Abstract
In order to investigate the effect of heat treatment on the mechanical properties of the Al-7.0Si-0.3Mg alloy at 20–60 °C under different heat treatment processes, the tensile mechanical properties of Al-7.0Si-0.3Mg at low temperature after heat treatment were explored. The microstructure of Al-7.0Si-0.3Mg was observed by scanning electron microscopy (SEM) and transmission electron microscopy. The results show that the resistance of the dislocation movement in α-Al increased in the low-temperature condition, which is beneficial for the number of Si phase fractures that increase to enhance the tensile strength and weaken the elongation of the Al-7.0Si-0.3Mg alloy. After the solution treatment, the particle size of the Si phase reduced, while the morphology became rounded. In the early stage of aging, a GP region is generated inside the α-Al. With the prolonging of aging time, the acicular β″ phase is formed and then grows into rod-shaped β′. In the overaging stage, β′ eventually grows into flaky β. Due to the different linear expansion coefficients of the α-Al and the Mg2Si phase in the Al-7.0Si-0.3Mg alloy, the α-Al is squeezed by the Mg2Si phase under the effect of low-temperature shrinkage.
1. Introduction
With the rapid development of the high-speed railway and automotive industry, the application range of Al-7.0Si-0.3Mg has been increasing gradually every year. Since the winter environment in northern China exhibits a long-term low-temperature state, the basic requirements of the mechanical properties of Al-7.0Si-0.3Mg at low temperature need to be researched and improved. Hence, the extensive research conducted on the aluminum alloy’s low-temperature mechanical properties has generally only concentrated on the effect of the thermal activation registration of the fault movement, such as the yield strength, tensile strength and elongation [1,2,3]. As the hardening and the uniform deformation of the aluminum matrix during the Al–Li alloy tension process takes place, the elongation of the wrought aluminum alloy increases, which can be revealed through the use of the Arrhenius equation of the thermal activation process during plastic deformation [4,5,6]. For the Al–Si alloy tension, the tension stress concentrates approximately at the tip of the crack, which is driven to induce the formation and expansion of micro cracks in the Si phase [7]. The micro cracks formation region in the Si phase and the stress concentration are greatly influence by the large number of dislocation movements at the low-temperature condition. The Si phase fracture is mainly determined by the size, the length-to-width ratio and the distribution of the Si phase [8]. The number of eutectic Si phases is proportional to the shape variable at room temperature [9]. As the temperature decreases, the number of corresponding Si phase ruptures increases, which has also been found to happen to the hypoeutectic Al–Si alloy [10,11]. The low-temperature plasticity property of Al–Si alloys is enhanced by the refinement of the Si phase [12]. During the plastic deformation at low-temperature conditions, the trend deformation of the Al–Si alloy gradually becomes homogeneous, which is the mainly reason for the increase in the number of fracture Si phases [13]. When the stress increasingly concentrates on the Si particles, the Si phase rapidly fractures, which leads to a decrease in the plastic deformation of the aluminum matrix. Hence, the quasi-cleavage fracture is more likely to occur in the aluminum matrix. In this way, the elongation of Al-7.0Si-0.3Mg decreases at low-temperature conditions [2,14].
In this paper, the low-temperature mechanical properties of the Al-7.0Si-0.3Mg alloy under different heat treatment processes are investigated by tensile experiments at temperature conditions ranging from −60 to approximately 20 °C. The fracture behavior of the Al-7.0Si-0.3Mg alloy under heat treatment at a low temperature is systematically analyzed through the use of a microstructure analysis. Hence, the fracture mechanism of the Al-7.0Si-0.3Mg alloy at low-temperature conditions after heat treatment is revealed, which is beneficial for the expansion of the application range of the Al-7.0Si-0.3Mg alloy in low-temperature conditions.
2. Experimental Section
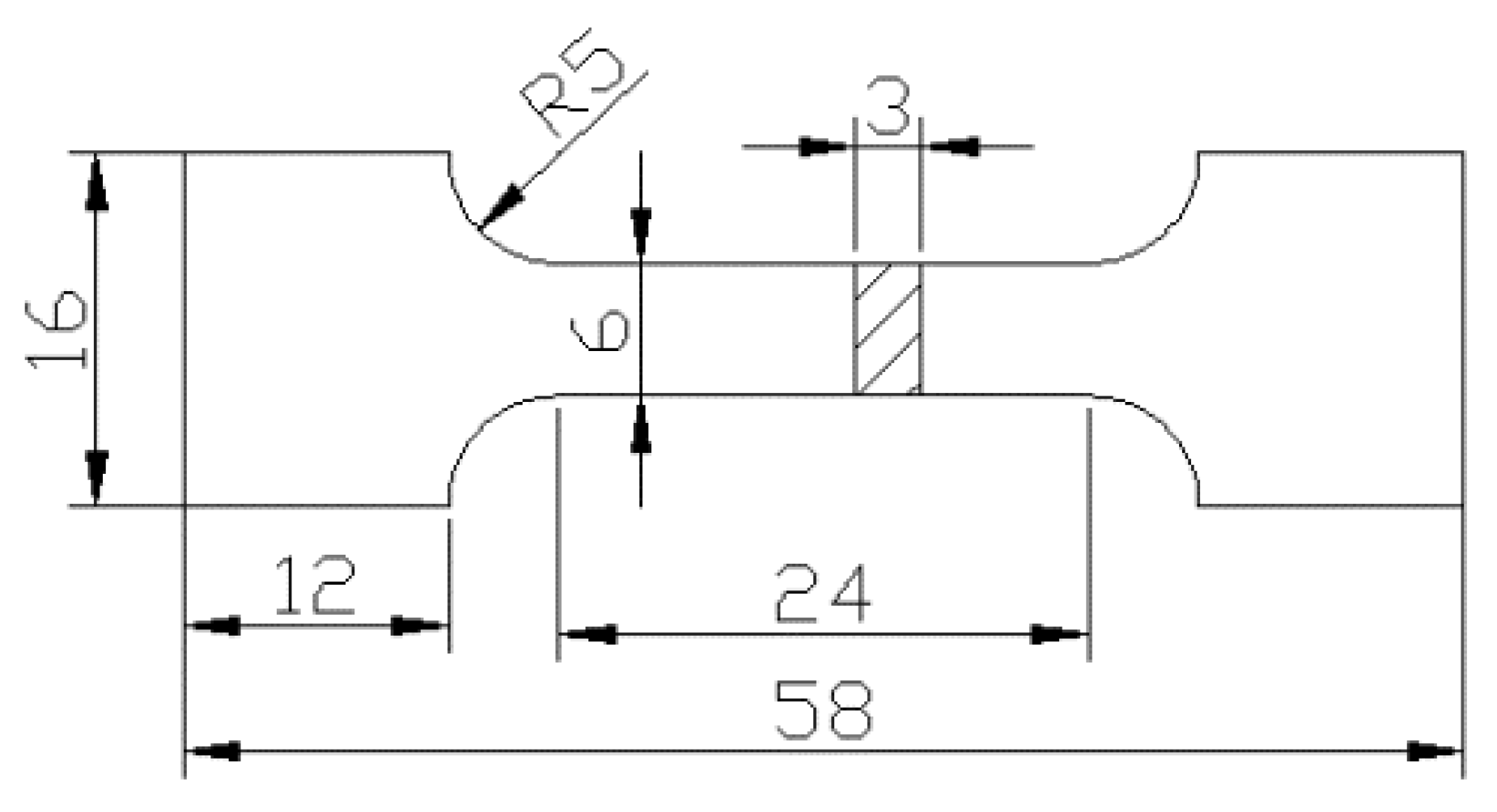
Al-7.0Si-0.3Mg alloy was used in this paper, of which the chemical composition is listed in Table 1. A resistance furnace was used for melting the Al-7.0Si-0.3Mg alloy at a temperature of 730 °C. The modification was conducted through the use of Sr. After refining with the use of the electromagnetic stirring method under argon atmosphere, the Al-7.0Si-0.3Mg alloy was cast into billet specimens. The heat treatment process was as follows: (1) Solution treatment for 0 h, 3 h, 6 h, 10 h, 12 h, 15 h at a temperature of 525 °C. (2) After 525 °C × 10 h solution treatment, aging treatment was conducted for 0 h, 1 h, 2 h, 4 h, 6 h, 8 h at a temperature of 165 °C. The experiment specimen was generated according to the 13239–2006 GB/T standard, which is shown in Figure 1. The temperature of experiment was approximately 20–60 °C. The tensile speed was 2 mm/min. The specimen was cooled in the cooling tank for 15 min. In order to reduce the error of results, 3 repeated experiments were necessary under same conditions. The microstructure of Al-7.0Si-0.3Mg was observed by TM3030 scanning electron microscope and OLYMPUS-GX51 optical microscope. To obtain the TEM (transmission electron microscope) images of tissue morphology and dislocation distribution, the sample was obtained from near the fracture surface through the use of 12 V Two Jet Thinning under −30 °C with corrosion liquid composition of 20% per chloric acid +80% ethanol.

Table 1.
Chemical composition of Al-7.0Si-0.3Mg alloy (mass fraction, %).
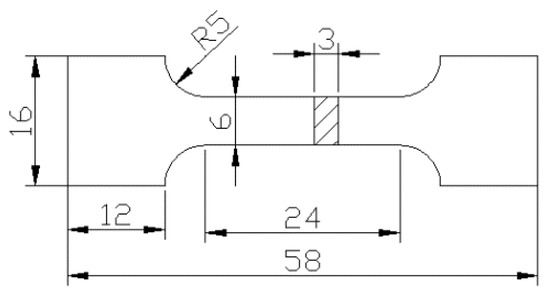
Figure 1.
Schematics of tensile specimens.
3. Results
3.1. Effect of Solution Treatment on Low-Temperature Mechanical Property
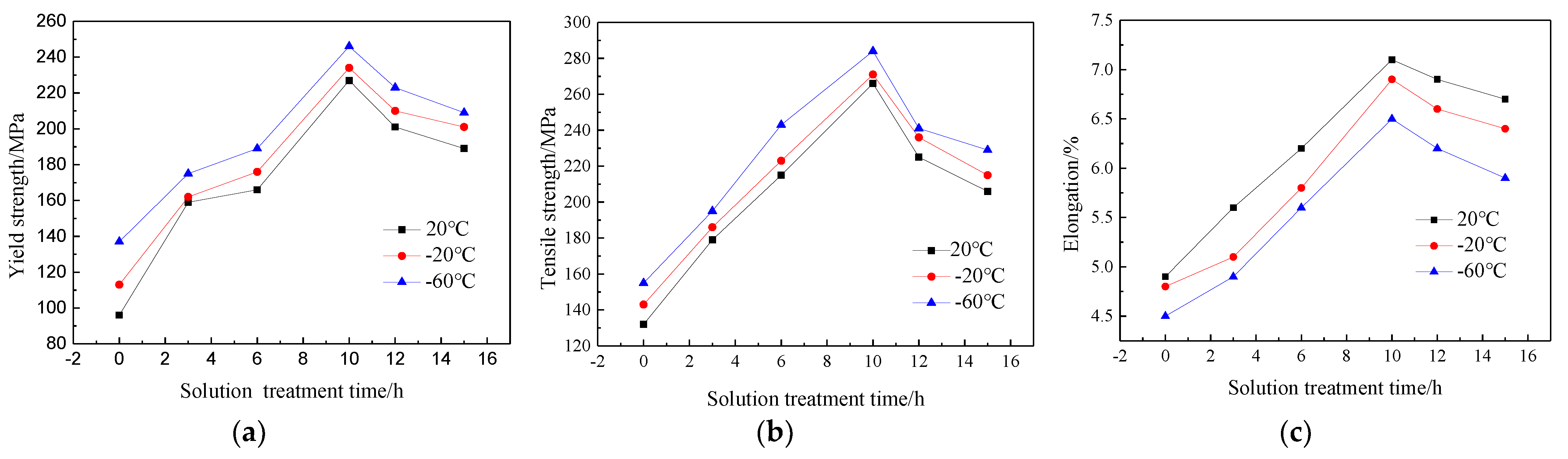
During the solution treatment process, the precipitates in the as-cast structure of the Al-7.0Si-0.3Mg alloy dissolved, so that the Mg element was fully dissolved in the α-Al, which was well prepared for the aging treatment [15,16]. At the same time, the Si phase morphology in the alloy matrix significantly changed due to the high-temperature diffusion, which had a significant impact on the mechanical properties of the Al-7.0Si-0.3Mg alloy [17,18]. The solution temperature was determined at 525 °C. The solution time was set as 0h, 3 h, 6 h, 10 h, 12 h, and 15 h, respectively. The tensile experiments and fracture analysis were conducted at 20 °C, −20 °C, and −60 °C, respectively, which are shown in Figure 2. It can be seen that the tensile strength, yield strength, and elongation of the Al-7.0Si-0.3Mg alloy all showed an upward trend when the solution time was 0~10 h, reaching a peak at 10 h and then decreasing significantly. With the decrease in the experimental temperature, the slopes of the tensile strength and elongation of the Al-7.0Si-0.3Mg alloy increased from 0 h to 6 h. The increasing trend of tensile strength and elongation became flat at 6~10 h. After reaching the peak value at 10 h, the decreasing trend of the mechanical properties of Al-7.0Si-0.3Mg became more obvious in the interval of 10~15 h. The yield strength of the Al-7.0Si-0.3Mg alloy did not change much at different experimental temperatures.
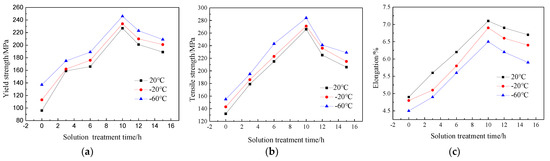
Figure 2.
Low-temperature tensile properties of Al-7.0Si-0.3Mg alloy after different solution treatments: (a) yield strength, (b) tensile strength, and (c) elongation.
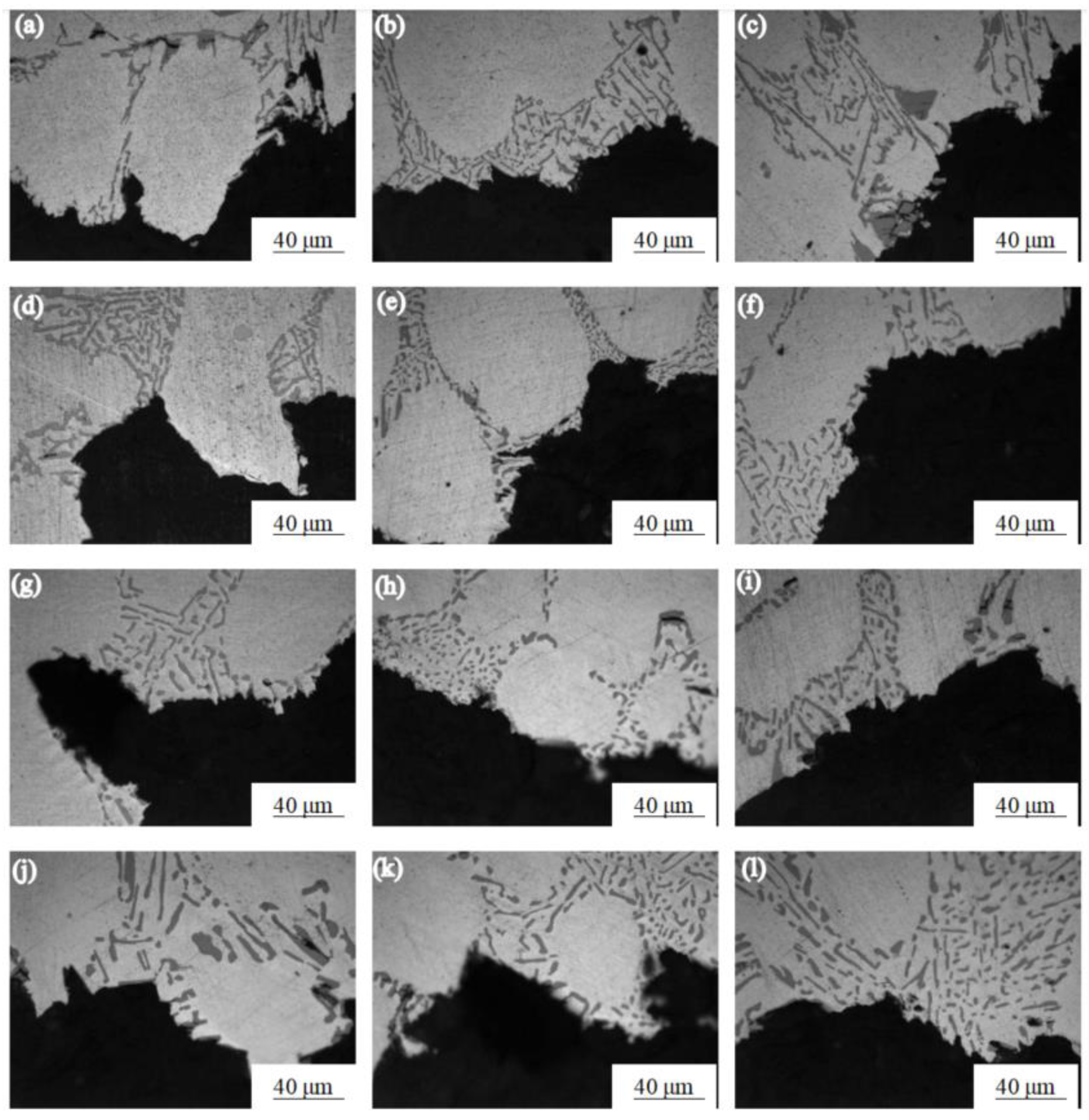
Figure 3 shows the microstructure near the tensile fracture of the Al-7.0Si-0.3Mg alloy treated with different solution times at different temperatures. It can be seen from Figure 3a–c that the light-gray rod-like morphology was the Si phase. The size and aspect ratio of the Si phase in the as-cast Al-7.0Si-0.3Mg alloy structure were large, and the fracture extended along the eutectic structure, causing the coarse Si phase to occur. By comparison with Figure 3d–f, the size and aspect ratio of the Si phase in the Al-7.0Si-0.3Mg alloy in the solution treatment for 6 h were obviously reduced, which led to the reduction in the cleavage area of the Si phase fracture. By comparison with Figure 3g–i, the Si phase in the α-Al reached the minimum size after 10 h of the solution treatment. The roundness of the Si phase particles was greatly improved. By comparison with Figure 3j–l, it can be seen that the size and aspect ratio of Si phase particles in the α-Al showed an increasing trend after the solution treatment for 15 h. Consequently, the particle morphology and size of the Si phase had a great influence on the tensile properties of the Al-7.0Si-0.3Mg alloy, while the effect of the Si phase morphology and size on the tensile properties of the Al-7.0Si-0.3Mg alloy intensified with the decrease in temperature.
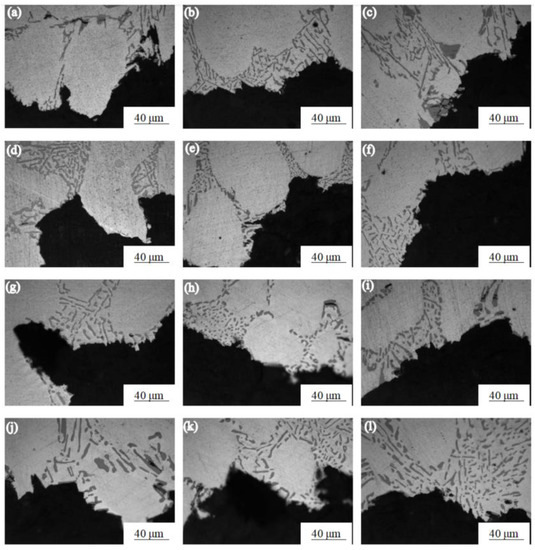
Figure 3.
Fracture micro-structures of Al-7.0Si-0.3Mg alloy after different solution treatments: (a) 0 h, 20 °C; (b) 0 h, −20 °C; (c) 0 h, −60 °C; (d) 6 h, 20 °C; (e) 6 h, −20 °C; (f) 6 h, −60 °C; (g) 10 h, 20 °C; (h) 10 h, −20 °C; (i) 10 h, −60 °C; (j) 15 h, 20 °C; (k) 15 h, −20 °C; (l) 15 h, −60 °C.
3.2. Effects of Aging Treatment on Low-Temperature Mechanical Property
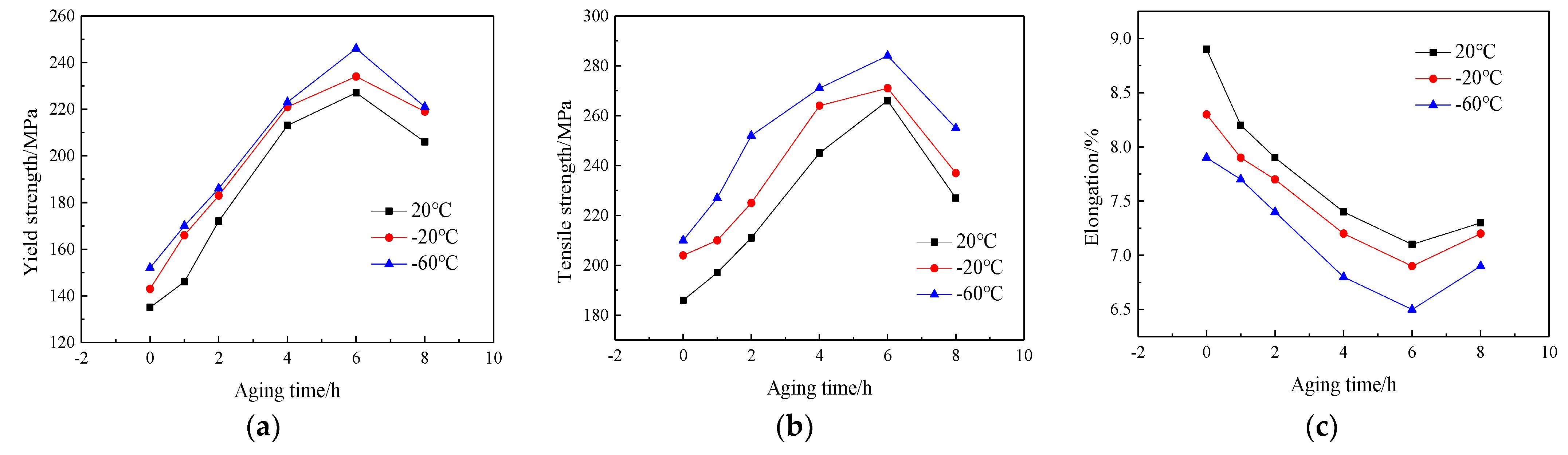
Figure 4 shows the curves of the mechanical properties of the Al-7.0Si-0.3Mg alloy after the aging treatment for different times at 20 °C, −20 °C, and −60 °C. The tensile strength and yield strength were obviously improved, while the elongation of the alloy showed a decreasing trend. As the aging time increased, the strength of the Al-7.0Si-0.3Mg alloy gradually increased, while the elongation gradually decreased. When the aging time was 6 h, the mechanical properties after the aging treatment of the Al-7.0Si-0.3Mg alloy, such as the tensile strength and yield strength, reached the peak value. However, the elongation reached the valley’s bottom. With the prolongation of aging time, the strength of the Al-7.0Si-0.3Mg alloy tended to decrease, while the elongation increased. The mechanical properties of the Al-7.0Si-0.3Mg alloy in the low-temperature environment showed the same trend as in the normal temperature environment. However, the tensile strength and yield strength increased significantly with the decrease in temperature, while the elongation decreased. Consequently, during the aging process, the strength and elongation exhibited opposite trends, but were the same effect as the temperature.
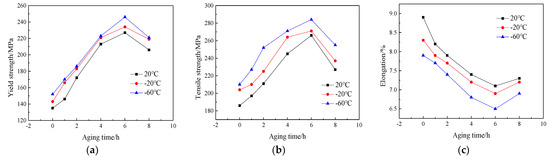
Figure 4.
Low-temperature tensile properties of Al-7.0Si-0.3Mg alloy after different aging processes: (a) yield strength, (b) tensile strength, and (c) elongation.
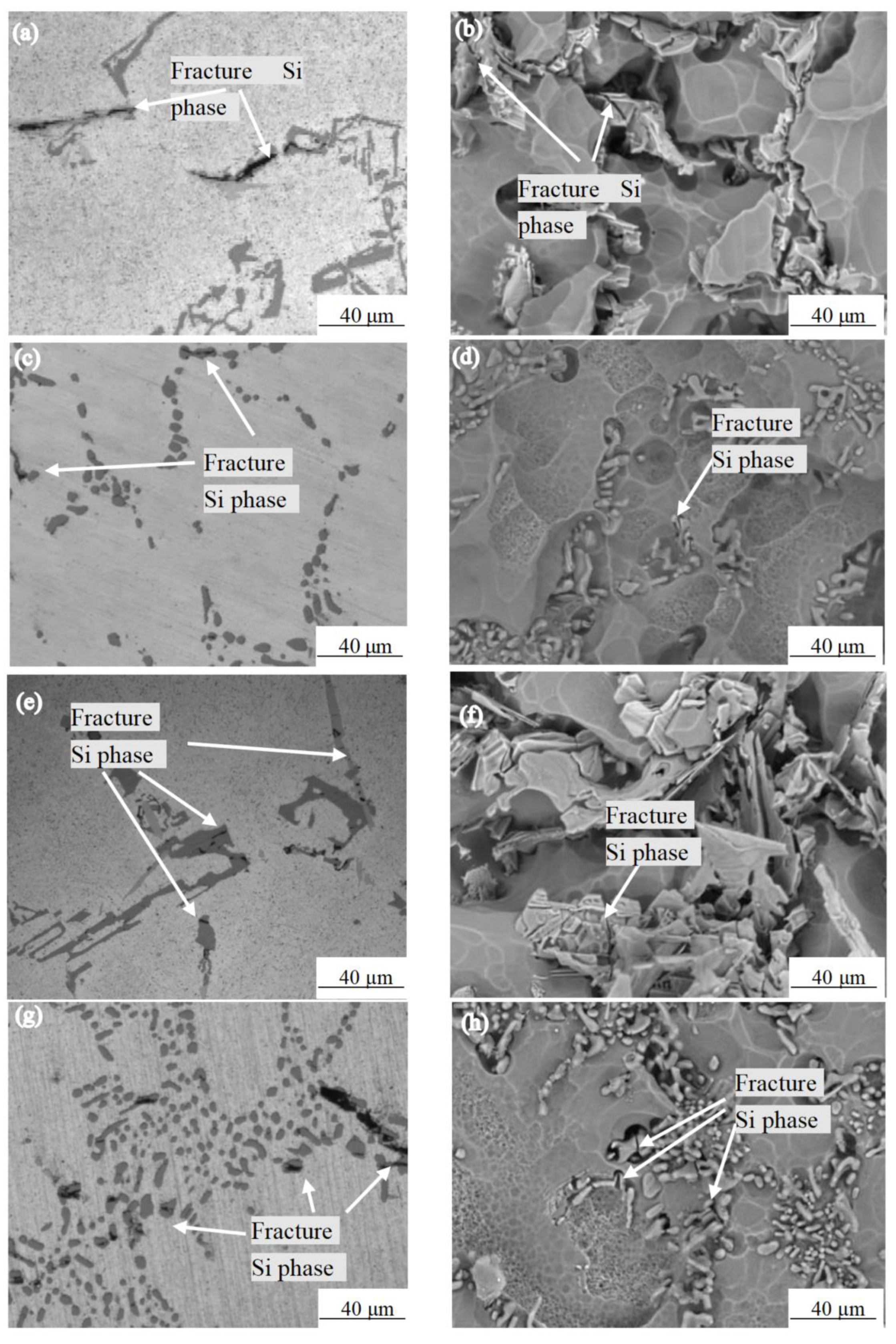
Mg is the most important alloying element in the Al-7.0Si-0.3Mg alloy. Mg and Si come from Mg2Si, which has a solubility of 1.85% in an α-Al solution at 595 °C [19]. Therefore, in Mg-containing Al–Si alloys, even the Mg2Si phase formed due to non-equilibrium solidification during the casting process dissolves into an α-Al solution at the appropriate temperature during the solid solution process. During the aging heat treatment, a large number of fine mass Mg2Si phases precipitated, which cause the alloy to significantly strengthen. Figure 5 shows the Si phase morphology near the tensile fracture of the as-cast Al-7.0Si-0.3Mg alloy and after 10 h of the solution treatment at different experimental temperatures. By comparison with Figure 5a–d, it can be seen that the number of fractured Si phases near the fracture morphology of the as-cast Al-7.0Si-0.3Mg alloy and after 10h of the solution treatment at 20 °C was relatively small. By comparison with Figure 5e–h, it can be seen that a large number of fractured Si phases appeared near the fracture surface of the as-cast Al-7.0Si-0.3Mg alloy and after 10 h of the solution treatment at −60 °C. By comparison with Figure 5a,b,e,f, the Si phase in the as-cast alloy structure was staggered and distributed in α-Al in the form of flakes or blocks. Near the grain boundary of the dendrite, a large number of dislocations were piled up in the α-Al dendrite during the stretching process, resulting in stress concentrating in the Si phase. Hence, the higher the stress, the more prone to the formation of fractures the large-area cleavage plane. By comparison with Figure 5c,d,g,h, after 10 h of the solution treatment, the structure of the Si phase was granularly distributed in the α-Al branch around the crystal. As the size of the Si phase reduced and it became more rounded, the dislocation stress received during the plastic deformation of the alloy was reduced. Therefore, it was not easy for the fracture to occur, which was an improvement of the tensile strength of the Al-7.0Si-0.3Mg alloy. Only small cracks were generated during the fracture. Hence, the fracture was dominated by dimples with small cleavage planes at the bottom, while the elongation of the Al-7.0Si-0.3Mg alloy was improved.
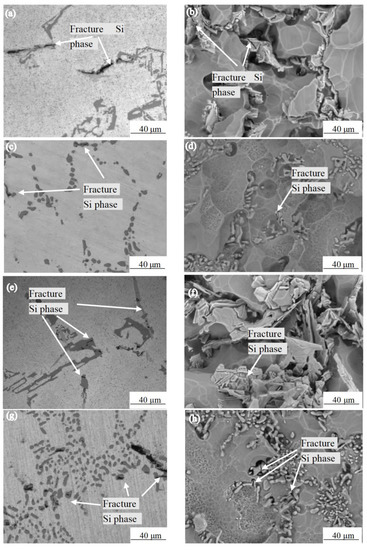
Figure 5.
Morphologies of Si particles after 0 h and 10 h solution treatment: (a) as-cast microstructure, 20 °C; (b) as-cast Si phase morphology,20 °C; (c) 10 h solution treatment microstructure, 20 °C; (d) 10 h solution treatment Si phase morphology, 20 °C; (e) as-cast microstructure, −60 °C; (f) as-cast Si phase morphology, −60 °C; (g) 10 h solution treatment microstructure, −60 °C; (h) 10 h solution treatment Si phase morphology, −60 °C.
4. Discussion
4.1. Effect of Heat Treatment on Fracture Behavior
The change to the Si phase during the solution treatment of the Al-7.0Si-0.3Mg alloy was divided into three stages: the initial stage of the solution treatment (1~6 h), the middle stage of the solution treatment (6~12 h), and the final stage of the solution treatment (after 12 h) [20,21]. At the initial stage of the solution treatment, due to the large diffusion coefficient of Si atoms at 525 °C and the large concentration gradient between the eutectic Si phase and α-Al, Si atoms in the alloy had a faster diffusion rate. As there was a “bottleneck” at the bifurcation of the Si phase and the specific parts, the crystal defects caused by the atomic dislocation in these parts led to an uneven energy state, necking, fuse and accompanying growth occurring firstly. In the middle stage of the solution treatment, the concentration of the Si phase in α-Al essentially reached saturation, while the dissolution and diffusion of the Si phase tended to balance. Under the combined effect of thermal and curvature effects, surface energy and the center of the stage with regularization and granulation of growth properties were reduced. In the final stage of the solution treatment, the decrease in the Al-7.0Si-0.3Mg alloy’s strength and hardness was related to the coarsening of Si phase particles and the deterioration of morphology. The growth of the Si phase was a process controlled by diffusion. Si atoms were transferred to the particles through a matrix diffusion and attached to the surface of the particles with a certain selectivity and preference, which was arranged in a certain orientation. Therefore, the angular facet feature was presented by the Si phase particles [22]. When the Al-7.0Si-0.3Mg alloy underwent plastic deformation, the dislocation slip in the α-Al was hindered by the Si phase particles, which were accumulated on the edge of the Si phase to generate large stress. When the stress increased to the critical value, the fracture of the Si phase particles occurred. According to the Griffith formula, the fracture critical stress of the Si phase with surface defects could be expressed as follow [23]:
where is the specific surface energy of the Si phase; is the Young’s modulus of the Si phase; is the defect length on the Si phase particles. It can be seen from Equation (1) that the fracture of the Si phase required not only a large number of dislocation accumulation, but also the existence of defects with sufficient lengths. Therefore, the fracture behavior of the Si phase conformed to the Weibull distribution.
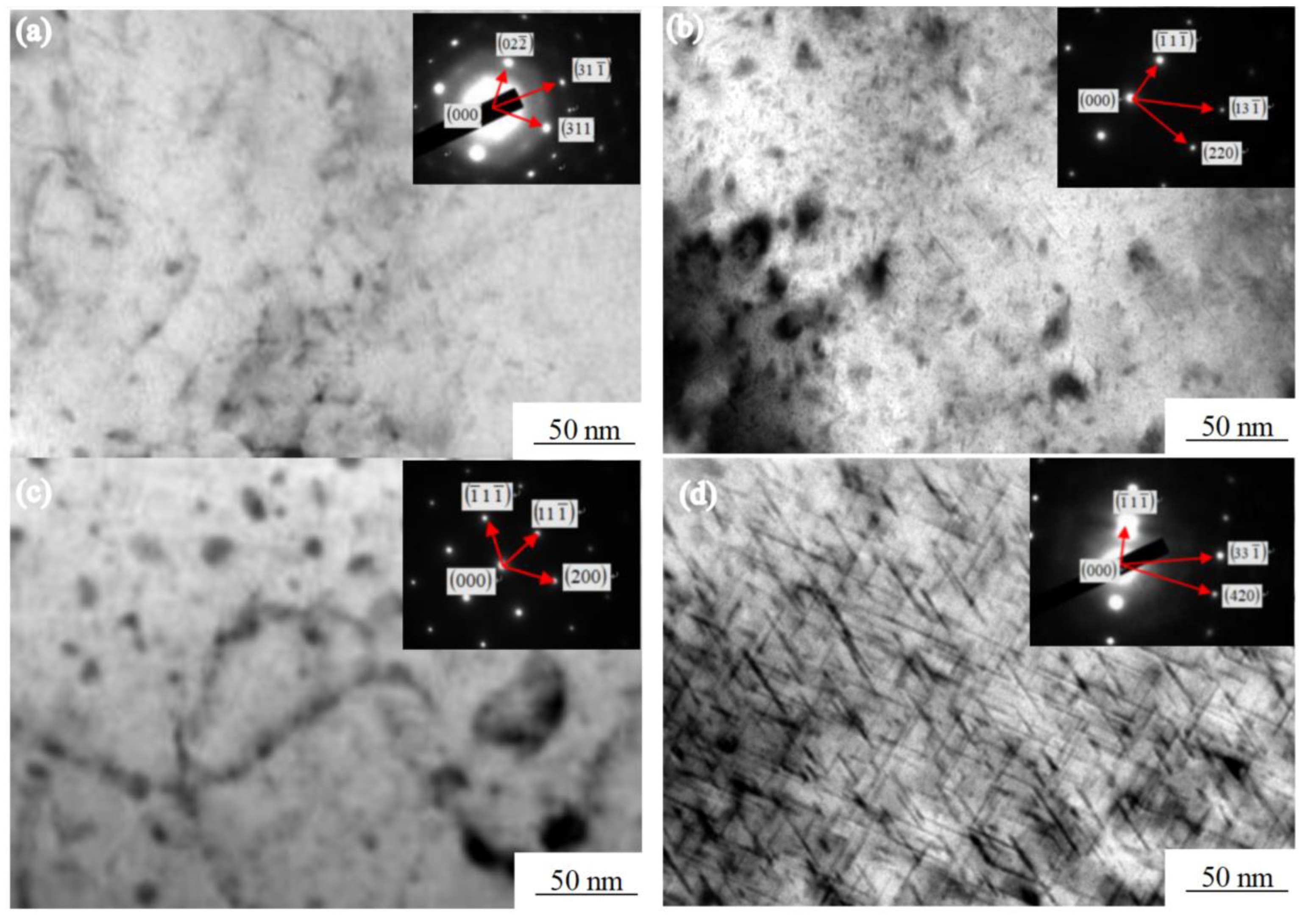
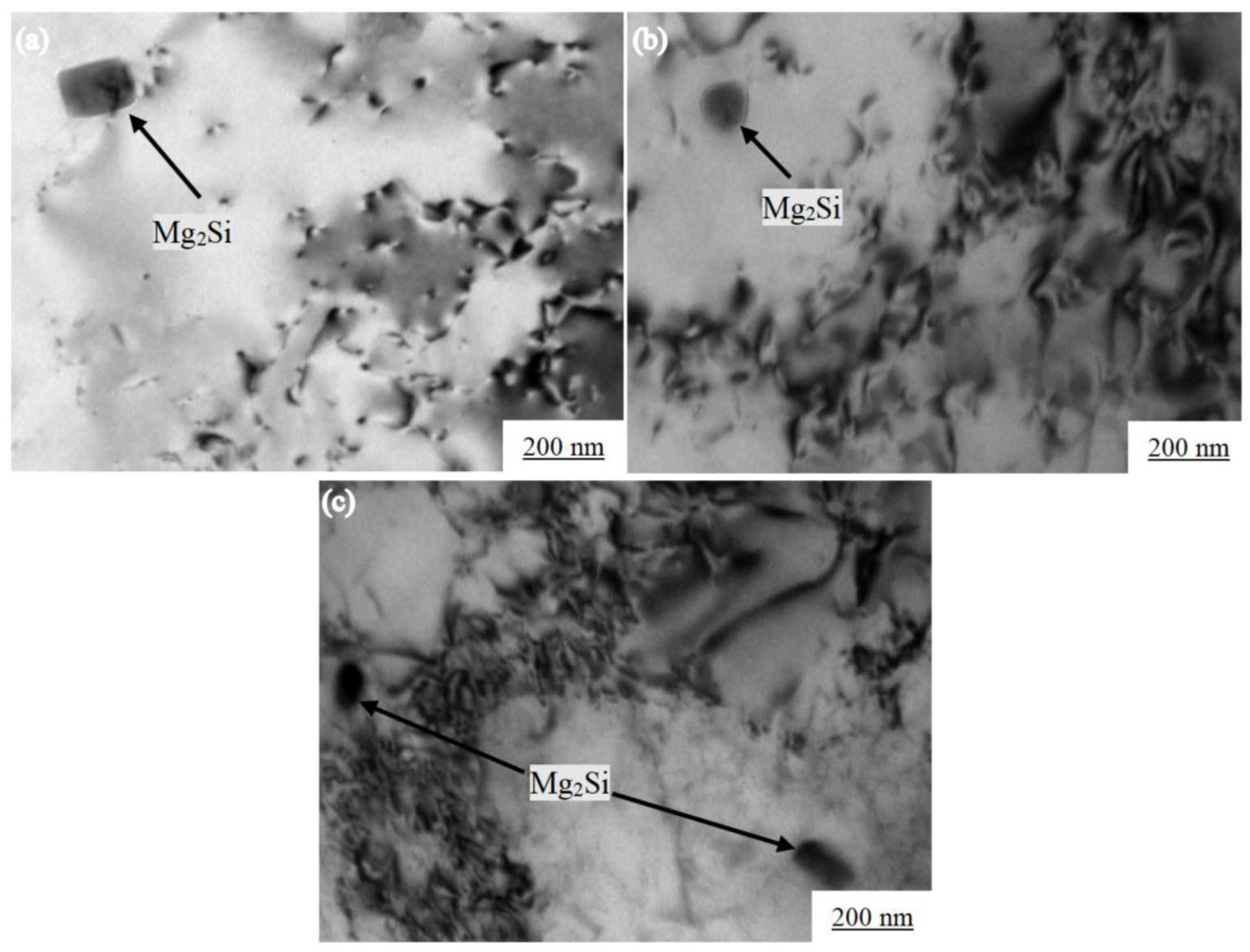
Figure 6 shows the various of the Mg2Si morphology of the Al-7.0Si-0.3Mg alloy during the aging treatment. The Mg2Si phase underwent the following transformation process during precipitation [24]: A spherical GP region was formed at the beginning of the aging precipitation, as shown in Figure 6a. A fine and diffuse point-like structure was observed in the α-Al dendrites in the early stage of aging. With the prolongation of aging time, the GP region grew along the [100] direction of α-Al and became a needle β″, as shown in Figure 6b. After 4h of aging, the internal point structure of α-Al dendrites gradually disappeared and grew into a large number of fine needle phases. The β″ and α-Al were in a coherent relationship. The orientation relationship between β″ and α-Al was: (111)β″‖(110)α;[110]β″‖[001]α. When the aging time reached 6 h, the needle β″ grew into a rod β′. The strength of the Al-7.0Si-0.3Mg alloy reached the maximum at this time, which was the peak aging, as shown in Figure 6c. β′ was in a semi-coherent relationship with α-Al. The orientation relationship between β′ and α-Al was: (001)β′‖(100)α;[100]β′‖[011]α. In the overaging stage, β′ finally grew into sheet β, as shown in Figure 6d. β did not have a coherent relationship with α-Al, while nucleates at the β′/matrix interface grew by consuming a large amount of β′ phases, which reduced the strength of the alloy. The orientation relationship between β and α-Al was: (100)β‖(100)α;[110]β‖[001]α.
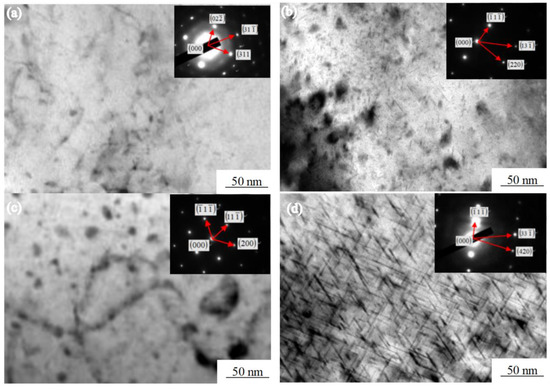
Figure 6.
Morphologies of Mg2Si phase in Al-7.0Si-0.3Mg in aging processes: (a) 1 h, (b) 4 h, (c) 6 h, and (d) 8 h.
4.2. Effect of Diffusion Enhancement on Dislocation Motion
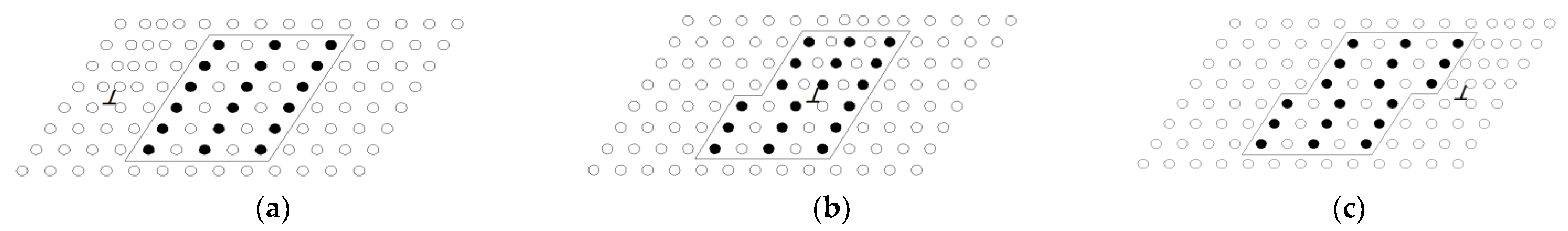
Age strengthening happened due to the formation of precipitates in the supersaturated solution treatment, hindering the dislocation motion. The strengthening effect mainly came from the hindering effect of the precipitates in α-Al on the dislocation movement of the surrounding elastic strain field. The precipitates were sheared by dislocations and the dislocations encircled the precipitate particles. The precipitated GP region in the supersaturated solution treatment, β″ phase, and β′ phase had sufficient mismatches with α-Al. Therefore, elastic deformation occurred between the matrix and the particles [25]. The existing stress field between the α-Al and particles interacted with the approaching dislocation stress field. Hence, an increasing force was necessary for the dislocation to cross the stress field. If the dislocation cut through the grain, the surface energy increased to the value, where the dislocation entered and left the particles due to the offset, as shown in Figure 7. Similarly, if the ordering of the precipitated phase was ordered, the energy was also required for the dislocations to move atoms into position where the atoms repelled each other. As a new particle–matrix interface was created in the passage of the dislocations, the atomic matching crossed into the slip plane in the particle changes. With the precipitation continuing, the particles and the stress of dislocations through the particles were enhanced due to the addition of the new matrix–particle interfaces and the number of mismatched atoms.
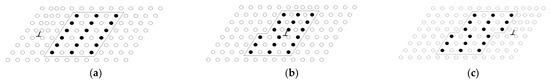
Figure 7.
Schematic summary of dislocation cutting through precipitate: (a) initial stage of dislocation cut, (b) middle stage of dislocation cut, and (c) final stage of dislocation cut.
When the alloy mainly existed in the GP region and β″ phase, the dislocations would cut through them. If the influence of the size and volume fraction of these phases were to be considered, their contribution to the yield strength of the alloy could be expressed as follow [26]:
where cb, m, and n are all constants. For the interaction of most dislocation solute atoms, m and n were set as 0.5. f is the volume fraction of the precipitated phase. r is the average radius of the precipitated phase. The f and r could be calculated by the aging time and temperature [27]:
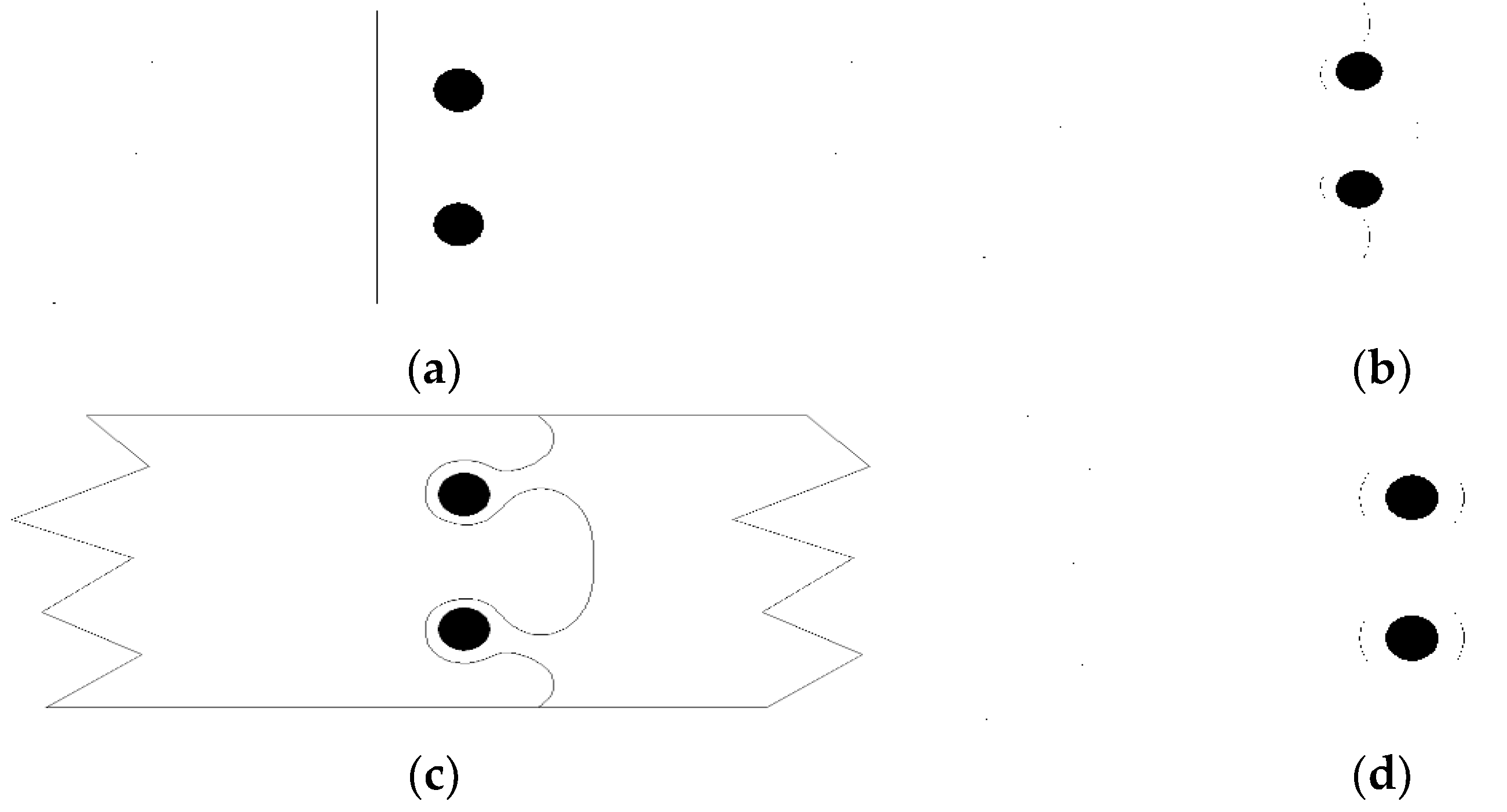
where f0 is the volume fraction of the precipitated phase at equilibrium. ci is the initial solute concentration. QS is the free energy of the solid solution. QA is the activation energy of atomic diffusion between the precipitated phases. Cm is a constant related to the matrix composition. In the final stage of aging, β′ and β phases were formed in the α-Al, which became hard particles in the soft matrix, hindering the dislocation movement [28,29]. If the stress was high enough, the dislocation would cut through or bypass the particles, and the dislocation would move from the slip plane to climb around the particles. However, the most likely process was the dislocations bowing around the particles, as shown in Figure 8. This was because the adjacent particles played a similar role to the Frank–Read source. The dislocation center experienced an elastic strain. The dislocation bowing increased the length of the strain center, which also increased the energy of the crystal. For dislocations to pass through the barrier, the stress had to increase to the strengthen of the Al-7.0Si-0.3Mg alloy. Their contribution to the yield strength Δσb was inversely proportional to the inter-particle distance r [30]:
Figure 8.
Movement of dislocation bowing through particles: (a) initial stage, (b) stage II, (c) stage III, and (d) final stage.
The closer the grains were, the more difficult it was for dislocations to bow out. Hence, the degree of strengthening was inversely proportional to the grain distance. When the particles started to coarsen, the average distance between the particles became larger, which was beneficial for reducing the strength of the Al-7.0Si-0.3Mg alloy. In addition, when the precipitation strengthening of the alloy occurred, the weakening of the solution treatment strengthening was caused by the reduction in the supersaturated concentration. Although this effect was smaller than the precipitation hardening, it had a definite influence on the hardness after long-term aging.
Figure 9 shows the dislocation morphologies near the tensile fracture of the heat-treated Al-7.0Si-0.3Mg alloy at different temperatures. The α-Al sustained a large plastic deformation during the room temperature tension process. A large number of dislocations in the α-Al near the short rod-shaped β′ phase were generated, as shown in Figure 9a. Due to the different thermal expansion coefficients of α-Al and β’ phases, the thermal motion activity of atoms decreased at −20 °C. The range of the elastic stress field strength near the β’ phase increased. Therefore, during the plastic deformation process of the β’ phase, the number of dislocations entangled nearby significantly increased, as shown in Figure 9b. At −60 °C, the elastic stress field near the β’ phase was further enhanced. Meanwhile, the number of entangled dislocations near the β’ phase reached the maximum. The bending degree of dislocations was the most obvious, as shown in Figure 9c. The Mg2Si phase had a cubic anti-fluorite (CaF2) structure, and Si occupied the octahedral sites to form an fcc arrangement, while Mg occupied the tetrahedral sites. With a high-strength and low-thermal expansion coefficient, the coefficient of linear expansion was 7.5 × 10−6 K−1, and the modulus of elasticity was 120 GPa [31]. However, the coefficient of the thermal expansion of Al was larger. The linear expansion coefficient of Al was 20.1 × 10−6/°C. The elasticity modulus of Al was 72 GPa at 200 K [32]. Hence, there was a sufficient degree of misfit between the Mg2Si phase and α-Al, resulting in a certain elastic strain. In the low-temperature environment, due to the difference in the thermal expansion coefficients of the α-Al and Mg2Si phase, the elastic strain further increased, which further increased the hindering effect of the stress field on dislocations. In order to simplify the analysis, the Mg2Si phase was regarded as a sphere with a radius r of 100 nm, of which the linear expansion coefficient and elastic modulus were approximately constant. When the ambient temperature dropped from 20 °C to −60 °C, then the radius reduction in the Mg2Si phase could be expressed as:
where ΔT is the amount of temperature change. On the other hand, the decreasing trend of the boundary radius of the Mg2Si phase was caused by the cooling shrinkage of the α-Al, expressed as follows:
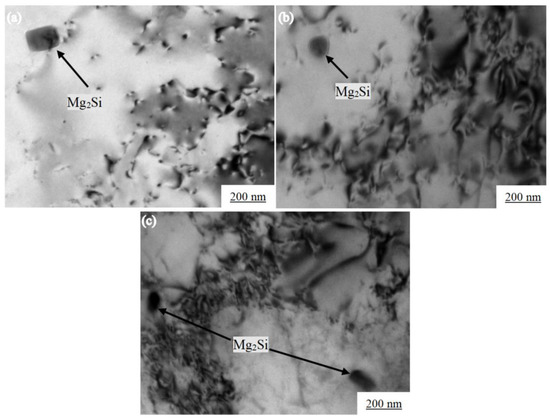
Figure 9.
Morphologies of dislocations in Al-7.0Si-0.3Mg alloy after heat treatment near tensile fracture at different temperatures: (a) 20 °C, (b) −20 °C, and (c) −60 °C.
As the Mg2Si phase had greater strength than the α-Al [2], the Mg2Si phase was squeezed by the α-Al to produce an elastic deformation at −60 °C. The radial deformation could be expressed as follows:
From Equations (7) to (9), we could draw the conclusion that the amount of elastic strain caused by cold shrinkage was proportional to the amount of temperature change.
5. Conclusions
In this paper, low-temperature tensile experiments were carried out for the Al-7.0Si-0.3Mg alloy with different solution treatments and aging treatments. The effects of microstructure changes on the mechanical properties of the Al-7.0Si-0.3Mg alloy under different heat treatment processes were studied, and the following main conclusions were obtained:
- (1)
- The resistance of the dislocation movement in α-Al increased in the low-temperature conditions, which was beneficial to the increase in the number of Si phase fractures, which enhanced the tensile strength and weakened the elongation of the Al-7.0Si-0.3Mg alloy. After the solution treatment, the particle size of the Si phase was reduced, while the morphology was rounded. The strength and elongation of the alloy significantly increased. In the final stage of the solution treatment, the strength and elongation of the alloy decreased due to the coarsening of the Si phase and the slight recovery of the aspect ratio.
- (2)
- In the early stage of aging, a GP region was generated inside the α-Al. When the dislocations were cut, the surface energy of the GP region increased. With the prolonging of aging time, the acicular β″ phase was formed, which produced a stress field that hindered the movement of dislocations. When aging for 10 h, β″ in the matrix grew into rod-shaped β′. The strength of the Al-7.0Si-0.3Mg alloy reached the maximum. In the overaging stage, β′ eventually grew into flaky β, which was induced to reduce the strength of the alloy.
- (3)
- Due to the different linear expansion coefficients of the α-Al and the Mg2Si phase in the Al-7.0Si-0.3Mg alloy, the α-Al was squeezed by the Mg2Si phase under the effect of low-temperature shrinkage. An elastic stress field was formed around the Mg2Si phase, which hindered the dislocation movement.
Author Contributions
Conceptualization, G.M.; methodology, G.M.; software, Z.G.; validation, Z.G., Y.D. and H.L.; formal analysis, Y.J.; investigation, Y.J.; resources, Y.D.; data curation, H.L.; writing—original draft preparation, G.M.; writing—review and editing, M.Y.; visualization, G.M.; supervision, Z.G.; project administration, Y.D.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
Natural Science Research General project of Universities in Jiangsu province in 2021(no.21KJB430006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to acknowledgement the financial support of the Natural Science Research General project of Universities in Jiangsu province in 2021 (no.21KJB430006).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saji, S.; Yasuhara, K.; Hori, S. Mechanical Properties of 7075 Aluminum Alloy at 6–130 K. Trans. Jpn. Inst. Met. 1987, 28, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Li, R.; Li, R. Effect of Mg2Si particles on low-temperature fracture behavior of Al-7.0Si-0.3Mg alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2016, 674, 666–671. [Google Scholar] [CrossRef]
- Ma, G.; Li, R.; Li, R. Effects of stress concentration on low-temperature fracture behavior of Al-7.0Si-0.3Mg alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2016, 667, 459–467. [Google Scholar] [CrossRef]
- Nouri, S.; Sahmani, S.; Hadavi, M.; Mirdamadi, S. Mechanical Properties Improvement of Al-Li 8090 Alloy by Using the New Proposed Method of Directional Quenching. Met. Mater. Int. 2020, 26, 1134–1143. [Google Scholar] [CrossRef]
- Dong, F.; Yi, Y.; Huang, S.; Wang, B.; He, H.; Huang, K.; Wang, C. Cryogenic formability and deformation behavior of 2060 Al-Li alloys with water-quenched and T4 aged temper. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2021, 823, 141722. [Google Scholar] [CrossRef]
- Isaev, N.V.; Zabrodin, P.A.; Rusakova, A.V. Localization of plastic deformation in ultra-fine grained Al and Al–Li at temperatures of 4.2–350 K. Low Temp. Phys. 2012, 38, 973–979. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.H.; Kahtani, S.A. Microstructure, tensile properties and fracture behavior of high temperature Al–Si–Mg–Cu cast alloys. Mater. Sci. Eng. A 2013, 577, 64–72. [Google Scholar] [CrossRef]
- Cao, F.; Li, Z.; Zhang, N.; Ding, H.; Yu, F.; Zuo, L. Superplasticity, flow and fracture mechanism in an Al-12.7Si-0.7Mg alloy. Mater. Sci. Eng. A 2013, 571, 167–183. [Google Scholar] [CrossRef]
- Caceres, C.H.; Griffiths, J.R. Damage by the cracking of silicon particles in an Al-7Si-0.4Mg casting alloy. Acta Mater. 1996, 44, 25–33. [Google Scholar] [CrossRef]
- Rincón, E.; López, H.F.; Cisneros, M.M.; Mancha, H.; Cisneros, M.A. Effect of temperature on the tensile properties of an as-cast aluminum alloy A319. Mater. Sci. Eng. A 2007, 452–453, 682–687. [Google Scholar] [CrossRef]
- Gokhale, A.M.; Dighe, M.D.; Horstemeyer, M. Effect of temperature on silicon particle damage in Al-7.0Si-0.3Mg alloy. Metall. Mater. Trans. A 1998, 29, 905–907. [Google Scholar] [CrossRef]
- Umezawa, O. Mechanical Properties of Thermomechanical Treated Hyper-Eutectic Al-Si-(Fe,Mn,Cu) Materials. Mater. Trans. 2005, 46, 2616–2623. [Google Scholar] [CrossRef]
- Ma, G.; Li, R.; Bai, Y.; Rongde, L. Effect of silicon phase on tensile fracture of Al-Si alloys at low temperature. Chin. J. Nonferrous Met. 2015, 35, 1121–1125. [Google Scholar]
- Ma, G.; Li, R.; Bai, Y.; Li, R. Crack Extension in ZL101 Alloy at Low Temperature. Foundry 2015, 64, 960–963. [Google Scholar]
- Samuel, F.H. Incipient melting of Al5Mg8Si6Cu2 and Al2Cu intermetallics in unmodified and strontium-modified Al-Si-Cu-Mg(319) alloys during solution heat treatment. J. Mater. Sci. 1998, 33, 2283–2297. [Google Scholar] [CrossRef]
- Sokolowski, J.H.; Sun, X.C. The removal of copper-phase segregation and the subsequent improvement in mechanical properties of cast 319 aluminum alloys by two-stage solution heat treatment. J. Mater. Process. Technol. 1995, 250, 58–82. [Google Scholar]
- Li, L.; Li, D.; Feng, J.; Zhang, Y.; Kang, Y. Effect of Cooling Rates on the Microstructure and Mechanical Property of La Modified Al7SiMg Alloys Processed by Gravity Die Casting and Semi-Solid Die Casting. Metals 2020, 10, 549. [Google Scholar] [CrossRef]
- Meyers, C.W. Solution heat treatment effects in A359 alloys. AFS Trans. 1985, 112, 741–744. [Google Scholar]
- Lu, S.; Du, R.; Liu, J.; Chen, L.; Wu, S. A new fast heat treatment process for cast Al-7.0Si-0.3Mg alloy motorcycle wheel hubs. China Foundry 2018, 15, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Sharma, A.; Pandel, U.; Ratke, L. Effect of heat treatment on microstructures and mechanical properties of Al-7.0Si-0.3Mg alloy cast through rapid slurry formation (RSF) process. Int. J. Cast Met. Res. 2017, 30, 283–292. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Q.; Liu, T.; Ye, B.; Jiang, H.; Ding, W. Effect of T6 heat treatment on microstructure and mechanical property of 6101/Al-7.0Si-0.3Mg bimetal fabricated by squeeze casting. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2017, 696, 208–215. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, L.; Liu, D.; Tang, Y.; Han, Y. Investigation of semi-solid microstructures of an Al-7.0Si-0.3Mg alloy containing rare-earth Gd during isothermal heat treatment. Int. J. Mater. Res. 2019, 110, 422–427. [Google Scholar] [CrossRef]
- Jien, W.; Wen, P. The cracking mechanism of silicon particles in an A357 aluminum alloy. Metall. Mater. Trans. A 1996, 27, 3558–3568. [Google Scholar]
- Kido, K.; Matsuda, K.; Kawabata, T.; Sato, T.; Ikeno, S. HRTEM observation of precipitates in Al-Mg-Si alloy containing copper at early stage during aging. Mater. Sci. Forum. 2002, 396–402, 953–958. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, K.; Elgallad, E.; Breton, F.; Chen, X.G. Differential Scanning Calorimetry Fingerprints of Various Heat-Treatment Tempers of Different Aluminum Alloys. Metals 2020, 10, 763. [Google Scholar] [CrossRef]
- Kang, H. Age-hardening characteristics of Al-Si-Cu-base cast alloy. AFS Trans. 1999, 27, 507–514. [Google Scholar]
- Kashyap, K.T.; Murall, S.; Raman, K.S.; Murthy, K.S.S. Casting and heat treatment variable of Al-7Si-Mg alloy. Mater. Sci. Technol. 1993, 9, 189–204. [Google Scholar] [CrossRef]
- Chen, K.; Chao, C. Effect of δ alumina fibera on the aging characterstics of 2024-based matel-matrix composites. Metall. Mater. Trans. A 1995, 26, 1035–1043. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.; Oh, K.; Lee, H. Suppression of Θ″formation in the SiC whisker-reinforced Al-4wt%Cu composites. J. Mater. Sci. 1992, 27, 2599–2605. [Google Scholar] [CrossRef]
- Colley, L.; Wells, M.; Poole, W. Microstructure-strength models for heat treatment of Al-Si-Mg casting alloys I: Microstructure evolution and precipitation kinetics. Can. Metall. Q. 2014, 53, 125–137. [Google Scholar] [CrossRef]
- Xu, J.; Pan, Y.; Lu, T.; Bo, B. Synergistic effects of composition and heat treatment on microstructure and properties of vacuum die cast Al-Si-Mg-Mn alloys. China Foundry 2018, 15, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Choi, S.; Kim, Y.; Kang, C. Increasing the thermal diffusivity of Al-Si-Mg alloys by heat treatment. J. Therm. Anal. Calorim. 2022, 147, 2139–2146. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).