Hot-Corrosion and Particle Erosion Resistance of Co-Based Brazed Alloy Coatings
Abstract
:1. Introduction
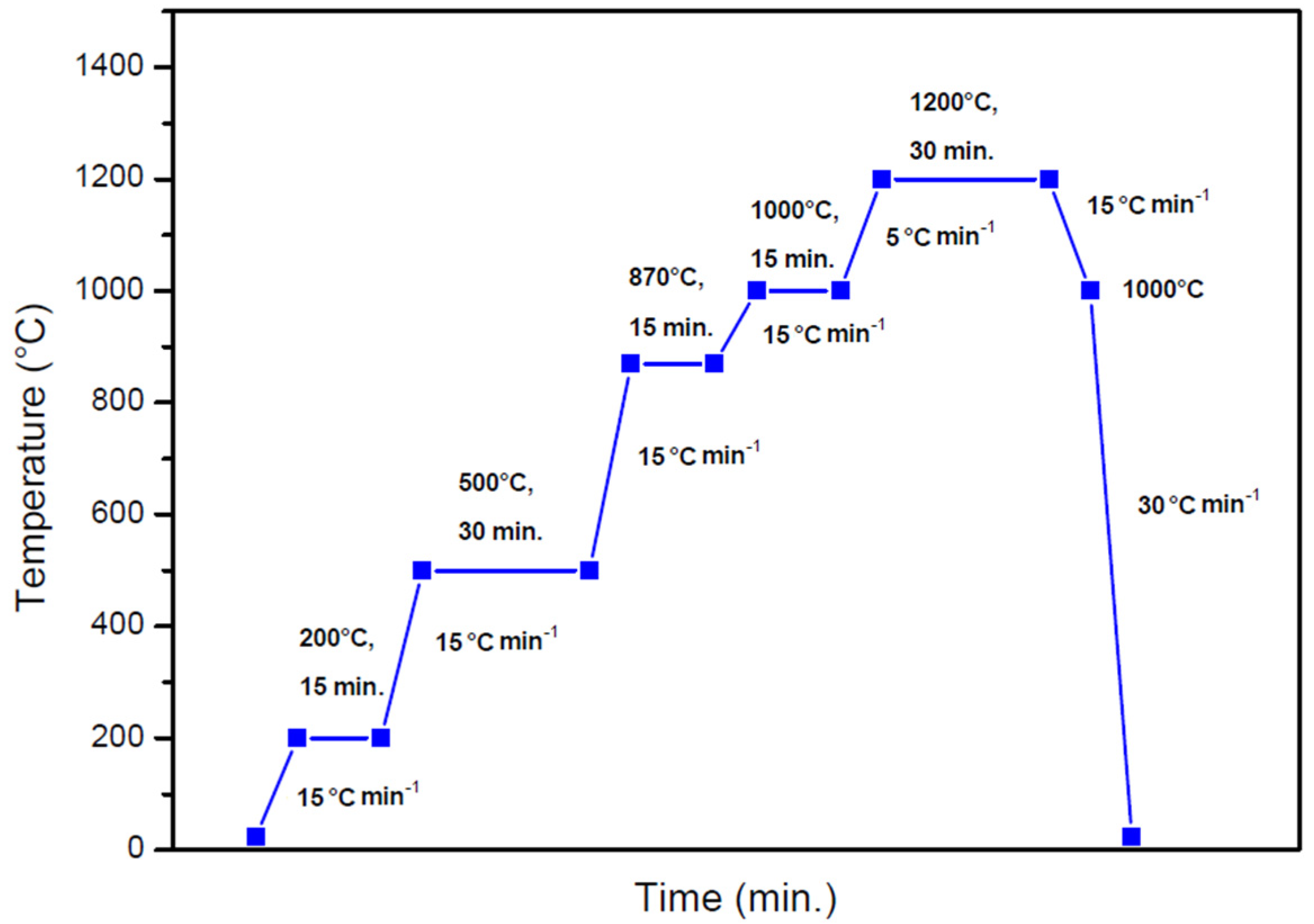
2. Materials and Methods
3. Data analysis and Preliminary Results
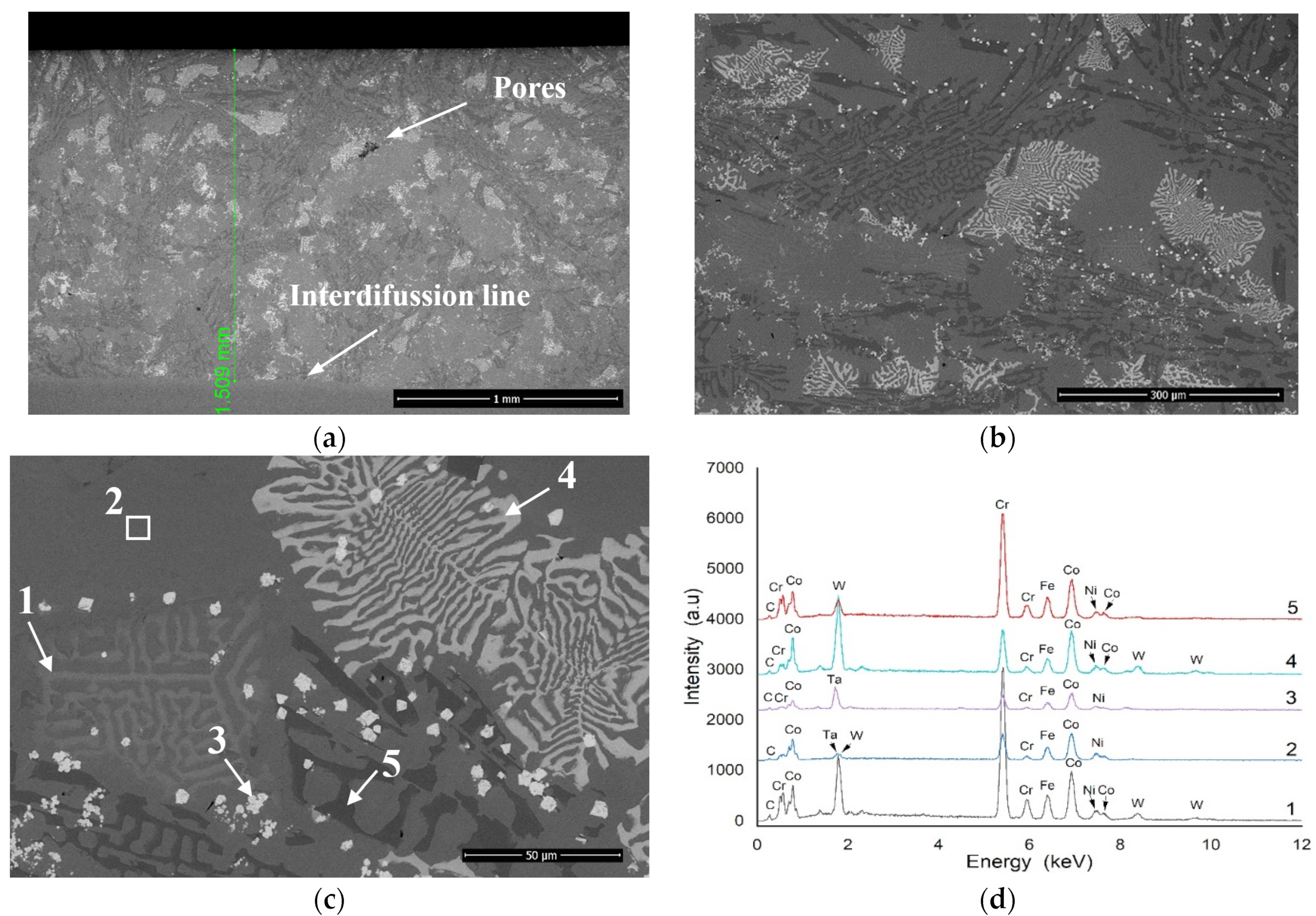
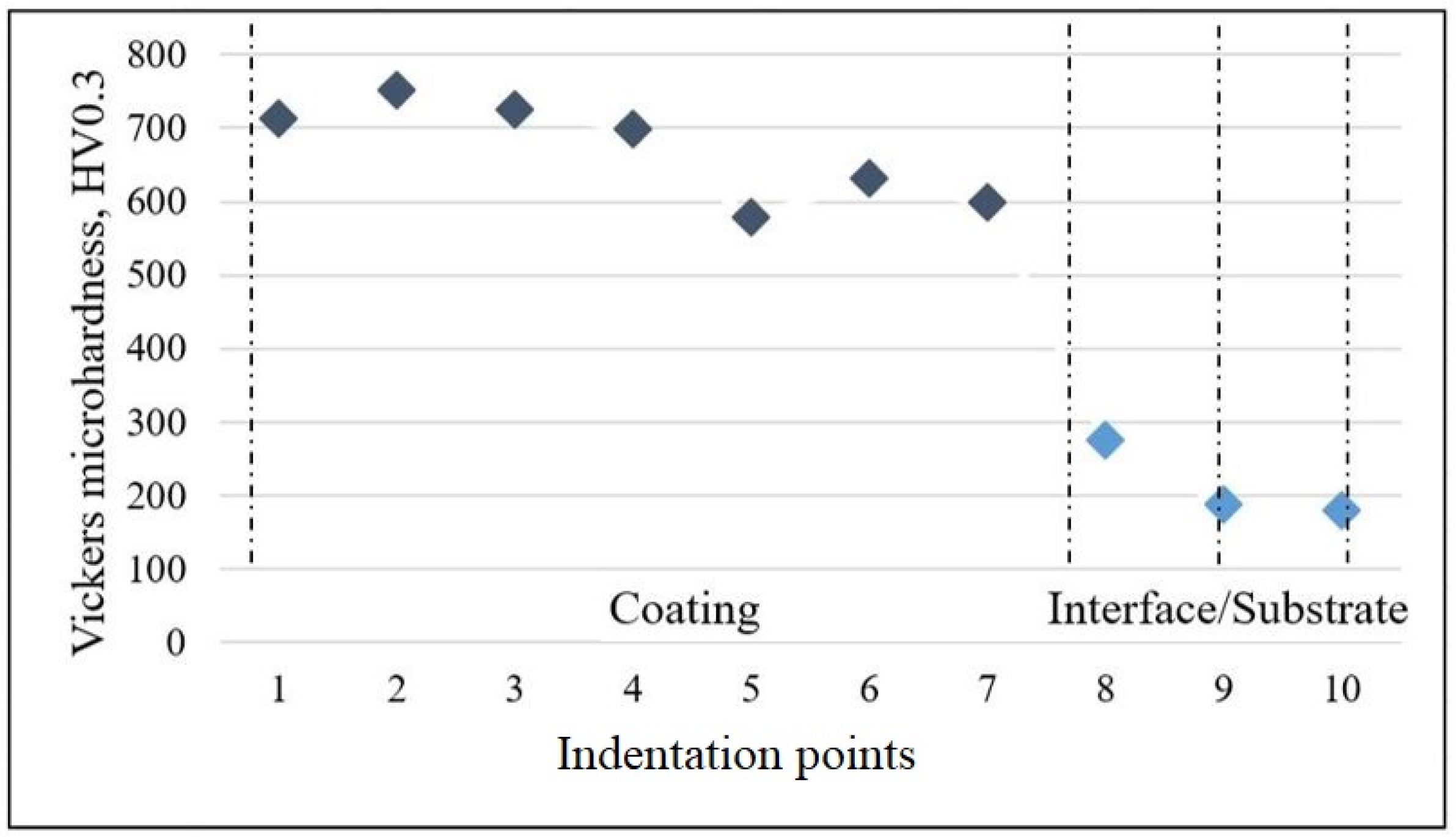
3.1. Microstructural Analysis
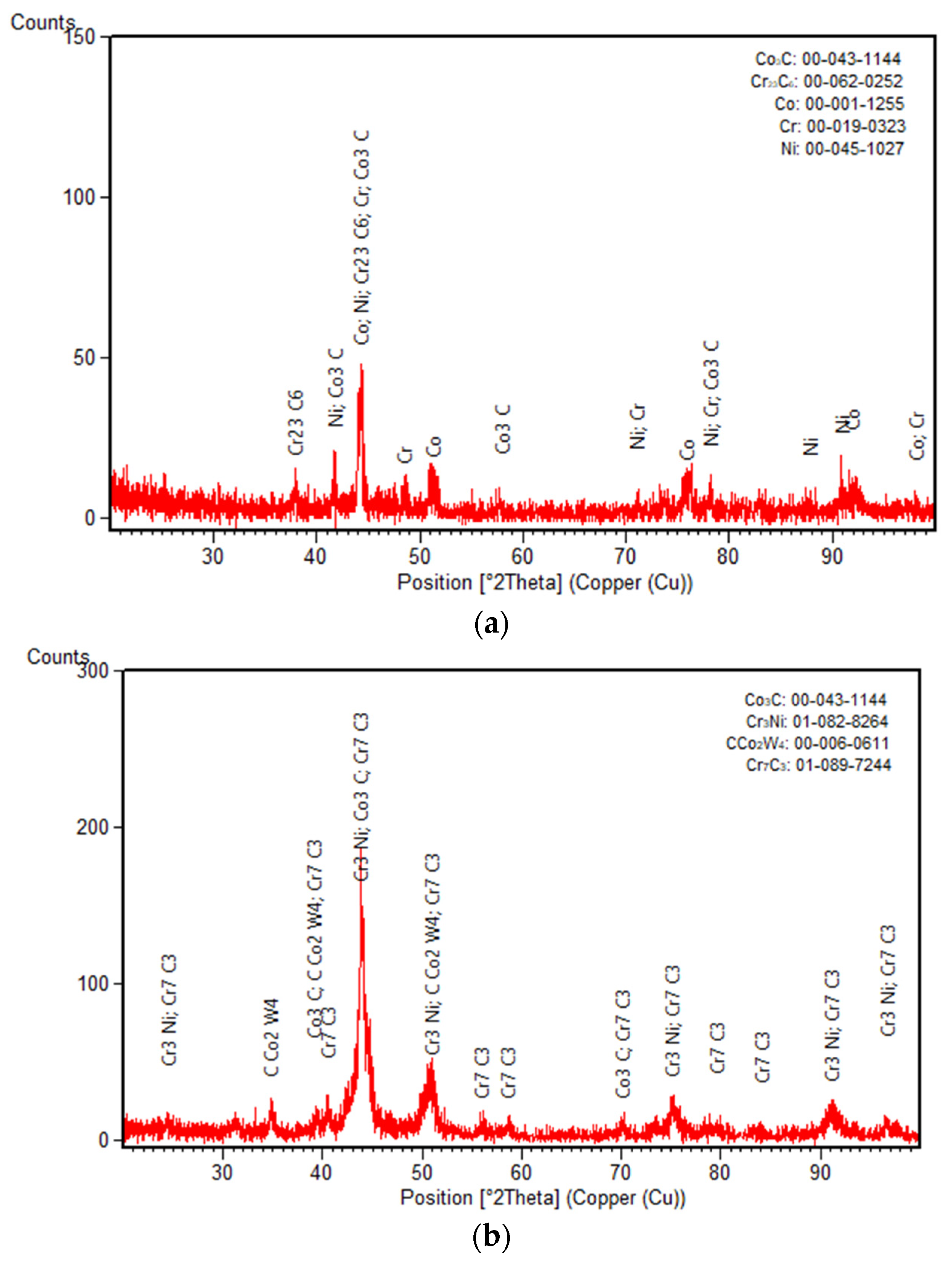
3.2. XRD Diffraction Measurements
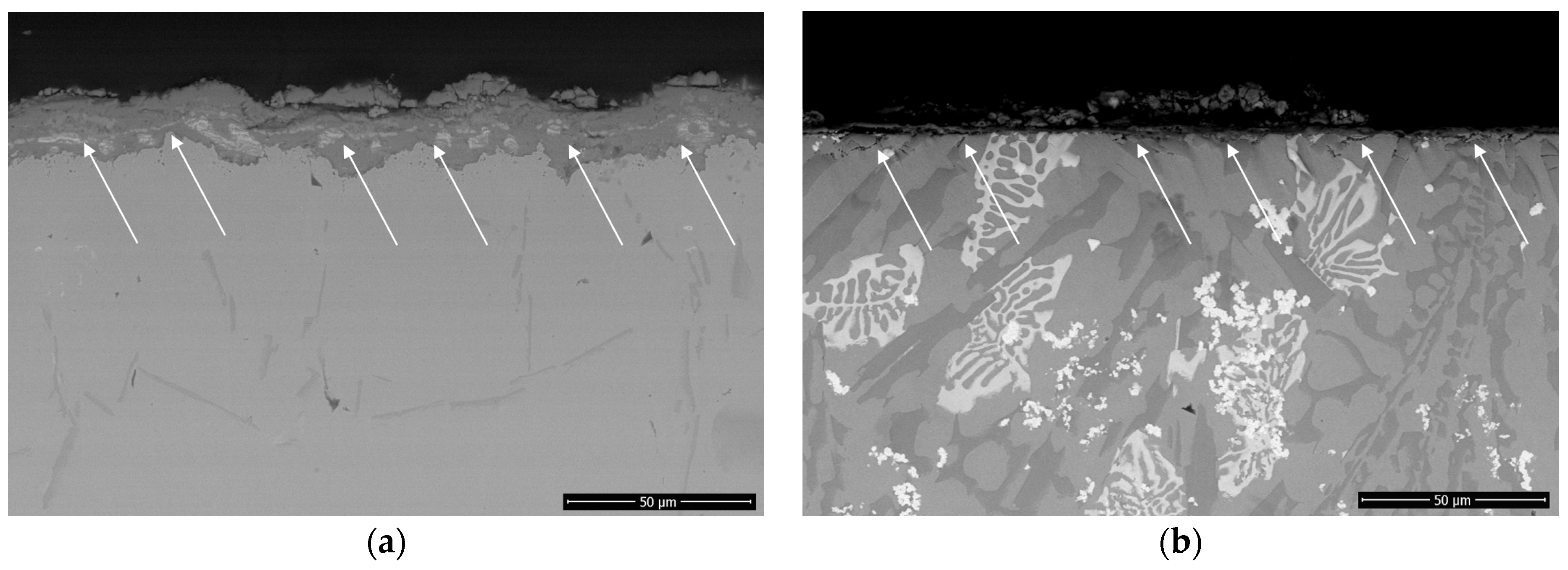
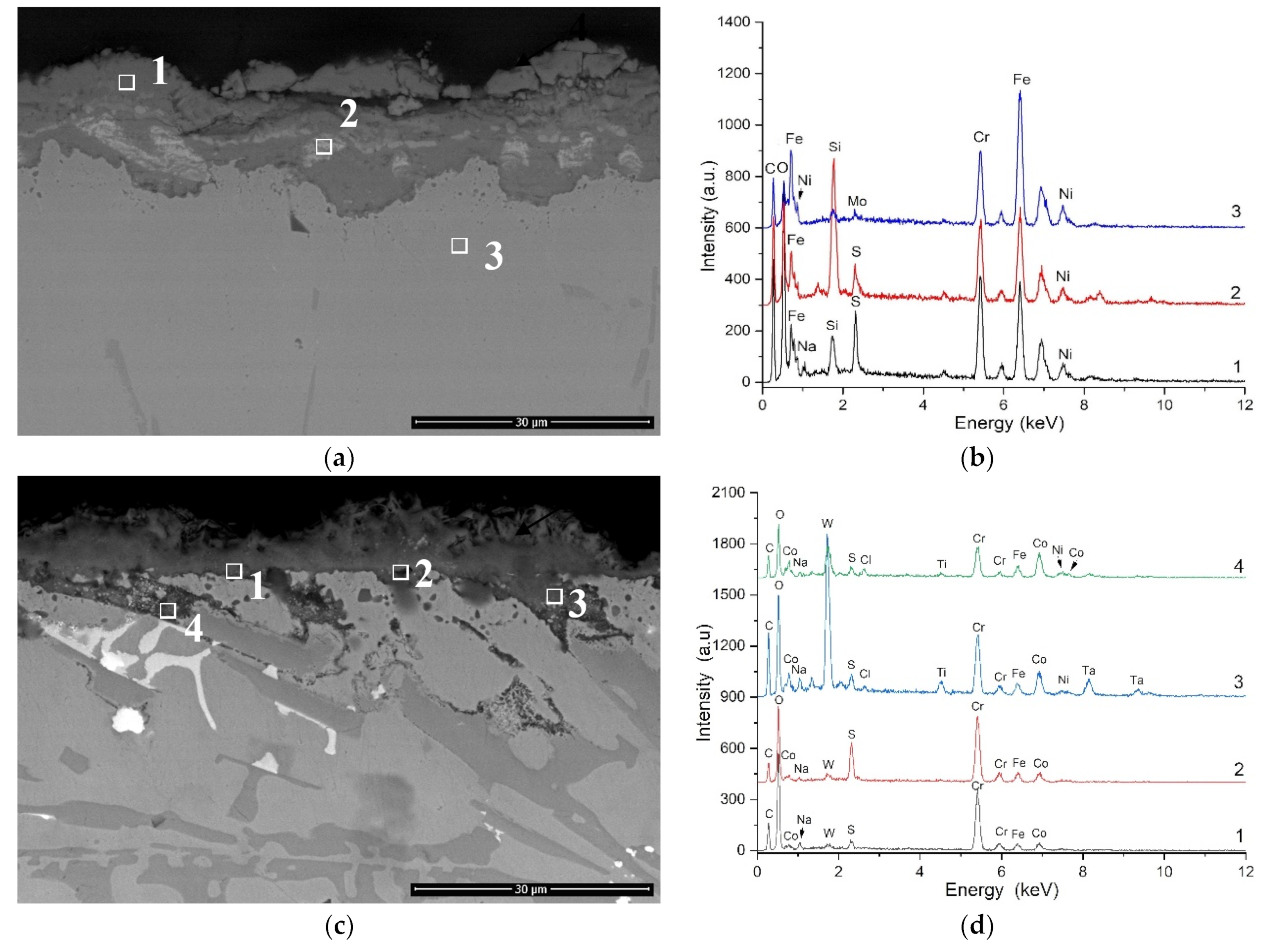
3.3. Hot Corrosion Behavior
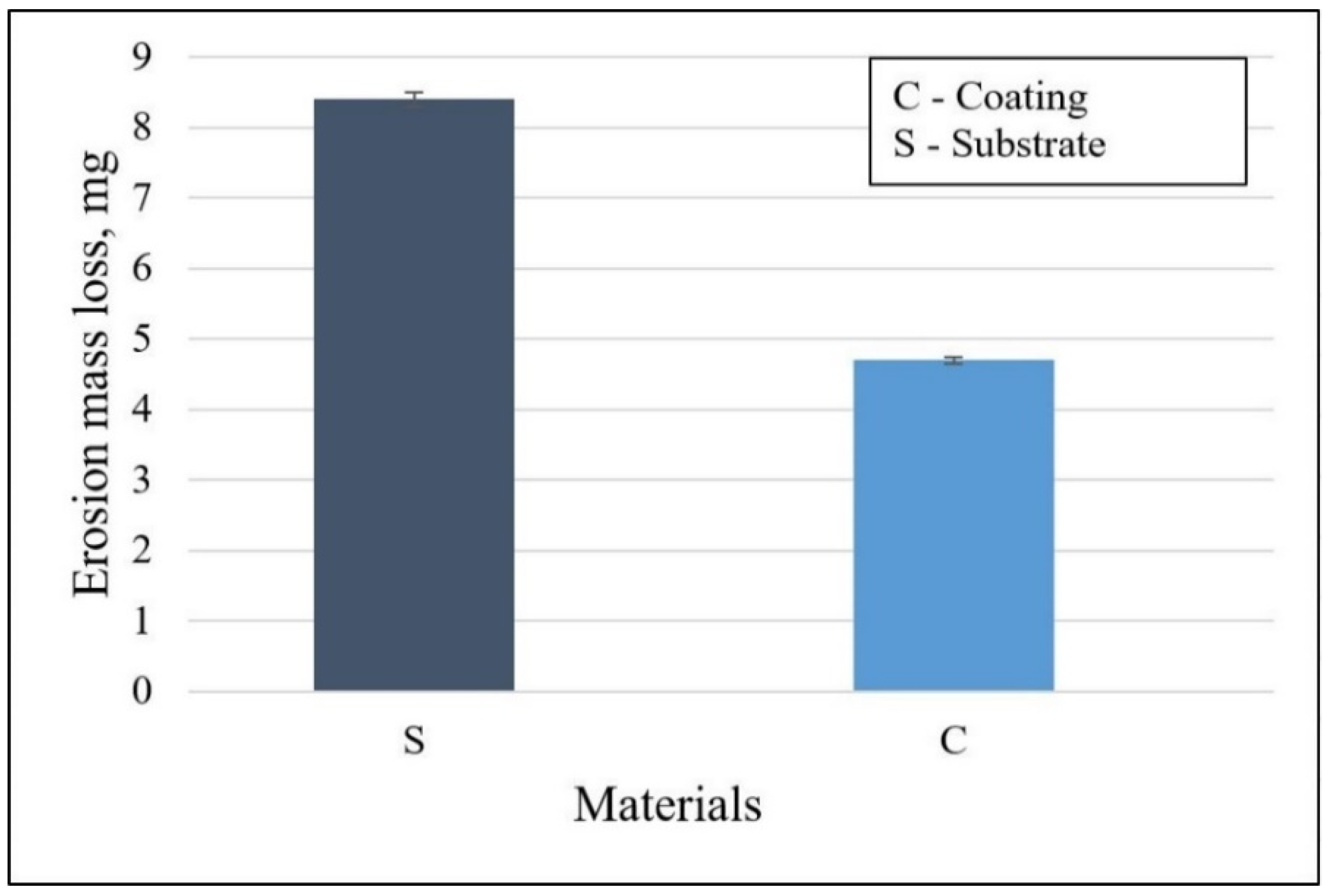
3.4. Solid Particle Erosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Chauhan, S.S.; Singh, G.; Sharma, M.; Singh, V.P.; Vaish, R. Anticorrosion and Electromagnetic Interference Shielding Behavior of Candle Soot-Based Epoxy Coating. J. Appl. Polym. Sci. 2020, 137, 48678. [Google Scholar] [CrossRef]
- Jiang, K. Effects of Heat Treatment on Microstructure and Wear Resistance of Stainless Steels and Superalloys; University of Ottawa: Ottawa, ON, Canada, 2013. [Google Scholar]
- Bell, T. Surface Engineering of Austenitic Stainless Steel. Surf. Eng. 2002, 18, 415–422. [Google Scholar] [CrossRef]
- Karafyllias, G.; Galloway, A.; Humphries, E. Erosion-Corrosion Assessment in Strong Acidic Conditions for a White Cast Iron and UNS S31600 Stainless Steel. Wear 2021, 484–485, 203665. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent Developments in Stainless Steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Graf, K.; Tetzlaff, U.A.W.; de Souza, G.B.; Scheid, A. Effect of Dilution on the Microstructure and Properties of CoCrMoSi Alloy Coatings Processed on High-Carbon Substrate. Mater. Res. 2019, 22. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.C.; Chen, C. Characteristics of Thin Surface Layers of Cobalt-Based Alloys Deposited by Laser Cladding. Surf. Coat. Technol. 2006, 200, 4557–4563. [Google Scholar] [CrossRef]
- Davis, J.R. Nickel, Cobalt, and Their Alloys; ASM International: Almere, The Netherlands, 2001; ISBN 0-87170-685-7. [Google Scholar]
- Wen, J.; Che, H.; Cao, R.; Dong, H.; Ye, Y.; Zhang, H.; Brechtl, J.; Gao, Y.; Liaw, P.K. Evolution of the Mechanical Properties of a Cobalt-Based Alloy under Thermal Shocks. Mater. Des. 2020, 188, 108425. [Google Scholar] [CrossRef]
- Liu, P.; Chen, D.; Wang, Q.; Xu, P.; Long, M.; Duan, H. Crystal Structure and Mechanical Properties of Nickel–Cobalt Alloys with Different Compositions: A First-Principles Study. J. Phys. Chem. Solids 2020, 137, 109194. [Google Scholar] [CrossRef]
- Cinca, N.; López, E.; Dosta, S.; Guilemany, J.M. Study of Stellite-6 Deposition by Cold Gas Spraying. Surf. Coat. Technol. 2013, 232, 891–898. [Google Scholar] [CrossRef]
- Yao, M.X.; Wu, J.B.C.; Yick, S.; Xie, Y.; Liu, R. High Temperature Wear and Corrosion Resistance of a Laves Phase Strengthened Co–Mo–Cr–Si Alloy. Mater. Sci. Eng. A 2006, 435–436, 78–83. [Google Scholar] [CrossRef]
- Hu, H.X.; Guo, X.M.; Zheng, Y.G. Comparison of the Cavitation Erosion and Slurry Erosion Behavior of Cobalt-Based and Nickel-Based Coatings. Wear 2019, 428–429, 246–257. [Google Scholar] [CrossRef]
- Kong, G.; Zhang, D.; Brown, P.D.; McCartney, D.G.; Harris, S.J. Microstructural Characterisation of High Velocity Oxyfuel Thermally Sprayed Stellite 6. Mater. Sci. Technol. 2003, 19, 1003–1011. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Puri, D.; Prakash, S. Mechanical and Metallurgical Properties of Plasma Sprayed and Laser Remelted Ni–20Cr and Stellite-6 Coatings. J. Mater. Processing Technol. 2005, 159, 347–355. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, W.; Yao, K.; Goussain, J.-C.; Mayer, C.; Becker, A. Microstructural Evolution in High Power Laser Cladding of Stellite 6+WC Layers. Surf. Coat. Technol. 2002, 157, 128–137. [Google Scholar] [CrossRef]
- Cui, G.; Han, B.; Zhao, J.; Li, M. Comparative Study on Tribological Properties of the Sulfurizing Layers on Fe, Ni and Co Based Laser Cladding Coatings. Tribol. Int. 2019, 134, 36–49. [Google Scholar] [CrossRef]
- Ahn, B. Recent Advances in Brazing Fillers for Joining of Dissimilar Materials. Metals 2021, 11, 1037. [Google Scholar] [CrossRef]
- Jia, J.H.; Wang, Z.H.; Yao, D.F.; Tu, S.-T. Brazing Coupling Performance of Piezoelectric Waveguide Transducers for the Monitoring of High Temperature Components. Sensors 2021, 21, 94. [Google Scholar] [CrossRef]
- Sharma, A.; Ahn, B. Brazeability, Microstructure, and Joint Characteristics of ZrO2/Ti-6Al-4V Brazed by Ag-Cu-Ti Filler Reinforced with Cerium Oxide Nanoparticles. Adv. Mater. Sci. Eng. 2019, 2019, 8602632. [Google Scholar] [CrossRef] [Green Version]
- Rybaulin, V.M.; Skorobatyuk, A.V.; Mikitas, A.V. Brazing of Absorbers of Planar Solar Heating Collectors Produced from Materials of the Cu–CuZn System. Weld. Int. 2016, 30, 142–149. [Google Scholar] [CrossRef]
- Pascal, D.-T.; Kazamer, N.; Muntean, R.; Valean, P.-C.; Serban, V.-A. Electrochemical Corrosion Behavior of High Temperature Vacuum Brazed WC-Co-NiP Functional Composite Coatings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012003. [Google Scholar] [CrossRef] [Green Version]
- Pascal, D.-T. Development of High Temperature Vacuum Brazed WC-Co-NiP Functional Composite Coatings. Ph.D. Thesis, University Politehnica Timisoara, Timisoara, Romania, 2017. [Google Scholar]
- Kalsi, S.S. Hot Corrosion and Its Mechanism: A Review. Int. J. Emerg. Technol. 2015, 7, 133–136. [Google Scholar]
- Petit, F.; Meier, G.H. Oxidation and Hot Corrosion of Superalloys. Supperalloys 1984, 85, 651–687. [Google Scholar] [CrossRef]
- Amdry MM509B-C Cobalt Braze Alloy Powder (Boron as Melt-Depressant). Available online: https://matmatch.com/materials/metc000058-amdry-mm509b-c-cobalt-braze-alloy-powder-boron-as-melt-depressant (accessed on 8 November 2021).
- Bohatch, R.G.; Graf, K.; Scheid, A. Effect of Track Overlap on the Microstructure and Properties of the CoCrMoSi PTA Coatings. Mater. Res. 2015, 18, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Scheid, A.; D’Oliveira, A.S.C.M. Effect of Processing on Microstructure and Properties of CoCrMoSi Alloy. Mater.Res. 2013, 16, 1325–1330. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Sergeev, D.; Dodge, M.F.; Fanicchia, F.; Müller, M.; Paul, S.; Dong, H. Microstructure and Thermal Analysis of Metastable Intermetallic Phases in High-Entropy Alloy CoCrFeMo0.85Ni. Materials 2021, 14, 1073. [Google Scholar] [CrossRef]
- Pilehrood, A.E.; Mashhuriazar, A.; Baghdadi, A.H.; Sajuri, Z.; Omidvar, H. Effect of Laser Metal Deposition Parameters on the Characteristics of Stellite 6 Deposited Layers on Precipitation-Hardened Stainless Steel. Materials 2021, 14, 5662. [Google Scholar] [CrossRef]
- Mora-García, A.G.; Ruiz-Luna, H.; Mosbacher, M.; Popp, R.; Schulz, U.; Glatzel, U.; Muñoz-Saldaña, J. Microstructural Analysis of Ta-Containing NiCoCrAlY Bond Coats Deposited by HVOF on Different Ni-Based Superalloys. Surf. Coat. Technol. 2018, 354, 214–225. [Google Scholar] [CrossRef]
- Rowe, A.P.; Bigelow, W.C.; Asgar, K. Effect of Tantalum Additions to a Cobalt-Chromium-Nickel Base Alloy. J Dent Res 1974, 53, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.-P.; Mao, W.; Xie, Y.-H.; Chen, B.; Guo, W.-L.; Li, X.-H.; Cheng, Y.-Y. Control of Interfacial Reactions and Strength of the SiC/SiC Joints Brazed with Newly-Developed Co-Based Brazing Alloy. J. Mater. Res. 2007, 22, 2727–2736. [Google Scholar] [CrossRef]
- Lin, H.; Sun, J.; Li, C.; He, H.; Qin, L.; Li, Q. A Facile Route to Synthesize WC–Co Nanocomposite Powders and Properties of Sintered Bulk. J. Alloy. Compd. 2016, 682, 531–536. [Google Scholar] [CrossRef]
- Guo, S.; Bao, R.; Yang, P.; Liu, L.; Yi, J. Morphology and Carbon Content of WC-6%Co Nanosized Composite Powders Prepared Using Glucose as Carbon Source. Trans. Nonferrous Met. Soc. China 2018, 28, 722–728. [Google Scholar] [CrossRef]
- Bartha, L.; Kotsis, I.; Laczko, L.; Harmat, P. High Temperature Reactions of WC-Co Nanopowders in Various Atmospheres. In Proceedings of the 15 th International Plansee Seminar 2001, Reutte, Austria, 25–29 May 2001; Plansee Holding AG: Reutte, Austria, 2001; pp. 97–105. [Google Scholar]
- Sharma, S.; Pandey, R.K.; Mishra, R.K.; Dixit, A. Improving Structure, Properties, and Abrasive Wear Resistance of the Thermally Sprayed Co-Based Coating by Means of La2O3 Addition. Powder Metall. Met. Ceram. 2016, 54, 672–678. [Google Scholar] [CrossRef]
- Zhang, P.; Li, M.; Yu, Z. Microstructures Evolution and Micromechanics Features of Ni-Cr-Si Coatings Deposited on Copper by Laser Cladding. Materials 2018, 11, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, E.; Amirjan, M.; Parvin, N. Preparation and Characterisation of Nickel-Based Brazing Powder: Cobalt and Chromium Addition Effects. Powder Metall. 2022, 1–13. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, L.; Qu, W.; Wang, J.; Chen, M.; Li, S.; Wang, F. Characteristics and Hot Corrosion Resistance of Co-Deposition Layers with Different Activators and Al-Cr Ratios. Corros. Sci. 2022, 202, 110320. [Google Scholar] [CrossRef]
- Liu, X.; Hu, K.; Zhang, S.; Xu, T.; Chen, L.; Byon, E.; Liu, D. Study of KCl-Induced Hot Corrosion Behavior of High Velocity Oxy-Fuel Sprayed NiCrAlY and NiCrBSi Coatings Deposited on 12CrMoV Boiler Steel at 700 °C. Corros. Sci. 2022, 203, 110351. [Google Scholar] [CrossRef]
- Esmaeili, N.; Ojo, O.A. Analysis of Brazing Effect on Hot Corrosion Behavior of a Nickel-Based Aerospace Superalloy. Metall. Mater. Trans. B 2018, 49, 912–918. [Google Scholar] [CrossRef]
- Fang, J.-H.; Ma, H.-W.; Xie, M.; Chen, Y.-T.; Yang, Y.-C.; Hu, J.-Q.; Wang, S. Effect of Joining Temperature and Bonding Time on Evolution of Interfacial Microstructure and Brazing Properties for 4J29/Ag–27Cu–4Ga/4J29 Brazed Joint. Vacuum 2019, 167, 459–470. [Google Scholar] [CrossRef]
- Prasanna, N.D.; Siddaraju, C.; Shetty, G.; Ramesh, M.R.; Reddy, M. Studies on the Role of HVOF Coatings to Combat Erosion in Turbine Alloys. Mater. Today Proc. 2018, 5, 3130–3136. [Google Scholar] [CrossRef]
- Sandhu, H.; Kumar, M. High-Termperature, Hardness and Wear Resistance of Cobalt-Based Tribaloy Alloys. Int. J. Eng. Sci. Comput. 2017, 17, 5411–5417. [Google Scholar]
- Ma, A.; Liu, D.; Zhang, X.; Liu, D.; He, G.; Yin, X. Solid Particle Erosion Behavior and Failure Mechanism of TiZrN Coatings for Ti-6Al-4V Alloy. Surf. Coat. Technol. 2021, 426, 127701. [Google Scholar] [CrossRef]

| Co wt. (%) | Cr wt. (%) | Ni wt. (%) | W wt. (%) | Ta wt. (%) | B wt. (%) | Ti wt. (%) | Zr wt. (%) | C wt. (%) |
|---|---|---|---|---|---|---|---|---|
| balance | 22.4–24.25 | 9.0–11.0 | 6.5–7.5 | 3.0–4.0 | 2.0–3.0 | 0.15–0.30 | 0.30–0.60 | 0.55–0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uțu, I.-D.; Hulka, I.; Kazamer, N.; Constantin, A.T.; Mărginean, G. Hot-Corrosion and Particle Erosion Resistance of Co-Based Brazed Alloy Coatings. Crystals 2022, 12, 762. https://doi.org/10.3390/cryst12060762
Uțu I-D, Hulka I, Kazamer N, Constantin AT, Mărginean G. Hot-Corrosion and Particle Erosion Resistance of Co-Based Brazed Alloy Coatings. Crystals. 2022; 12(6):762. https://doi.org/10.3390/cryst12060762
Chicago/Turabian StyleUțu, Ion-Dragoș, Iosif Hulka, Norbert Kazamer, Albert Titus Constantin, and Gabriela Mărginean. 2022. "Hot-Corrosion and Particle Erosion Resistance of Co-Based Brazed Alloy Coatings" Crystals 12, no. 6: 762. https://doi.org/10.3390/cryst12060762
APA StyleUțu, I.-D., Hulka, I., Kazamer, N., Constantin, A. T., & Mărginean, G. (2022). Hot-Corrosion and Particle Erosion Resistance of Co-Based Brazed Alloy Coatings. Crystals, 12(6), 762. https://doi.org/10.3390/cryst12060762










