20 K. We note that due to the small size of the hexagonal phase crystal with a thickness of only a few tens of microns, only the magnetization curves in ab plane were obtained. In the hexagonal phase Lu0.67Sc0.33FeO3, the M-T curve in Figure 4c reveals a typical paramagnetic-to-ferromagnetic transition at Curie temperature TC ~ 160 K. It can be seen from the M-H curve that as the temperature decreases, the remnant magnetization first increases, then decreases below 75 K, while the coercivity continues to increase from 300 K to 2 K, which is consistent with the literature [13,22] with a scenario of spin rearrangement at low temperature.
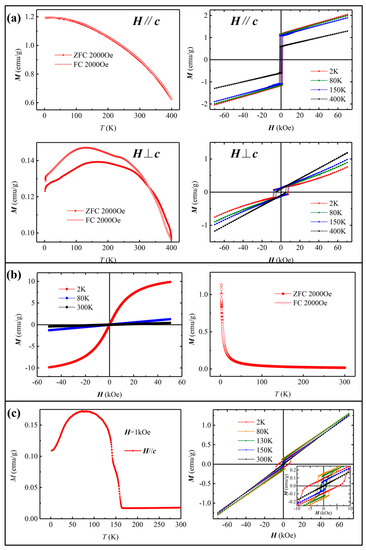
Figure 4.
Magnetic properties of the three single crystals. (a) The temperature-dependent magnetization curve M (T) and the field-dependent magnetization curve M (H) of orthorhombic Lu0.96Sc0.04FeO3. (b) M (T) and M (H) of cubic Lu0.20Sc0.80FeO3. (c) M (T) and M (H) of hexagonal Lu0.67Sc0.33FeO3.
Figure 5 shows the SEM morphologies and the corresponding EDX characterizations of the series of Lu1−xScxFeO3 single crystals. While the surfaces of orthorhombic Lu0.96Sc0.04FeO3 is smooth and featureless (see Figure 5a), both the Lu0.67Sc0.33FeO3 and Lu0.20Sc0.80FeO3 exhibit some patterns on the surfaces. It is noteworthy that Figure 5b shows a typical cloverleaf-like pattern on the surface of the hexagonal sample Lu0.67Sc0.33FeO3 that observed by scanning electron microscopy (SEM), which reflects the surface topologies of ferroelectric domains. As we soaked the sample with dilute nitric acid in the process of removing the flux solution, the surface of the sample was corroded depending on the ferroelectric domain structures. The corrosion rates of the parts in different polarization directions are different, thus the corrosion lines in different depth are left on the surface of the sample. These cloverleaf-like patterns closely reflect the distribution of vortex ferroelectric domains on the surface of the sample, whose appearance resembles the images by piezo-response force microscopy (PFM) [23]. We note that the cloverleaf-like patterns of vortex ferroelectric domains that observed by SEM had been reported in YMnO3 [24].
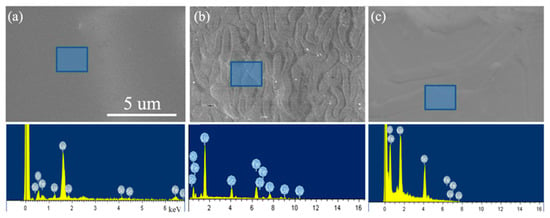
Figure 5.
SEM and EDX analysis of Lu1−xScxFeO3 single crystals. (a) Lu096Sc0.04FeO3. (b) Lu0.67Sc0.33FeO3. (c) Lu0.2Sc0.8FeO3.
We further characterized these typical ferroelectric domain microstructures in Lu0.67Sc0.33FeO3 at the atomic scale using high-angle annual-dark-field (HAADF) in the STEM mode. In the HAADF imaging, the recorded contrast arises primarily from Rutherford scattering and thermal diffuse scattering. Under thin specimen approximation, the HAADF intensity scales to a good approximation with the atomic number Z and specimen thickness. Figure 6a,b are the electron diffraction pattern and the corresponding HAADF atomic image of a [001]-oriented Lu0.67Sc0.33FeO3 thin specimen, and Figure 6c,d are the ones of a [110]-oriented specimen. In Figure 6d, the brightest dots can be attributed to the heavy Lu ions and the less bright dots to the Fe ions. Figure 6e displays an atomic HAADF image across typical ferroelectric domains and their respective polarizations are marked by “P”. Moreover, we found that additional diffraction spots appeared in the electron diffraction pattern of the as-obtained sample (as Figure 7 shows), which may be due to the structure distortions caused by the oxygen vacancy. After annealing treatment in the presence of oxygen atmosphere at 800 y for 12 h, these additional diffraction spots vanish, suggesting that thermal annealing process with oxygen is an effective means of greatly improving the quality of the LuxSc1−xFeO3 samples.
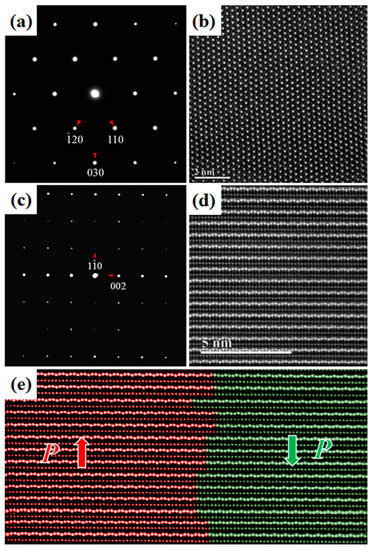
Figure 6.
Electron diffraction and Z-contrast atomic image via high-angle annual dark-field HAADF imaging. (a) [001]-oriented electron diffraction. (b) [001]-oriented Z-contrast atomic image. (c) [110]-oriented electron diffraction. (d) [110]-oriented Z-contrast atomic image. (e) Polarization domain wall along [110].
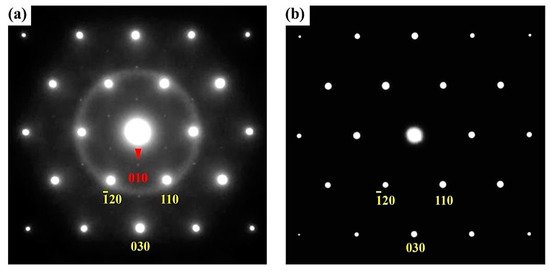
Figure 7.
Electron diffraction pattern along [001] axis of h-Lu0.67Sc0.33FeO3 single crystal before and after annealing in oxygen atmosphere. (a) Before annealing; (b) After annealing.
4. Conclusions
In summary, using a conventional flux-assisted solid-state reaction method, we systematically explored the influence of flux solution on the growth of high quality Lu1−xScxFeO3 single crystals. We obtained three types of Lu1−xScxFeO3 single crystals, namely, orthorhombic Lu0.96Sc0.04FeO3, hexagonal Lu0.67Sc0.33FeO3 and cubic Lu0.20Sc0.80FeO3 through appropriate choice of flux solution K2CO3-B2O3-Bi2O3 mixtures. We found that with higher Bi2O3 content, the as-obtained product is mainly comprised of cubic Lu0.2Sc0.8FeO3 bixbyite phase. With lower Bi2O3 content, the products are dominated by the ferrite phase with orthorhombic structure. A high yield of hexagonal single crystal samples can be obtained using K2CO3-B2O3-Bi2O3 mixed flux solution with molar ratio of 2:2:1.
The temperature- and field-dependent magnetization of the three types of LuxSc1−xFeO3 with distinct structure and composition were investigated. The orthorhombic phase Lu0.96Sc0.04FeO3 is a hard magnetic material at room temperature, the cubic phase Lu0.2Sc0.8FeO3 is a paramagnetic material down to very low temperature, and a spin rearrangement occurs at low temperature in the hexagonal phase Lu0.67Sc0.33FeO3. Since the hexagonal Lu0.67Sc0.33FeO3 is of particular research interest, we further carried out electron microscopy characterization of the ferroelectric domain topologies via SEM and the atomic configurations at ferroelectric domain walls via STEM. In SEM measurements, we observed the characteristic cloverleaf-like patterns of vortex ferroelectric domains in Lu0.67Sc0.33FeO3 surfaces. In the atomically-resolved STEM measurements, we obtained the atomic configuration at the ferroelectric domain walls and the polarization distribution across the ferroelectric domains in Lu0.67Sc0.33FeO3. In addition, we applied thermal annealing treatment in the presence of oxygen atmosphere for the hexagonal Lu0.67Sc0.33FeO3 single crystal. As a result of thermal treatment, structural defects and local lattice distortions caused by oxygen vacancy is considerably reduced in the post-annealed Lu0.67Sc0.33FeO3. Our results illustrate a feasible strategy to obtain multiferroic oxide based single crystals via the flux-assisted solid-state reaction.
Author Contributions
Conceptualization, C.X. and J.L. (Jianqi Li); methodology, C.X., J.L. (Jun Li), H.T. and Z.-A.L.; writing—original draft preparation, C.X. and Z.-A.L.; supervision, H.Y. and J.L. (Jianqi Li); All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangdong Major Scientific Research Project (2018KZDXM061), the National Key Research and Development Program of China (Grant Nos. 2017YFA0504703, 2017YFA0302904, 2017YFA0303000 and 2016YFA0300303), the National Natural Science Foundation of China (Grant Nos. 11974019, and 11804381, 12074408), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant Nos. XDB25000000 and XDB33010100), the Scientific Instrument Developing Project of the Chinese Academy of Sciences (Grant Nos. YJKYYQ20200055, ZDKYYQ2017000, 22017BA10), Beijing Municipal Science and Technology major project (Z201100001820006), IOP Hundred Talents Program (Y9K5051) and the Postdoctoral Support Program of China (No. 2020M670501) the China Postdoctoral Science Foundation funded project (Grant No. BX20180351).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Xingye Lu and Zhen Tao of Beijing Normal University for their assistance in X-ray Laue diffraction.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fiebig, M.; Lottermoser, T.; Meier, D.; Trassin, M. The evolution of multiferroics. Nat. Rev. Mater. 2016, 1, 16046. [Google Scholar] [CrossRef]
- Li, X.; Nan, C. Multiferroic Materials. Sci. Front. 2018, 13, 45–47. [Google Scholar]
- Ortega, N.; Kumar, A.; Scott, J.F.; Katiyar, R.S. Multifunctional magnetoelectric materials for device applications. J. Phys. Condens. Matter 2015, 27, 504002. [Google Scholar] [CrossRef]
- Onuta, T.D.; Wang, Y.; Long, C.J.; Takeuchi, I. Energy harvesting properties of all-thin-film multiferroic cantilevers. Appl. Phys. Lett. 2011, 99, 203506. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Tan, H.; Duan, W. Hexagonal rare-earth manganites and ferrites: A review of improper ferroelectricity, magnetoelectric coupling, and unusual domain walls. Phys. Chem. Chem. Phys. 2020, 22, 14415–14432. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef]
- Kimura, T.; Goto, T.; Shintani, H.; Ishizaka, K.; Arima, T.; Tokura, Y. Magnetic control of ferroelectric polarization. Nature 2003, 426, 55–58. [Google Scholar] [CrossRef]
- Fontcuberta, J. Multiferroic RMnO3 thin films. Comptes Rendus Phys. 2015, 16, 204–226. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, A.S.; Knight, K.S.; Lightfoot, P. High-temperature phase transitions of hexagonal YMnO3. Phys. Rev. B 2011, 87, 094111. [Google Scholar] [CrossRef] [Green Version]
- Picozzi, S.; Ederer, C. First Principles Studies of Multiferroic Materials. J. Phys. Condens. Matter Inst. Phys. J. 2009, 21, 303201. [Google Scholar] [CrossRef]
- Song, S.; Han, H.; Jang, H.M.; Kim, Y.T.; Lee, N.-S.; Park, C.G.; Kim, J.R.; Noh, T.W.; Scott, J.F. Implementing Room-Temperature Multiferroism by Exploiting Hexagonal-Orthorhombic Morphotropic Phase Coexistence in LuFeO3 Thin Films. Adv. Mater. 2016, 28, 7430–7435. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.L.; Green, W.; Lofland, S.E.; Ramanujachary, K.V.; Das, D.; Ganguli, A.K. Study on the solid solution of YMn1−xFexO3: Structural, magnetic and dielectric properties. J. Solid State Chem. 2008, 181, 61. [Google Scholar] [CrossRef]
- Masuno, A.; Ishimoto, A.; Moriyoshi, C.; Hayashi, N.; Kawaji, H.; Kuroiwa, Y.; Inoue, H. Weak Ferromagnetic Transition with a Dielectric Anomaly in Hexagonal Lu0.5Sc0.5FeO3. Inorg. Chem. 2013, 52, 11889–11894. [Google Scholar] [CrossRef]
- Leiner, J.C.; Kim, T.; Park, K.; Oh, J.; Perring, T.G.; Walker, H.C.; Xu, X.; Wang, Y.; Cheong, S.-W.; Park, J.-G. Magnetic excitations in the bulk multiferroic two-dimensional triangular lattice antiferromagnet (Lu,Sc)FeO3. Phys. Rev. B 2018, 98, 134412. [Google Scholar] [CrossRef] [Green Version]
- Markelova, M.; Nygaard, R.; Tsymbarenko, D.; Shurkina, A.; Abramov, A.; Amelichev, V.; Makarevich, A.; Vasiliev, A.; Kaul, A. Multiferroic h-LuFeO3 Thin Films on (111) and (100) Surfaces of YSZ Substrates: An Experimental and Theoretical Study. ACS Appl. Electron. Mater. 2021, 3, 1015–1022. [Google Scholar] [CrossRef]
- Liu, F.; Xu, C.; Shen, S.; Li, N.; Guo, H.; Lü, X.; Xiang, H.; Bellaiche, L.; Zhao, J.; Yin, L.; et al. Pressure-induced large enhancement of Néel temperature and electric polarization in the hexagonal multiferroic Lu0.5Sc0.5FeO3. Phys. Rev. B 2019, 100, 214408. [Google Scholar] [CrossRef]
- Wen, T.; Wang, Y.; Li, C.; Jiang, D.; Jiang, Z.; Qu, S.; Yang, W.; Wang, Y. Site-Specific Pressure-Driven Spin-Crossover in Lu1−xScxFeO3. J. Phys. Chem. Lett. 2020, 11, 8549–8553. [Google Scholar] [CrossRef]
- Atsunobu, M.; Atsushi, I.; Moriyoshi, C.; Kawaji, H.; Kuroiwa, Y.; Inoue, H. Expansion of the Hexagonal Phase-Forming Region of Lu1−xScxFeO3 by Containerless Processing. Inorg. Chem. 2015, 54, 9432–9437. [Google Scholar]
- Hamasaki, Y.; Shimizu, T.; Yasui, S.; Taniyama, T.; Sakata, O.; Itoh, M. Crystal ssomers of ScFeO3. Cryst. Growth Des. 2016, 16, 5214–5222. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Onodera, H.; Yamauchi, H.; Kagawa, M.; Syono, Y.; Hirai, T. Moessbauer spectra and magnetic susceptibilities of ultrafine hexagonal RFeO3 (R ≠ Eu, Yb) particles formed by the spray inductively coupled plasma technique. Mater. Sci. Eng. A 1996, 217–218, 164–166. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Takemura, H.; Yamashita, M.; Hayashida, A. Formation of Yttrium Iron Oxides Derived from Alkoxides. J. Electrochem. Soc. 1991, 138, 1492–1494. [Google Scholar] [CrossRef]
- Bréard, Y.; Fjellvåg, H.; Hauback, B. Investigation of bixbyite type scandium oxides involving a magnetic cation: Sc2−xFexO3. Solid State Commun. 2011, 151, 223–226. [Google Scholar] [CrossRef]
- Du, K.; Gao, B.; Wang, Y.; Xu, X.; Kim, J.; Hu, R.; Huang, F.-T.; Cheong, S.-W. Vortex ferroelectric domains, large-loop weak ferromagnetic domains, and their decoupling in hexagonal (Lu,Sc)FeO3. npj Quantum Mater. 2018, 3, 33. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Chen, D.; Yu, Y.; Hao, W.; Cheng, Z.; Dou, S.X. Manipulation of domain wall mobility by oxygen vacancy ordering in multiferroic YMnO3. Phys. Chem. Chem. Phys. 2013, 15, 20010–20015. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).