Enhancement of Protein Crystallization Using Nano-Sized Metal–Organic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis, Characterization of HKUST-1
2.3. Preparation of Nano-Sized HKUST-1
2.4. Sample Preparation
2.5. Crystallization Experiment
3. Results
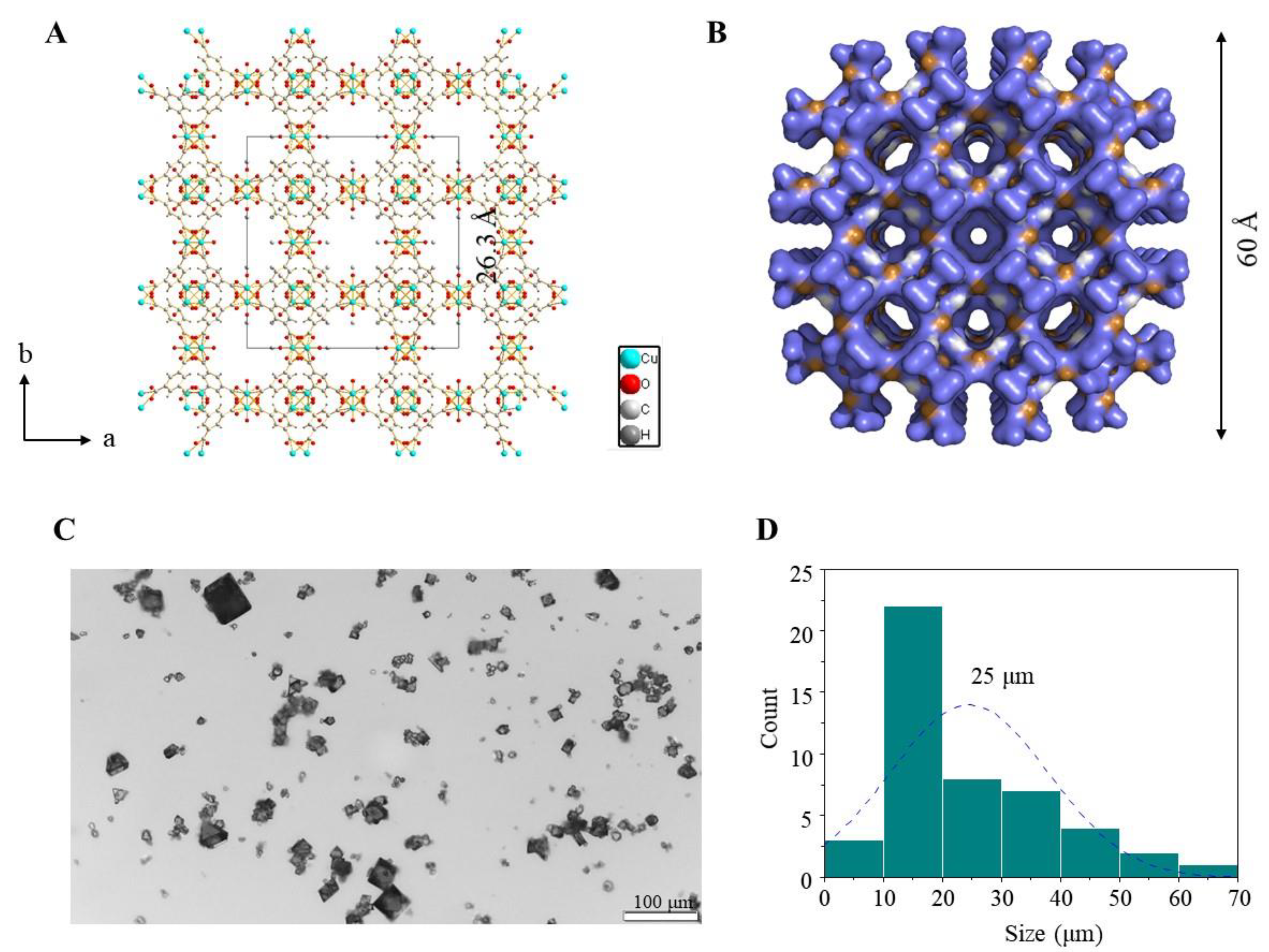
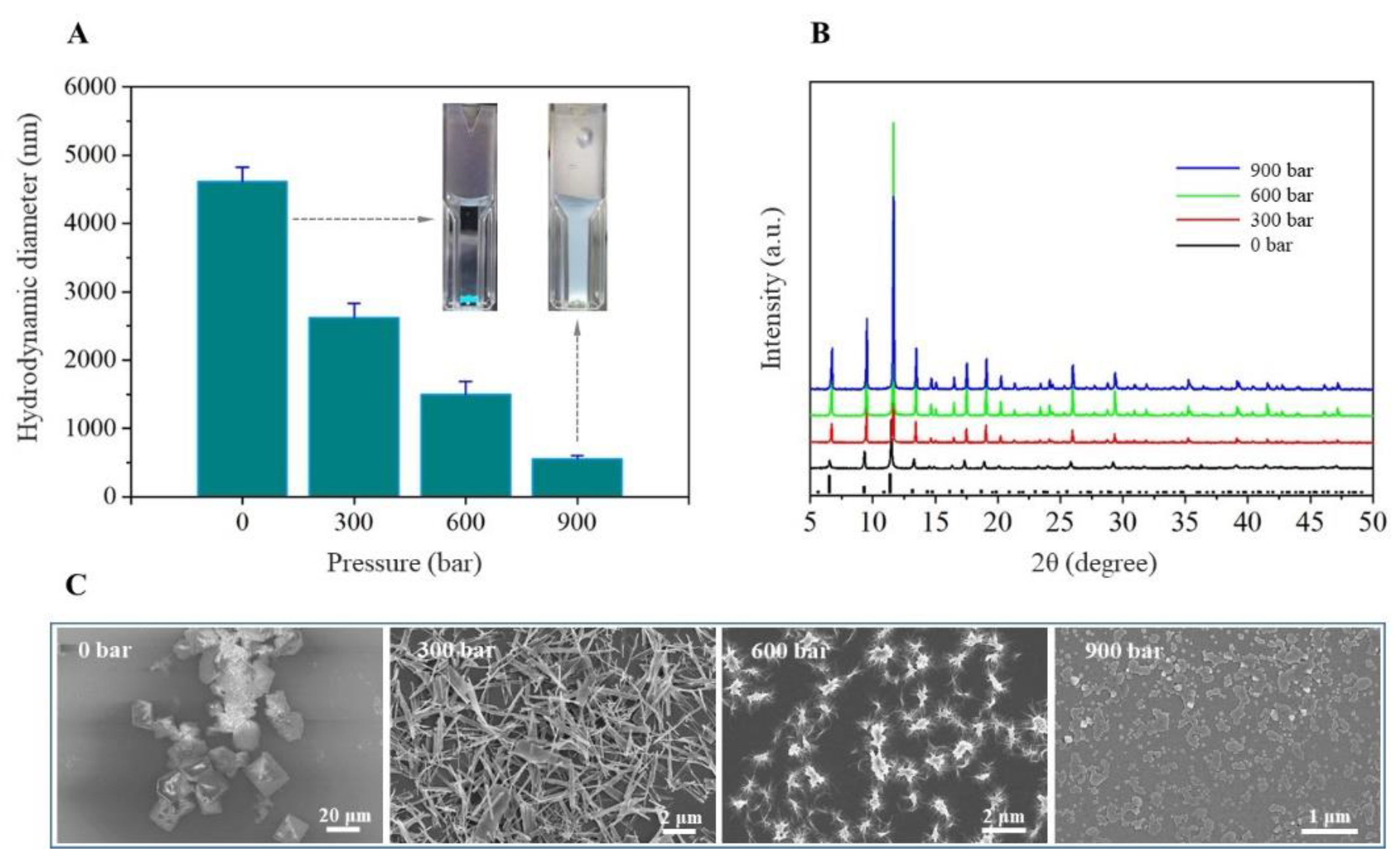
3.1. Preparation of Nano-Sized HKUST-1
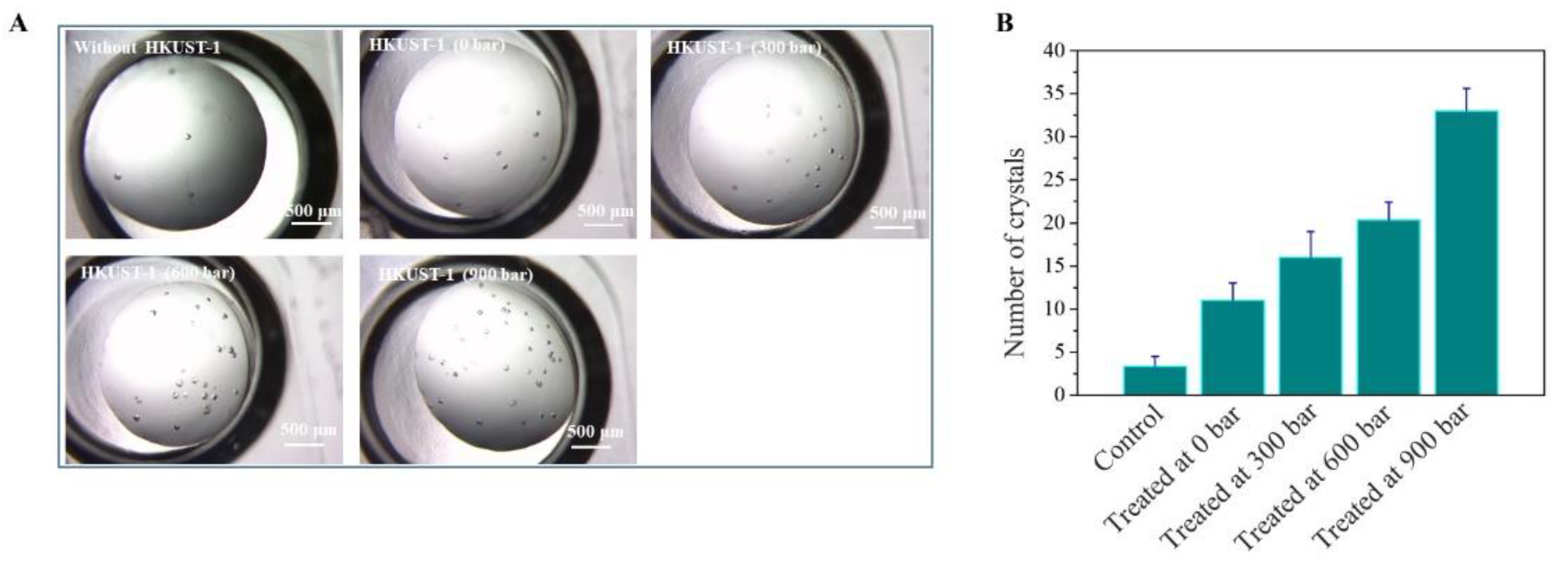
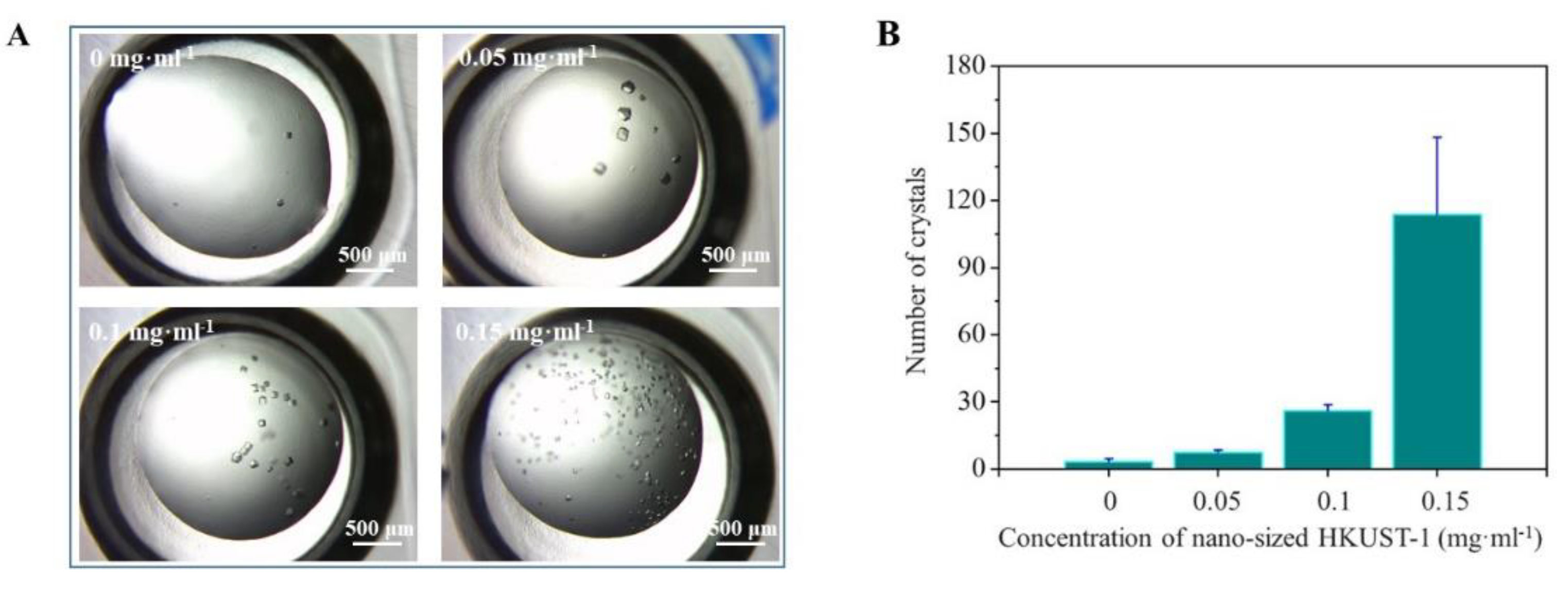
3.2. Crystallization Enhanced by Nano-Sized HKUST-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zundert, G.; Moriarty, N.W.; Sobolev, O.V.; Adams, P.D.; Borrelli, K.W. Macromolecular refinement of X-ray and cryoelectron microscopy structures with Phenix/OPLS3e for improved structure and ligand quality. Structure 2021, 29, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Gauto, D.F.; Estrozi, L.F.; Schwieters, C.D.; Effantin, G.; Macek, P.; Sounier, R.; Sivertsen, A.C.; Schmidt, E.; Kerfah, R.; Mas, G.; et al. Integrated NMR and cryo-EM atomic-resolution structure determination of a half-megadalton enzyme complex. Nat. Commun. 2019, 10, 2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Huang, L. Cross-Linking Mass Spectrometry: An Emerging Technology for Interactomics and Structural Biology. Anal. Chim. 2018, 90, 144–165. [Google Scholar] [CrossRef] [PubMed]
- Ancarik, J.; Kim, S.H. Sparse matrix sampling: A screening method for crystallization of proteins. J. Appl. Crystallogr. 1991, 24, 409–411. [Google Scholar] [CrossRef]
- Gorrec, F. The MORPHEUS protein crystallization screen. J. Appl. Crystallogr. 2009, 42, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Tarver, C.L.; Yuan, Q.; Pusey, M.L. Ionic Liquids as Protein Crystallization Additives. Crystals 2021, 11, 1166. [Google Scholar] [CrossRef]
- Santesson, S.; Cedergren-Zeppezauer, E.S.; Johansson, T.; Laurell, T.; Nilsson, J.; Nilsson, S. Screening of nucleation conditions using levitated drops for protein crystallization. Anal. Chim. 2003, 75, 1733–1740. [Google Scholar] [CrossRef]
- Vekilov, P.G. Nucleation of protein crystals. Prog. Cryst. Growth Charact. 2016, 62, 136–154. [Google Scholar] [CrossRef]
- Georgieva, D.G.; Kuil, M.E.; Oosterkamp, T.H.; Zandbergen, H.W.; Abrahams, J.P. Heterogeneous nucleation of three-dimensional protein nanocrystals. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 564–570. [Google Scholar] [CrossRef]
- Sleutel, M.; Van Driessche, A. Nucleation of protein crystals—A nanoscopic perspective. Nanoscale 2018, 10, 12256–12267. [Google Scholar] [CrossRef]
- Chayen, N.E.; Saridakis, E.; El-Bahar, R.; Nemirovsky, Y. Porous silicon: An effective nucleation-inducing material for protein crystallization. J. Mol. Biol. 2001, 312, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Saridakis, E.; Govada, L.; Chayen, N.E. Porous nucleating agents for protein crystallization. Nat. Protoc. 2014, 9, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E.; Saridakis, E.; Sear, R.P. Experiment and theory for heterogeneous nucleation of protein crystals in a porous medium. Proc. Natl. Acad. Sci. USA 2006, 103, 597–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanev, C.; Govada, L.; Chayen, N.E. Theoretical and experimental investigation of protein crystal nucleation in pores and crevices. IUCrJ 2021, 8, 270–280. [Google Scholar] [CrossRef]
- Chen, W.; Park, S.J.; Kong, F.; Li, X.; Heng, J.; Yang, H. High protein-loading silica template for heterogeneous protein crystallization. Cryst. Growth Des. 2019, 20, 866–873. [Google Scholar] [CrossRef]
- Stewart, P.S.; Kolek, S.A.; Briggs, R.A.; Chayen, N.E.; Baldock, P. Random microseeding: A theoretical and practical exploration of seed stability and seeding techniques for successful protein crystallization. Cryst. Growth Des. 2011, 11, 3432–3441. [Google Scholar] [CrossRef]
- Nanev, C.N.; Saridakis, E.; Chayen, N.E. Protein crystal nucleation in pores. Sci. Rep. 2017, 7, 35821. [Google Scholar] [CrossRef]
- Marchesan, S.; Prato, M. Under the lens: Carbon nanotube and protein interaction at the nanoscale. Chem. Commun. 2015, 51, 4347–4359. [Google Scholar] [CrossRef]
- Asanithi, P.; Saridakis, E.; Govada, L.; Jurewicz, I.; Brunner, E.W.; Ponnusamy, R.; Cleaver, J.A.; Dalton, A.B.; Chayen, N.E.; Sear, R.P. Carbon-nanotube-based materials for protein crystallization. ACS Appl. Mater. Interfaces 2009, 1, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Kertis, F.; Khurshid, S.; Okman, O.; Kysar, J.W.; Govada, L.; Chayen, N.; Erlebacher, J. Heterogeneous nucleation of protein crystals using nanoporous gold nucleants. J. Mater. Chem. 2012, 22, 21928–21934. [Google Scholar] [CrossRef]
- Leite, J.; Rodrigues, D.; Ferreira, S.; Figueira, F.; Paz, F.; Gales, L. Mesoporous metal–organic frameworks as effective nucleating agents in protein crystallography. Cryst. Growth Des. 2019, 19, 1610–1615. [Google Scholar] [CrossRef]
- Stolyarova, S.; Saridakis, E.; Chayen, N.E.; Nemirovsky, Y. A model for enhanced nucleation of protein crystals on a fractal porous substrate. Biophys. J. 2006, 91, 3857–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Morey, J.R.; McDevitt, C.A.; Kobe, B. Heterogeneous nucleation is required for crystallization of the ZnuA domain of pneumococcal AdcA. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 1459–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, M.; Asada, Y.; Morikawa, Y.; Kageyama, Y.; Kunishima, N. Nucleant-mediated protein crystallization with the application of microporous synthetic zeolites. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Kang, M.; Lee, G.; Jang, K.; Jeong, D.W.; Lee, J.; Kim, H.; Kim, Y.J. Graphene Quantum Dots as Nucleants for Protein Crystallization. Cryst. Growth Des. 2022, 22, 269–276. [Google Scholar] [CrossRef]
- Leese, H.S.; Govada, L.; Saridakis, E.; Khurshid, S.; Menzel, R.; Morishita, T.; Clancy, A.J.; White, E.R.; Chayen, N.E.; Shaffer, M. Reductively PEGylated carbon nanomaterials and their use to nucleate 3D protein crystals: A comparison of dimensionality. Chem. Sci. 2016, 7, 2916–2923. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.R.; Garcia, N.A.; Vitelli, V.; Lorenzana, J.; Vega, D.A. Phase nucleation in curved space. Nat. Commun. 2015, 6, 6856. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, Z.; Zhou, J.; Xing, X.; Li, L. Enhancement of Protein Crystallization Using Nano-Sized Metal–Organic Framework. Crystals 2022, 12, 578. https://doi.org/10.3390/cryst12050578
Zhang X, Xu Z, Zhou J, Xing X, Li L. Enhancement of Protein Crystallization Using Nano-Sized Metal–Organic Framework. Crystals. 2022; 12(5):578. https://doi.org/10.3390/cryst12050578
Chicago/Turabian StyleZhang, Xianfang, Zhengtao Xu, Jiahai Zhou, Xiwen Xing, and Long Li. 2022. "Enhancement of Protein Crystallization Using Nano-Sized Metal–Organic Framework" Crystals 12, no. 5: 578. https://doi.org/10.3390/cryst12050578
APA StyleZhang, X., Xu, Z., Zhou, J., Xing, X., & Li, L. (2022). Enhancement of Protein Crystallization Using Nano-Sized Metal–Organic Framework. Crystals, 12(5), 578. https://doi.org/10.3390/cryst12050578






