The Rigidity of the (BH4)-Anion Dispersed in Halides AX, A = Na, K; X = Cl, Br, I, and in MBH4 with M = Na, K, Rb, Cs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Study of BH4− Anion Isolated in AX Halides
2.2. First Principles Calculations
3. Results and Discussion
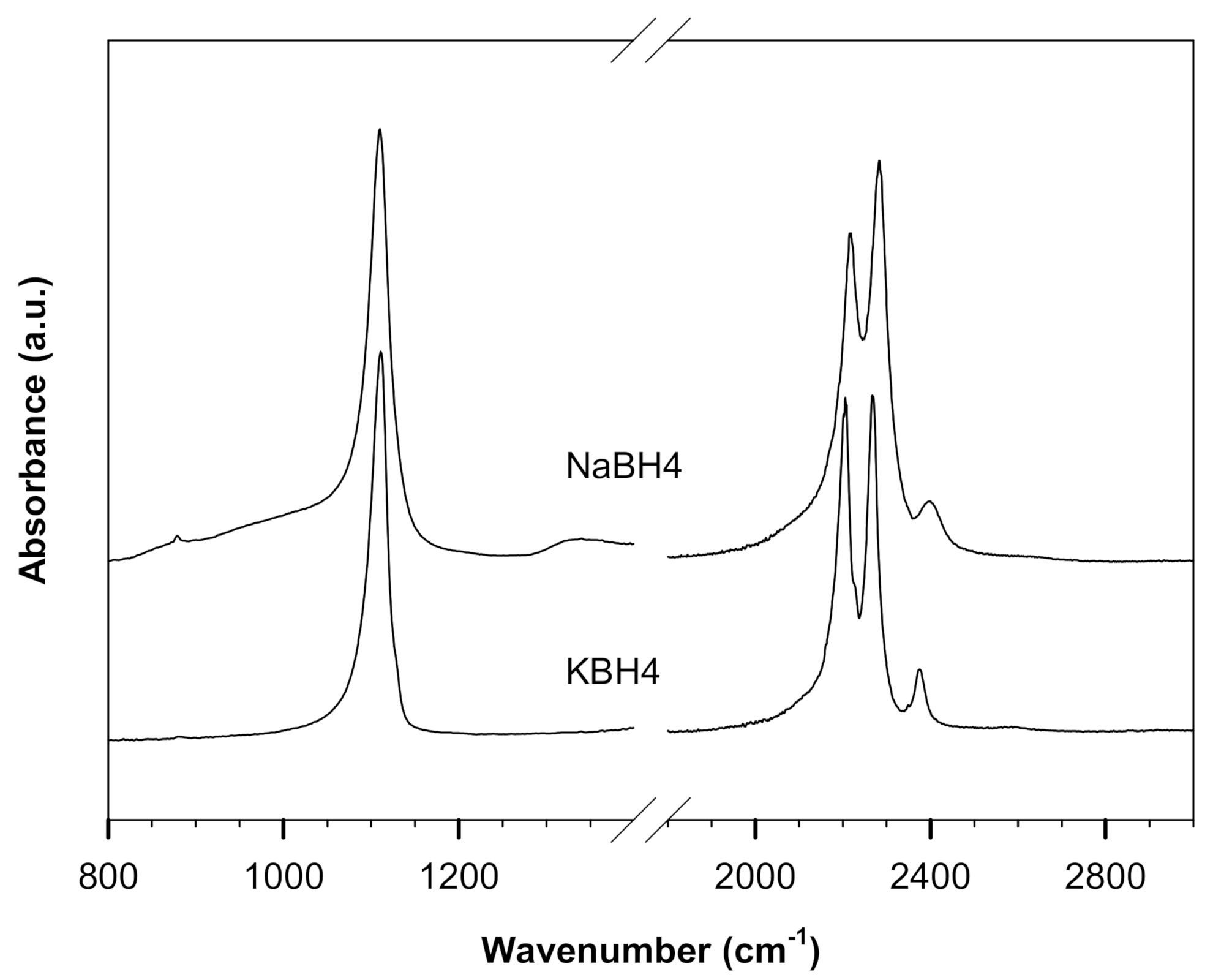
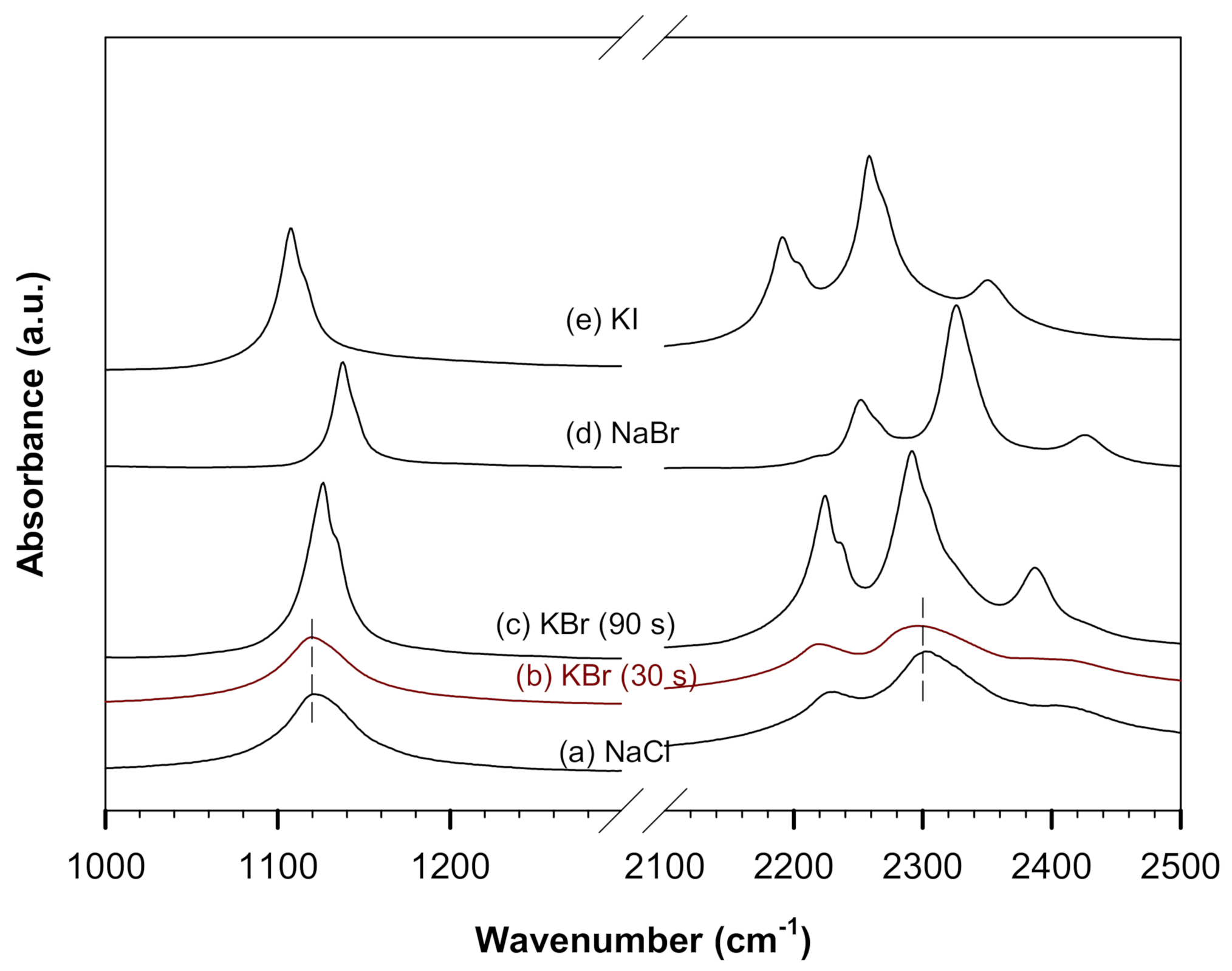
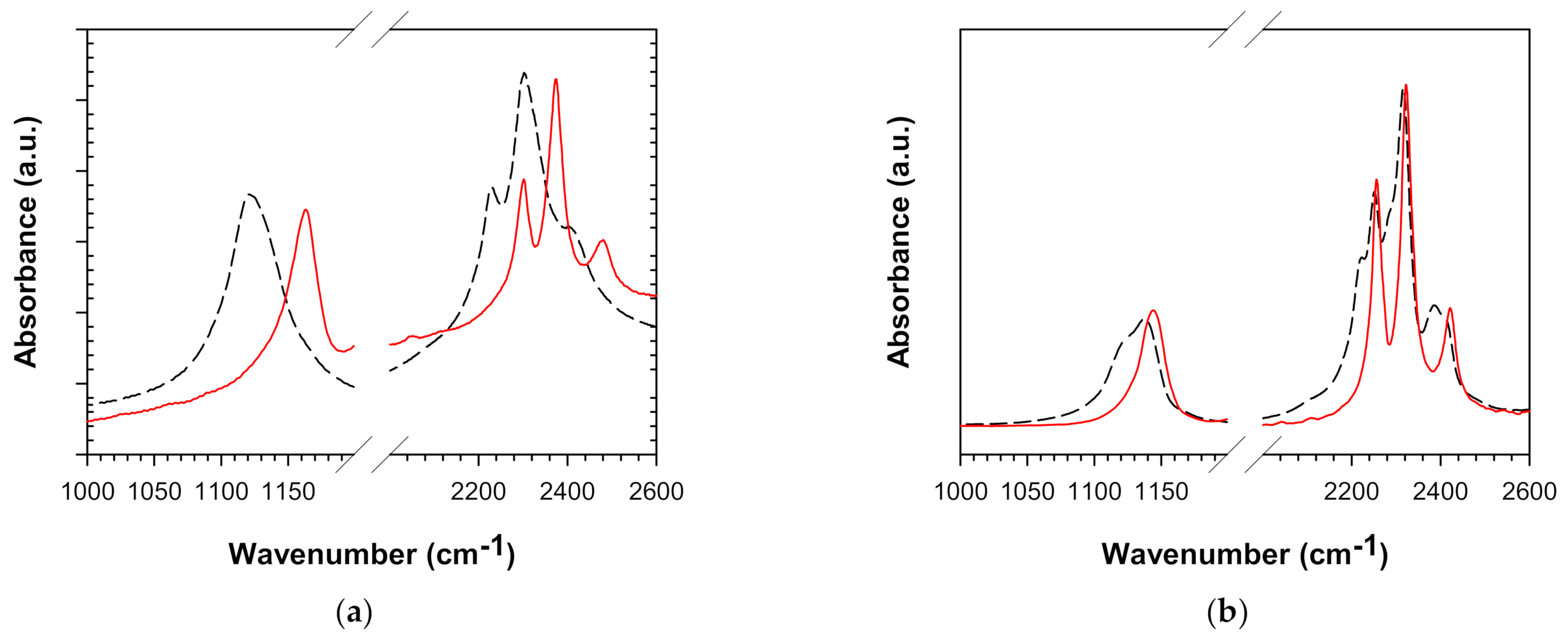
3.1. Spectra of NaBH4/KBH4 in ATR and Pressed in Various Halides
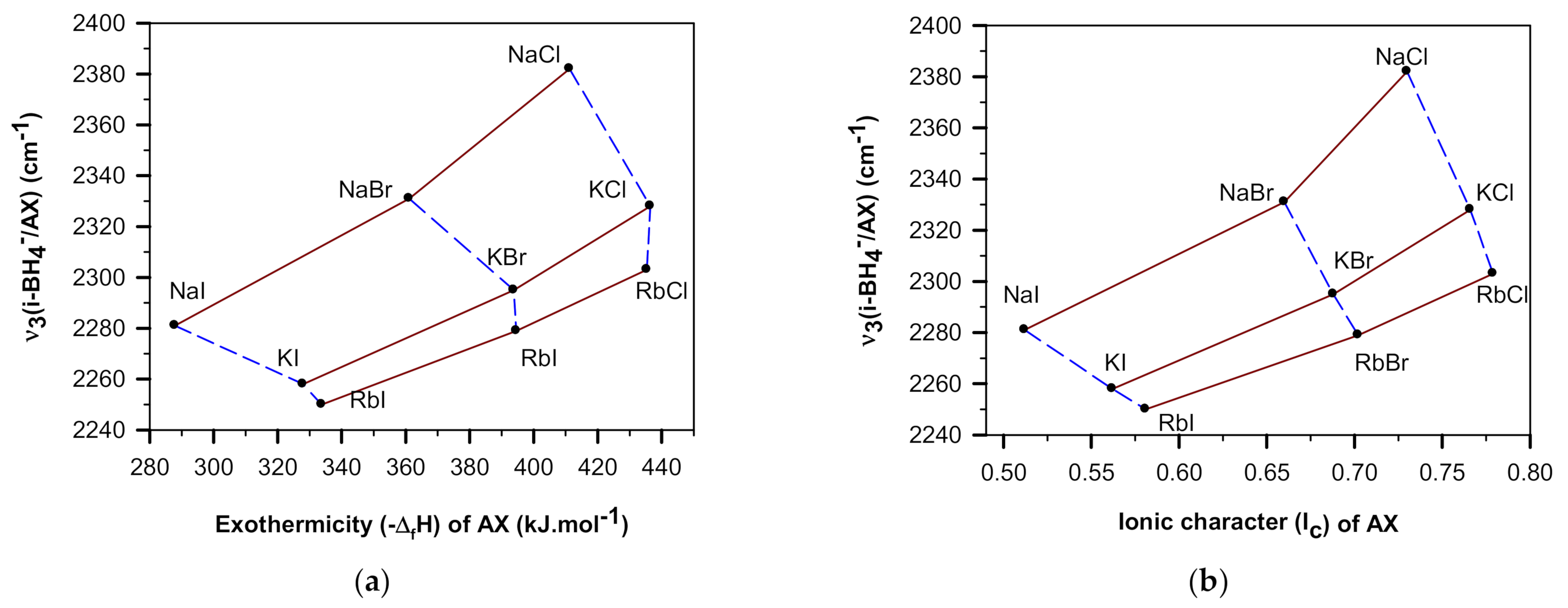
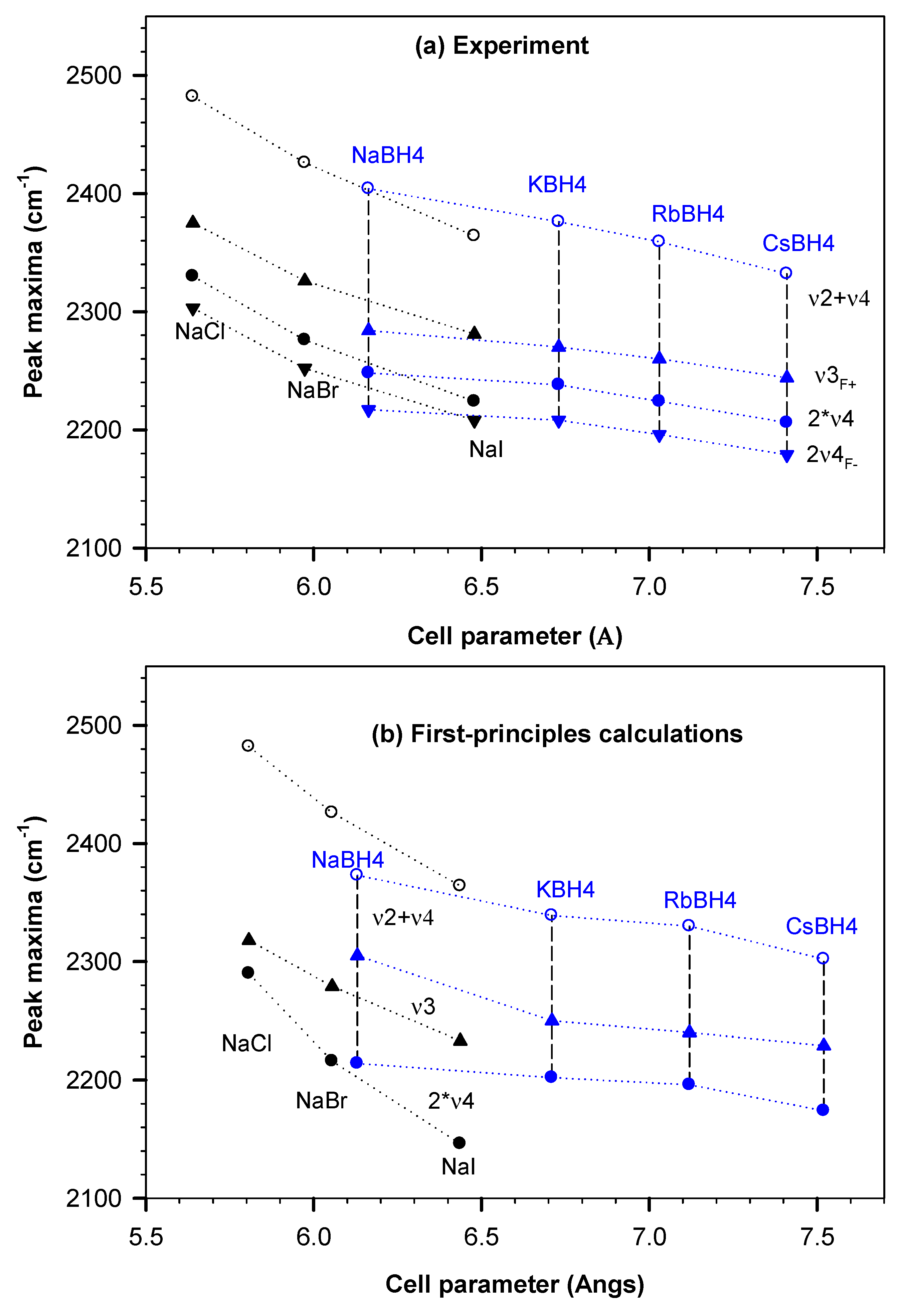
3.2. BH4-Frequency Variations Dependences on Halide Parameters
3.3. BH4-Frequency Variations Dependences on Fundamental Parameter Sets in DFT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Züttel, A.; Borgschulte, A.; Orimo, S.I. Tetrahydroborates as new hydrogen storage materials. Scr. Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Demirci, U.B.; Akdim, O.; Andrieux, J.; Hannauer, J.; Chamoun, R.; Miele, P. Sodium borohydride hydrolysis as hydrogen generator: Issues, state of the art and applicability upstream froma fuel cell. Fuel Cells 2010, 10, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Urgnani, J.; Torres, F.J.; Palumbo, M.; Baricco, M. Hydrogen release from solid state NaBH4. Int. J. Hydrogen Energy 2008, 33, 3111–3115. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Nevirkovets, I.P.; Liu, H.K.; Dou, S.X. Hydrogen De-/absorption improvement of NaBH4 catalyzed by titanium-based additives. J. Phys. Chem. C 2012, 116, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- Humphries, T.D.; Kalantzopoulos, G.N.; Llamas-Jansa, I.; Olsen, J.E.; Hauback, B.C. Reversible hydrogenation studies of NaBH4 milled with Ni-containing additives. J. Phys. Chem. C 2013, 117, 6060–6065. [Google Scholar] [CrossRef] [Green Version]
- Ngene, P.; Van Den Berg, R.; Verkuijlen, M.H.W.; De Jong, K.P.; De Jongh, P.E. Reversibility of the hydrogen desorption from NaBH4 by confinement in nanoporous carbon. Energy Environ. Sci. 2011, 4, 4108–4115. [Google Scholar] [CrossRef] [Green Version]
- Peru, F.; Garroni, S.; Campesi, R.; Milanese, C.; Marini, A.; Pellicer, E.; Baro, M.D.; Mulas, G. Ammonia-free infiltration of NaBH4 into highly-ordered mesoporous silica and carbon matrices for hydrogen storage. J. Alloys Compd. 2013, 580, S309–S312. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, X.; Fan, X.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Fast hydrogen release under moderate conditions from NaBH4 destabilized by fluorographite. RSC Adv. 2014, 4, 2550–2556. [Google Scholar] [CrossRef]
- Rude, L.H.; Filsø, U.; D’Anna, V.; Spyratou, A.; Richter, B.; Hino, S.; Jensen, T.R. Hydrogen-fluorine exchange in NaBH4-NaBF4. Phys. Chem. Chem. Phys. 2013, 15, 18185–18194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.G.; Wang, H.; Zhu, M. Hydrogen release from sodium borohydrides at low temperature by the addition of zinc fluoride. Int. J. Hydrogen Energy 2011, 36, 8203–8208. [Google Scholar] [CrossRef]
- Buhl, J.C.; Gesing, T.M.; Rüscher, C.H. Synthesis, crystal structure and thermal stability of tetrahydroborate sodalite Na8[AlSiO4]6(BH4)2. Microporous Mesoporous Mater. 2005, 80, 57–63. [Google Scholar] [CrossRef]
- Buhl, J.C.; Schomborg, L.; Rüscher, C.H. Enclosure of Sodium Tetrahydroborate (NaBH4) in Solidified Aluminosilicate Gels and Microporous Crystalline Solids for Fuel Processing. In Hydrogen Storage; InTechOpen: London, UK, 2012. [Google Scholar]
- Rüscher, C.H. Boronhydride-geopolymer composites. J. Ceram. Sci. Technol. 2017, 8, 399–410. [Google Scholar] [CrossRef]
- Schneider, A.G.; Bredow, T.; Schomborg, L.; Rscher, C.H. Structure and IR vibrational spectra of Na8[AlSiO4]6(BH4)2: Comparison of theory and experiment. J. Phys. Chem. A 2014, 118, 7066–7073. [Google Scholar] [CrossRef]
- Rüscher, C.H.; Schomborg, L.; Bredow, T. Experiments on the thermal activation of hydrogen release of NaBH4-sodalites characterized by IR- and MAS-NMR spectroscopy. Int. J. Hydrogen Energy 2022. under review. [Google Scholar]
- Mesmer, R.E.; Jolly, W.L. The Hydrolysis of Aqueous Hydroborate. Inorg. Chem. 1962, 1, 608–612. [Google Scholar] [CrossRef]
- Kreevoy, M.M.; Hutchins, J.E.C. H2BH3 as an Intermediate in Tetrahydridoborate Hydrolysis. J. Am. Chem. Soc. 1972, 94, 6371–6376. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.I.; Orimo, S.I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B—Condens. Matter Mater. Phys. 2006, 74, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nakamori, Y.; Li, H.W.; Kikuchi, K.; Aoki, M.; Miwa, K.; Towata, S.I.; Orimo, S.I. Thermodynamical stabilities of metal-borohydrides. J. Alloys Compd. 2007, 446–447, 296–300. [Google Scholar] [CrossRef]
- Matsunaga, T.; Buchter, F.; Miwa, K.; Towata, S.; Orimo, S.; Züttel, A. Magnesium borohydride: A new hydrogen storage material. Renew. Energy 2008, 33, 193–196. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hinze, J. The concept of electronegativity of atoms in molecules. Theor. Comput. Chem. 1999, 6, 189–212. [Google Scholar] [CrossRef]
- Ravnsbæk, D.; Filinchuk, Y.; Cerenius, Y.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A Series of Mixed-Metal Borohydrides. Angew. Chem. 2009, 121, 6787–6791. [Google Scholar] [CrossRef]
- Brinks, H.W.; Fossdal, A.; Hauback, B.C. Adjustment of the stability of complex hydrides by anion substitution. J. Phys. Chem. C 2008, 112, 5658–5661. [Google Scholar] [CrossRef]
- Yin, L.; Wang, P.; Fang, Z.; Cheng, H. Thermodynamically tuning LiBH4 by fluorine anion doping for hydrogen storage: A density functional study. Chem. Phys. Lett. 2008, 450, 318–321. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Rude, L.H.; Jensen, T.R. Chloride substitution in sodium borohydride. J. Solid State Chem. 2011, 184, 1858–1866. [Google Scholar] [CrossRef]
- Olsen, J.E.; Sørby, M.H.; Hauback, B.C. Chloride-substitution in sodium borohydride. J. Alloys Compd. 2011, 509, L228–L231. [Google Scholar] [CrossRef]
- Rude, L.H.; Zavorotynska, O.; Arnbjerg, L.M.; Ravnsbæk, D.B.; Malmkjær, R.A.; Grove, H.; Jensen, T.R. Bromide substitution in lithium borohydride, LiBH4-LiBr. Int. J. Hydrogen Energy 2011, 36, 15664–15672. [Google Scholar] [CrossRef] [Green Version]
- Rude, L.H.; Groppo, E.; Arnbjerg, L.M.; Ravnsbaek, D.B.; Malmkjaer, R.A.; Filinchuk, Y.; Jensen, T.R. Iodide substitution in lithium borohydride, LiBH4-LiI. J. Alloys Compd. 2011, 509, 8299–8305. [Google Scholar] [CrossRef] [Green Version]
- Vajeeston, P.; Ravindran, P.; Kjekshus, A.; Fjellvåg, H. Structural stability of alkali boron tetrahydrides ABH4 (A=Li, Na, K, Rb, Cs) from first principle calculation. J. Alloys Compd. 2005, 387, 97–104. [Google Scholar] [CrossRef]
- Renaudin, G.; Gomes, S.; Hagemann, H.; Keller, L.; Yvon, K. Structural and spectroscopic studies on the alkali borohydrides MBH4 (M = Na, K, Rb, Cs). J. Alloys Compd. 2004, 375, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Harvey, K.B.; McQuaker, N.R. Infrared and Raman Spectra of Potassium and Sodium Borohydride. Can. J. Chem. 1971, 49, 3272–3281. [Google Scholar] [CrossRef]
- Heyns, A.M.; Schutte, C.J.H.; Scheuermann, W. Low-temperature infrared studies. IX. The infrared and Raman spectra of sodium d4-borohydride NaBD4. J. Mol. Struct. 1971, 9, 271–281. [Google Scholar] [CrossRef]
- Memon, M.I.; Wilkinson, G.R.; Sherman, W.F. Vibrational studies of BH4- and BD4- isolated in alkali halides. J. Mol. Struct. 1982, 80, 113–116. [Google Scholar] [CrossRef]
- Memon, M.I.; Sherman, W.F.; Wilkinson, G.R. Fermi resonances in the Raman spectra of alkali halide/BH4-. J. Mol. Struct. 1984, 115, 213–216. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Civalleri, B.; Roetti, C.; Saunders, V.R.; Zicovich-Wilson, C.M. CRYSTAL: A computational tool for the ab initio study of the electronic properties of crystals. Z. Für. Krist.–Cryst. Mater. 2005, 220, 571–573. [Google Scholar] [CrossRef]
- Dovesi, R.; Roberto, O.; Alessandro, E.; Claudio, M.Z.-W.; Bartolomeo, C.; Silvia, C.; Lorenzo, M.; Matteo, F.; Marco, D.L.P.; Philippe, D.; et al. CRYSTAL14 User’s Manual; University of Turin: Torino, Italy, 2014; p. 382. [Google Scholar]
- Perdew, J.P.; Yue, W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B Condens. Matter 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Dovesi, R.; Roetti, C.; Freyria-Fava, C.; Prencipe, M.; Saunders, V.R. On the elastic properties of lithium, sodium and potassium oxide. An ab initio study. Chem. Phys. 1991, 156, 11–19. [Google Scholar] [CrossRef]
- Leininger, T.; Nicklass, A.; Küchle, W.; Stoll, H.; Dolg, M.; Bergner, A. The accuracy of the pseudopotential approximation: Non-frozen-core effects for spectroscopic constants of alkali fluorides XF (X = K, Rb, Cs). Chem. Phys. Lett. 1996, 255, 274–280. [Google Scholar] [CrossRef]
- Dovesi, R.; Ermondi, C.; Ferrero, E.; Pisani, C.; Roetti, C. Hartree-Fock study of lithium hydride with the use of a polarizable basis set. Phys. Rev. B 1984, 29, 3591–3600. [Google Scholar] [CrossRef]
- Orlando, R.; Dovesi, R.; Roetti, C.; Saunders, V.R. Ab initio Hartree-Fock calculations for periodic compounds: Application to semiconductors. J. Phys. Condens. Matter 1990, 2, 7769–7789. [Google Scholar] [CrossRef]
- Apra, E.; Causa, M.; Prencipe, M.; Dovesi, R.; Saunders, V.R. On the structural properties of {NaCl}: An ab initio study of the B1-B2 phase transition. J. Phys. Condens. Matter 1993, 5, 2969–2976. [Google Scholar] [CrossRef]
- Peintinger, M.F.; Oliveira, D.V.; Bredow, T. Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J. Comput. Chem. 2013, 34, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Stoll, H.; Metz, B.; Dolg, M. Relativistic energy-consistent pseudopotentials--recent developments. J. Comput. Chem. 2002, 23, 767–778. [Google Scholar] [CrossRef] [PubMed]
- The Inorganic Crystal Structure Database (ICSD). Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 9 March 2022).
- Hanson, R.M. Jmol—A paradigm shift in crystallographic visualization. J. Appl. Crystallogr. 2010, 43, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Hisatsune, I.C.; Suarez, N.H. Infrared Spectra of Metaborate Monomer and Trimer Ions. Inorg. Chem. 1964, 3, 168–174. [Google Scholar] [CrossRef]
- Ketelaar, J.A.A.; Schutte, C.J.H. The borohydride ion (BH4-) in a face-centred cubic alkali-halide lattice. Spectrochim. Acta 1961, 17, 1240–1243. [Google Scholar] [CrossRef]
- Nasar, A. Correlation between standard enthalpy of formation, structural parameters and ionicity for alkali halides. J. Serb. Chem. Soc. 2013, 78, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Bryant, J.I.; Turrell, G.C. Infrared Spectra of the Azide Ion in Alkali-Halide Lattices. J. Chem. Phys. 1962, 37, 1069–1077. [Google Scholar] [CrossRef]
- Sherman, W.F.; Wilkinson, G.R. Infrared and raman studies on the vibrational spectra of impurities in ionic and covalent crystals. In Vibrational Spectroscopy of Trapped Species; Wiley: London, UK, 1973; p. 291. [Google Scholar]
- Field, G.R.; Sherman, W.F. Cyanide ion—Environmental perturbation of its vibrational and rotational motion when isolated in alkali halides. J. Chem. Phys. 1967, 47, 2378–2389. [Google Scholar] [CrossRef]
- Strasheim, A.; Buijs, K. Infrared absorption of nitrate ions dissolved in solid alkali halides. J. Chem. Phys. 1961, 34, 691–692. [Google Scholar] [CrossRef]
- Seitz, F. The Modern Theory of Solids; McGraw-Hill Book Company, Incorporated: New York, NY, USA, 1940. [Google Scholar]
- Orimo, S.; Nakamori, Y.; Züttel, A. Material properties of MBH4 (M = Li, Na, and K). Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 108, 51–53. [Google Scholar] [CrossRef]
- Zhang, X.D.; Hou, Z.F.; Jiang, Z.Y.; Hou, Y.Q. Elastic properties of MBH4 (M = Na, K, Rb, Cs). Phys. B Condens. Matter 2011, 406, 2196–2199. [Google Scholar] [CrossRef]


| Atom | Basis Set |
|---|---|
| Na | 8-511G [40] |
| K | 86-511G [40] |
| Rb | ECP28MWB [41] |
| Cs | ECP46MWB [41] |
| H | 5-11G * [42] |
| B | 6-21G * [43] |
| Cl | 86-311G [44] |
| Br | Pob-TZVP [45] |
| I | ECP [46] |
| ν4 | 2ν4 | ν3 | ν2 + ν4 | ||
|---|---|---|---|---|---|
| ATR | NaBH4 | 1110 (1110) | 2217 (2217) | 2283 (2284) | 2397 (2404) |
| KBH4 | 1111 (1112) | 2205 (2208) | 2268 (2270) | 2375 (2376) | |
| Transmission (pellets) | NaBH4/NaCl | 1121 | 2229 | 2302 | 2403 |
| NaBH4/KBr (<30 s pressed) | 1120 | 2220 | 2299 | 2398 | |
| NaBH4/KBr (90 s pressed) | 1126 | 2224 | 2291 | 2387 |
| AX | IR Frequencies of i-BH4− in AX | Structural Parameters of AX | ||||
|---|---|---|---|---|---|---|
| ν4 | 2ν4 | ν3 | ν2 + ν4 | a0 | R = rA/rX | |
| NaCl | 1165 | 2303 | 2375 | 2482 | 5.6402 | 0.5635 |
| 1166 * | 2307 * | 2373 * | 2486 * | |||
| 1167 ** | 2311 ** | 2382 ** | 2493 ** | |||
| NaBr | 1138 | 2252 | 2326 | 2426 | 5.9738 | 0.5204 |
| 1136 * | 2254 * | 2328 * | 2427 * | |||
| 1135 ** | 2257 ** | 2331 ** | 2430 ** | |||
| NaI | 1112 | 2208 | 2281 | 2364 | 6.479 | 0.4636 |
| 1111 * | 2202 * | 2278 * | ||||
| KCl | 1144 | 2257 | 2323 | 2422 | 6.290 | 0.7624 |
| 1144 * | 2257 * | 2324 * | 2422 * | |||
| 1146 ** | 2262 ** | 2328 ** | 2428 ** | |||
| KBr | 1127 | 2226 | 2293 | 2390 | 6.598 | 0.7041 |
| 1128 * | 2226 * | 2293 * | 2387 * | |||
| 1128 ** | 2229 ** | 2295 ** | 2391 ** | |||
| KI | 1107 | 2191 | 2258 | 2350 | 7.064 | 0.6273 |
| 1108 * | 2191 * | 2258 * | 2341 * | |||
| 1108 ** | 2192 ** | 2258 ** | 2350 ** | |||
| RbCl | 1132 ** | 2236 ** | 2303 ** | 2395 ** | 6.582 | 0.8398 |
| RbBr | 1118 ** | 2212 ** | 2279 ** | 2369 ** | 6.8768 | 0.7755 |
| RbI | 1104 ** | 2181 ** | 2250 ** | 2335 ** | 7.3291 | 0.6909 |
| A-Groups | α (cm−1 Å−1) | β (cm−1) | R2 |
|---|---|---|---|
| Na-group | −119.9275 | 3054.0962 | 0.9848 |
| K-group | −88.8481 | 2884.8024 | 0.9928 |
| Rb-group | −69.1765 | 2757.6490 | 0.9956 |
| X-groups | |||
| Cl-group | −83.1449 | 2850.9477 | 0.9999 |
| Br-group | −56.6220 | 2668.9545 | 0.9999 |
| I-group | −35.7190 | 2511.7233 | 0.9957 |
| AX | Repulsive | Dipole-Dipole | Dipole-Quadrupole | Short Range |
|---|---|---|---|---|
| NaCl | −23.5 | 5.2 | 0.1 | −18.2 |
| NaBr | −20.6 | 5.5 | 0.1 | −15 |
| NaI | −17.1 | 6.3 | 0.1 | −10.7 |
| KCl | −21.5 | 7.1 | 0.1 | −14.3 |
| KBr | −18.6 | 6.9 | 0.1 | −11.6 |
| KI | −15.9 | 7.1 | 0.1 | −8.7 |
| RbCl | −19.9 | 7.9 | 0.1 | −11.9 |
| RbBr | −17.6 | 7.9 | 0.1 | −9.6 |
| RbI | −15.4 | 7.9 | 0.1 | −7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assi, Z.; Schneider, A.G.; Ulpe, A.C.; Bredow, T.; Rüscher, C.H. The Rigidity of the (BH4)-Anion Dispersed in Halides AX, A = Na, K; X = Cl, Br, I, and in MBH4 with M = Na, K, Rb, Cs. Crystals 2022, 12, 510. https://doi.org/10.3390/cryst12040510
Assi Z, Schneider AG, Ulpe AC, Bredow T, Rüscher CH. The Rigidity of the (BH4)-Anion Dispersed in Halides AX, A = Na, K; X = Cl, Br, I, and in MBH4 with M = Na, K, Rb, Cs. Crystals. 2022; 12(4):510. https://doi.org/10.3390/cryst12040510
Chicago/Turabian StyleAssi, Zeina, Alexander Gareth Schneider, Anna Christina Ulpe, Thomas Bredow, and Claus Henning Rüscher. 2022. "The Rigidity of the (BH4)-Anion Dispersed in Halides AX, A = Na, K; X = Cl, Br, I, and in MBH4 with M = Na, K, Rb, Cs" Crystals 12, no. 4: 510. https://doi.org/10.3390/cryst12040510
APA StyleAssi, Z., Schneider, A. G., Ulpe, A. C., Bredow, T., & Rüscher, C. H. (2022). The Rigidity of the (BH4)-Anion Dispersed in Halides AX, A = Na, K; X = Cl, Br, I, and in MBH4 with M = Na, K, Rb, Cs. Crystals, 12(4), 510. https://doi.org/10.3390/cryst12040510





