Advanced Catalysts for the Water Gas Shift Reaction
Abstract
:1. Introduction
2. Catalysts for the WGSR
Sulphur-Resistant Catalysts for the WGS Reaction
3. Experiments
3.1. Laboratory Apparatus
3.2. Selected Catalyst and Catalyst Preparation
3.2.1. Copper-Based Catalyst
3.2.2. Cobalt-Based Catalyst
3.3. Catalyst Characterisation
3.3.1. X-ray Fluorescence Spectroscopy
3.3.2. Specific Surface Area and Pore Volume
3.4. Catalytic Activity Tests
3.5. Product Analysis
3.5.1. Online Produced Gas Analysis
3.5.2. Offline Determination of Hydrocarbons, Permanent Gases, and Sulphur Compounds
3.5.3. Analysis of Sulphur in Condensed Water
4. Results and Discussion
4.1. Catalytic Tests of Nickel-Based Catalyst
4.1.1. Nickel-Based Catalyst Characterisation
X-ray Fluorescence, BET Surface Area, Pore Volume, and Pore Distribution
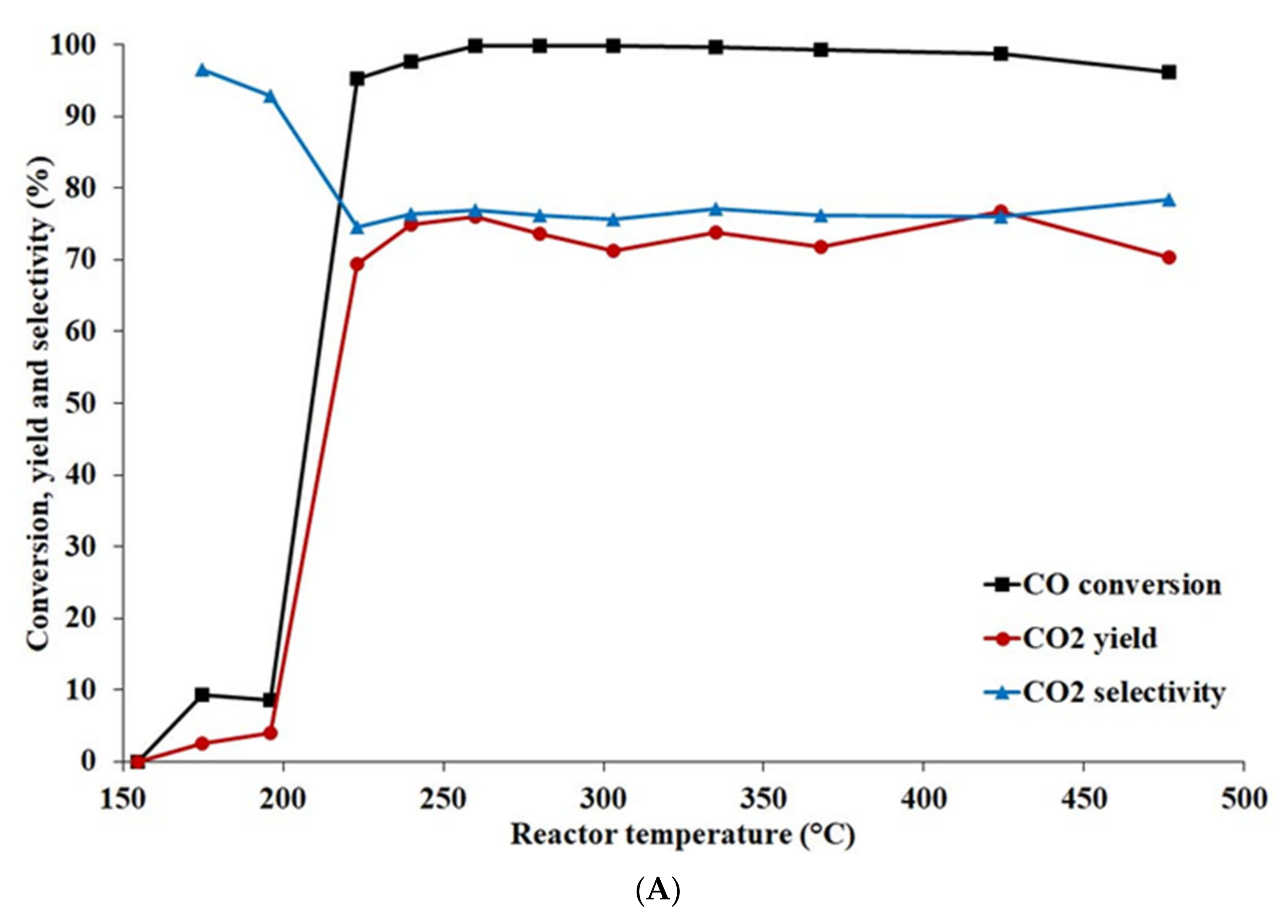
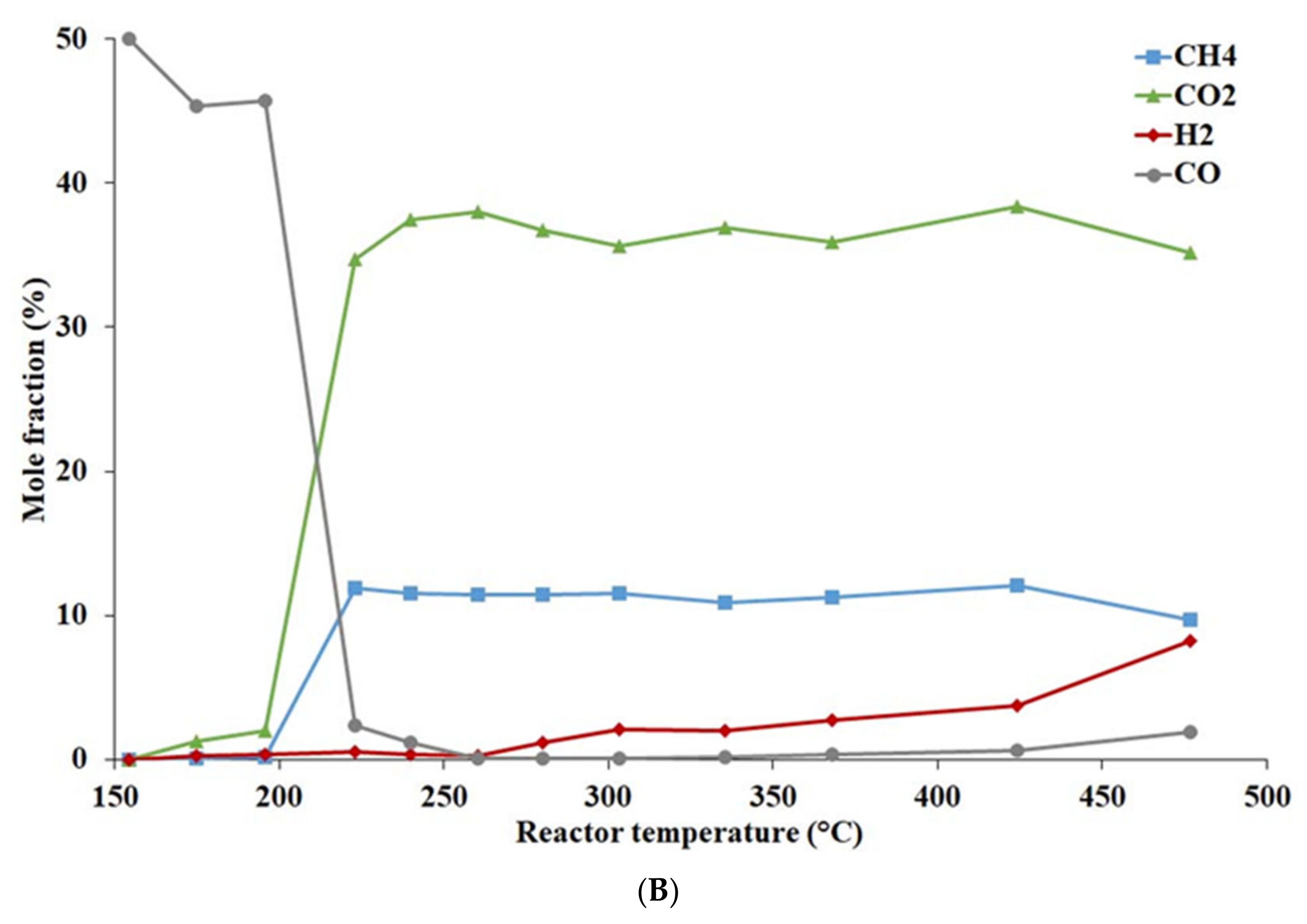
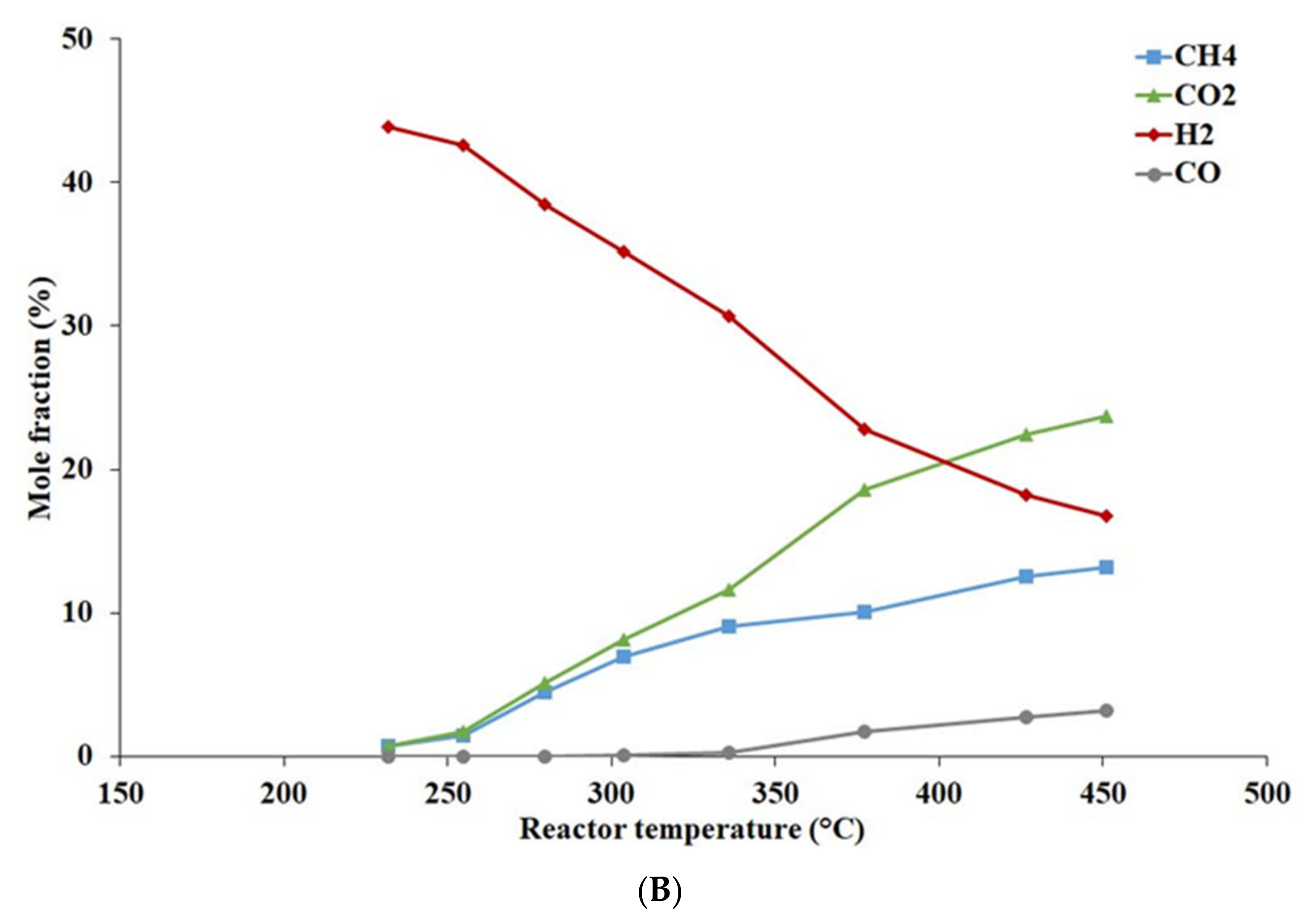
4.1.2. Effect of Temperature and Pressure on Catalyst Activity
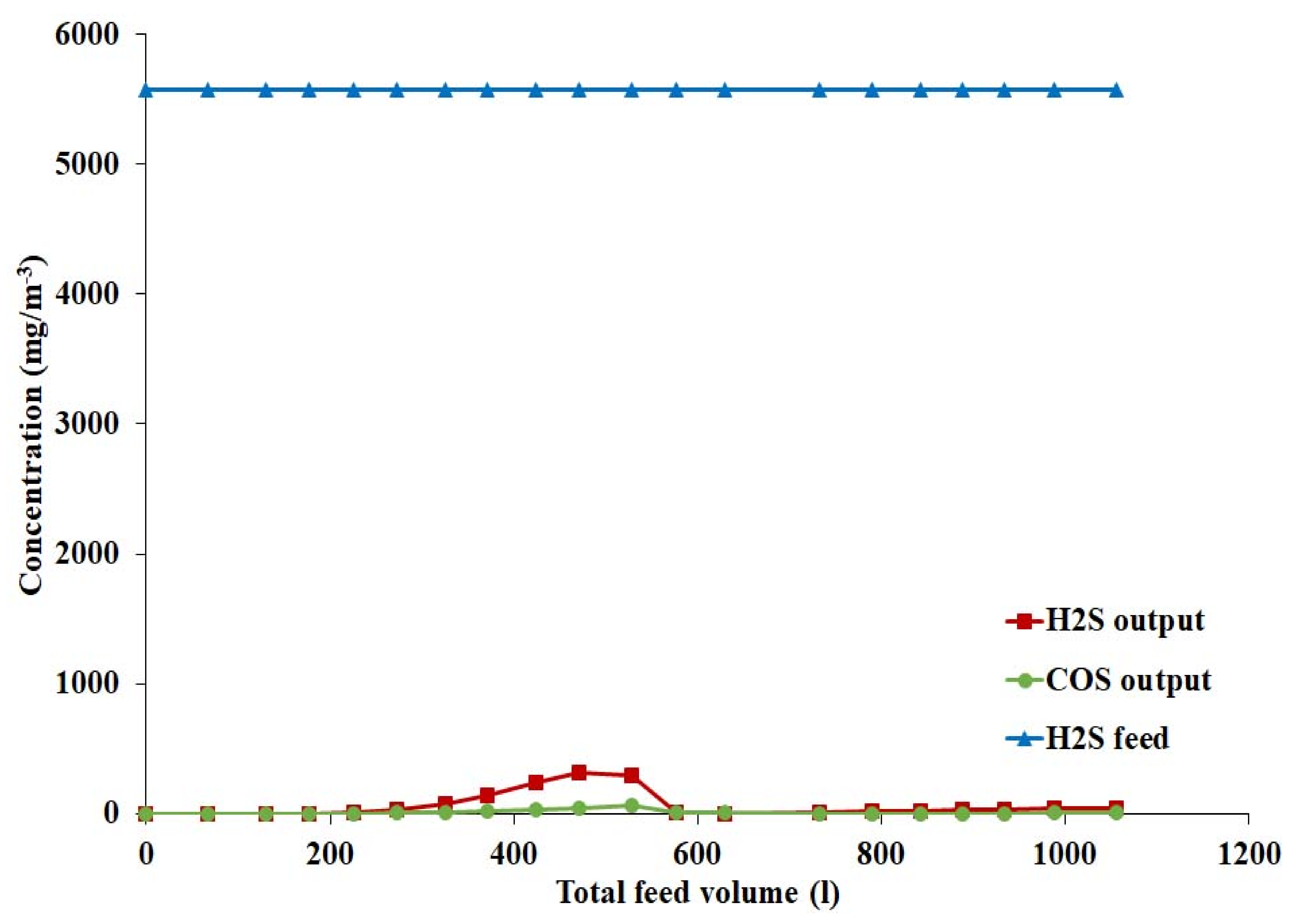
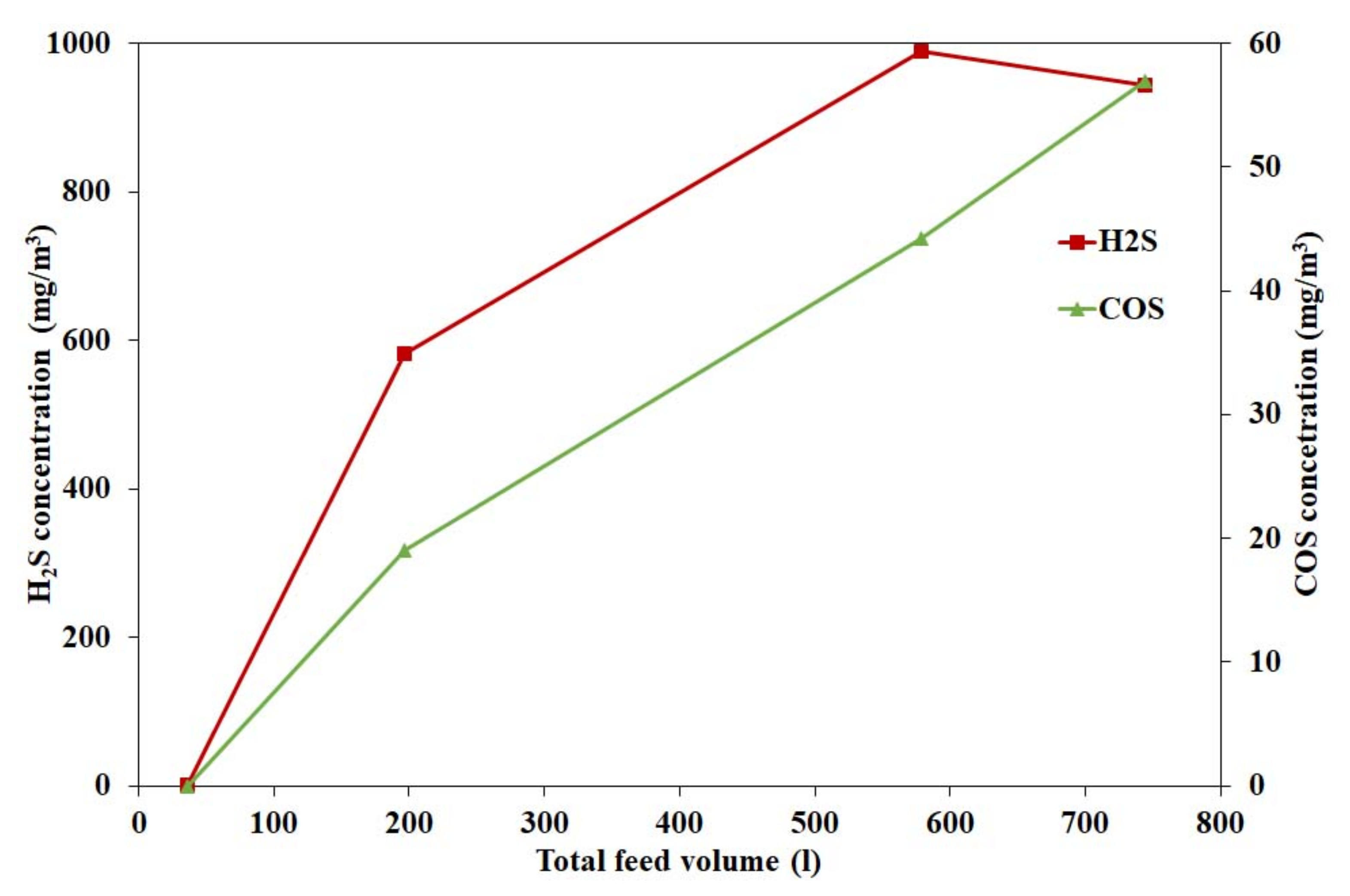
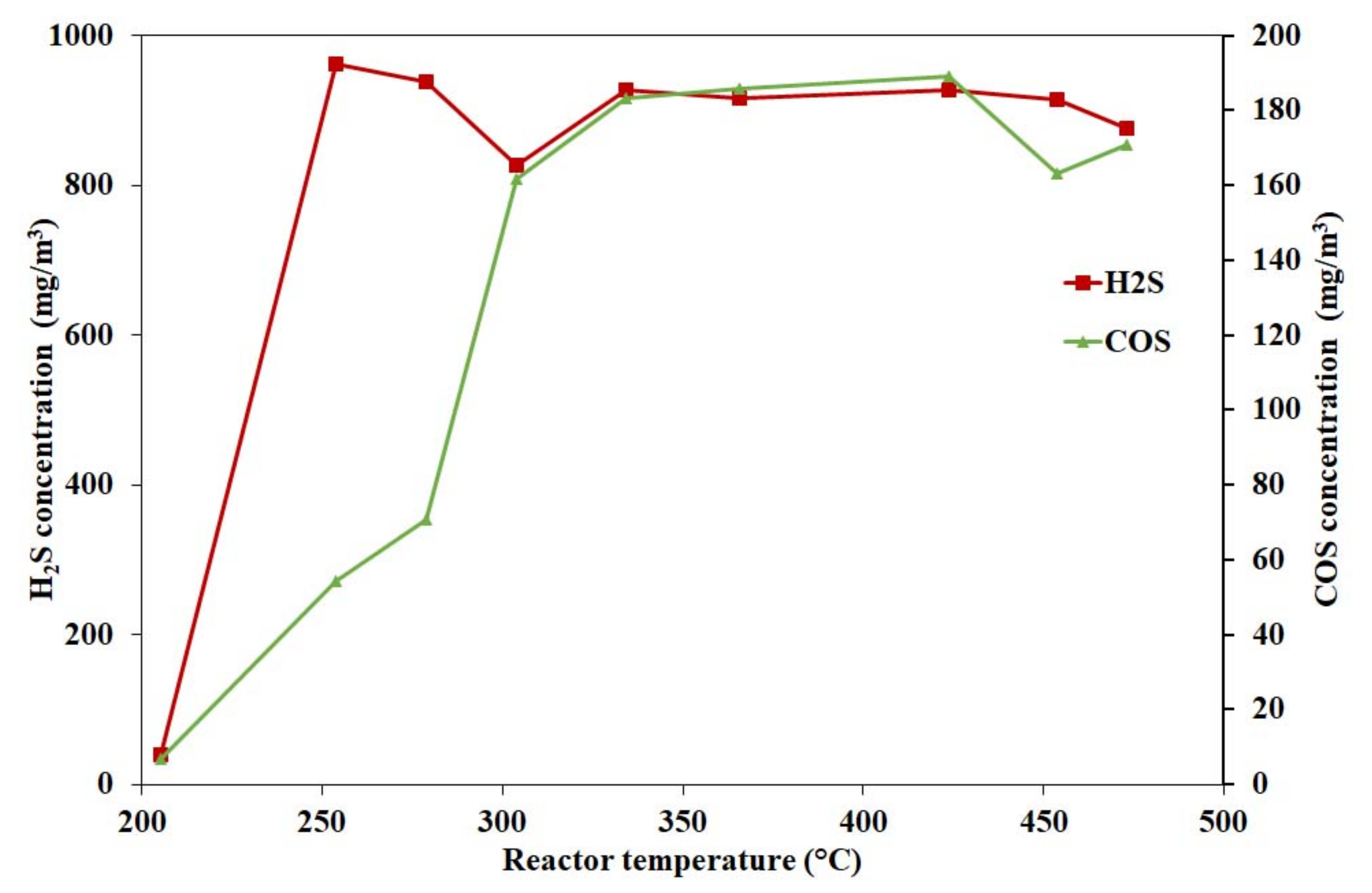
4.1.3. Effect of Hydrogen Sulphide on Catalyst Activity
4.2. Catalytic Tests of Copper-Based Catalyst
4.2.1. Support Characterisation
4.2.2. Copper-Based Catalyst Characterisation
4.2.3. Effect of Hydrogen Activation on Catalyst Activity
4.2.4. Effect of Support Calcination Temperature on Catalyst Activity
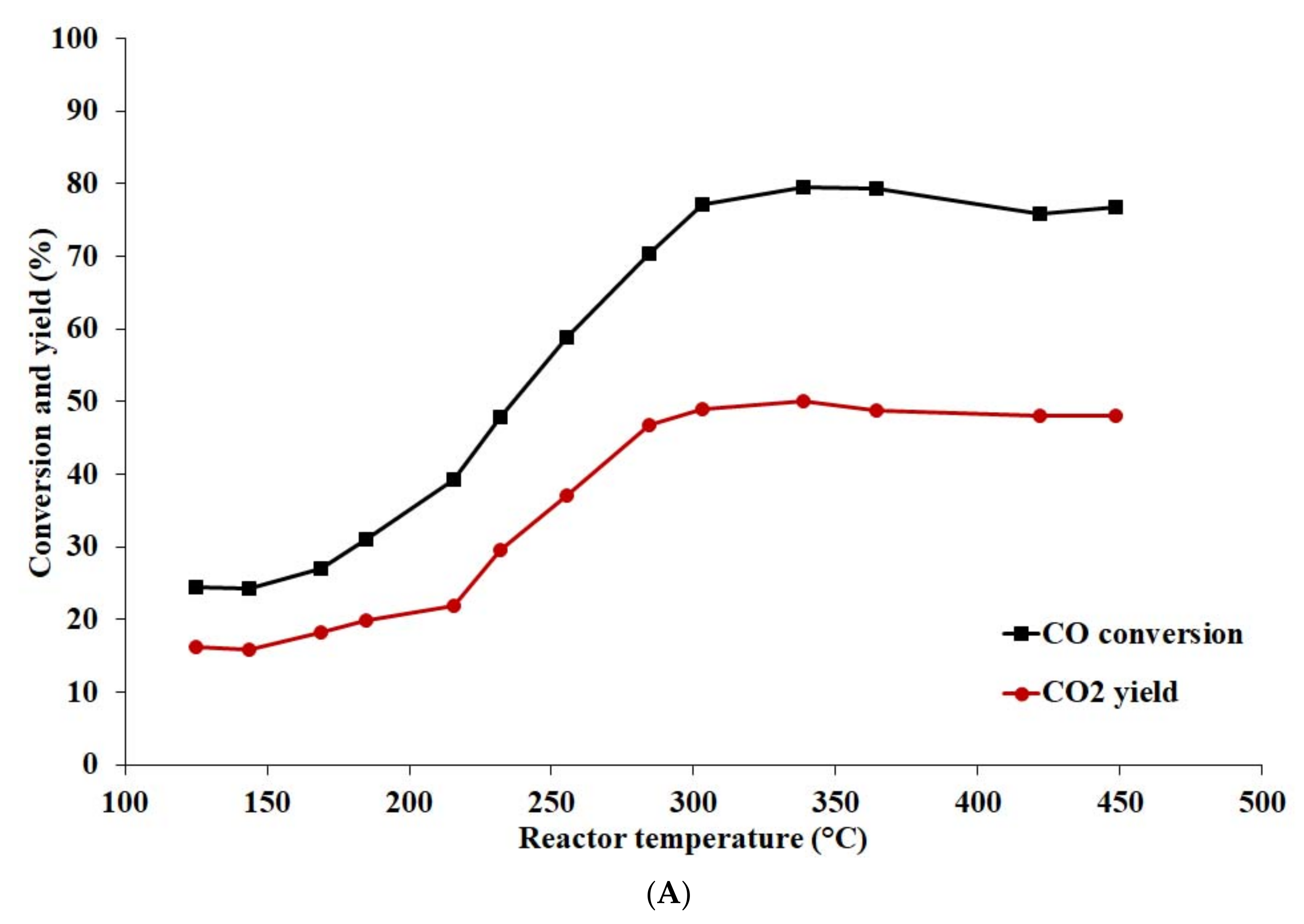
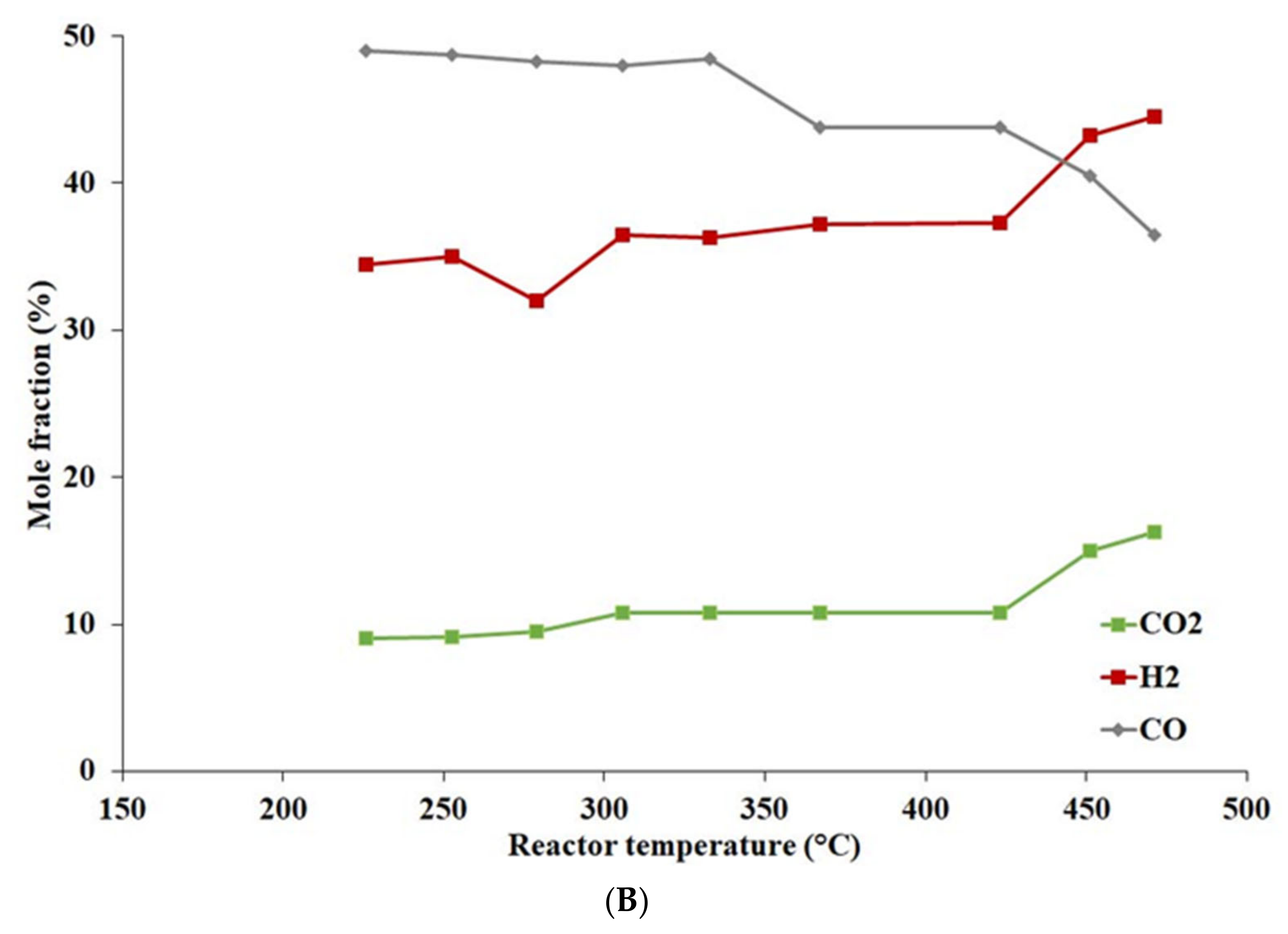
4.2.5. Effect of Temperature and Pressure on Catalyst Activity
4.2.6. Effect of Flow Rate on Catalyst Activity
4.2.7. Effect of Copper Content on Catalyst Activity
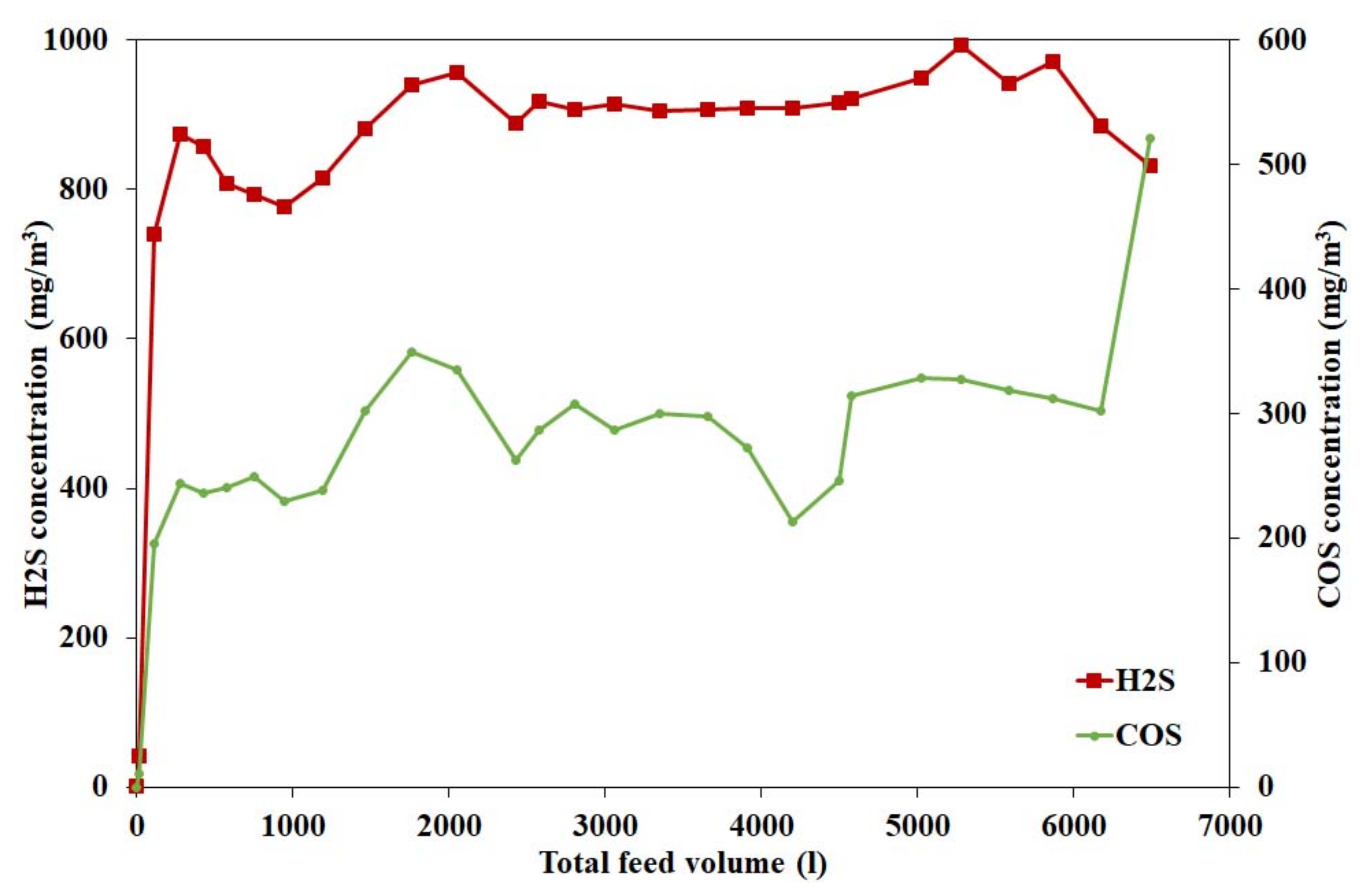
4.2.8. Effect of Hydrogen Sulphide on Catalyst Activity
4.3. Catalytic Tests of Cobalt-Based Catalyst
4.3.1. Cobalt-Based Catalyst Characterisation
4.3.2. Water-Gas Shift Reaction over Cobalt-Based Catalyst
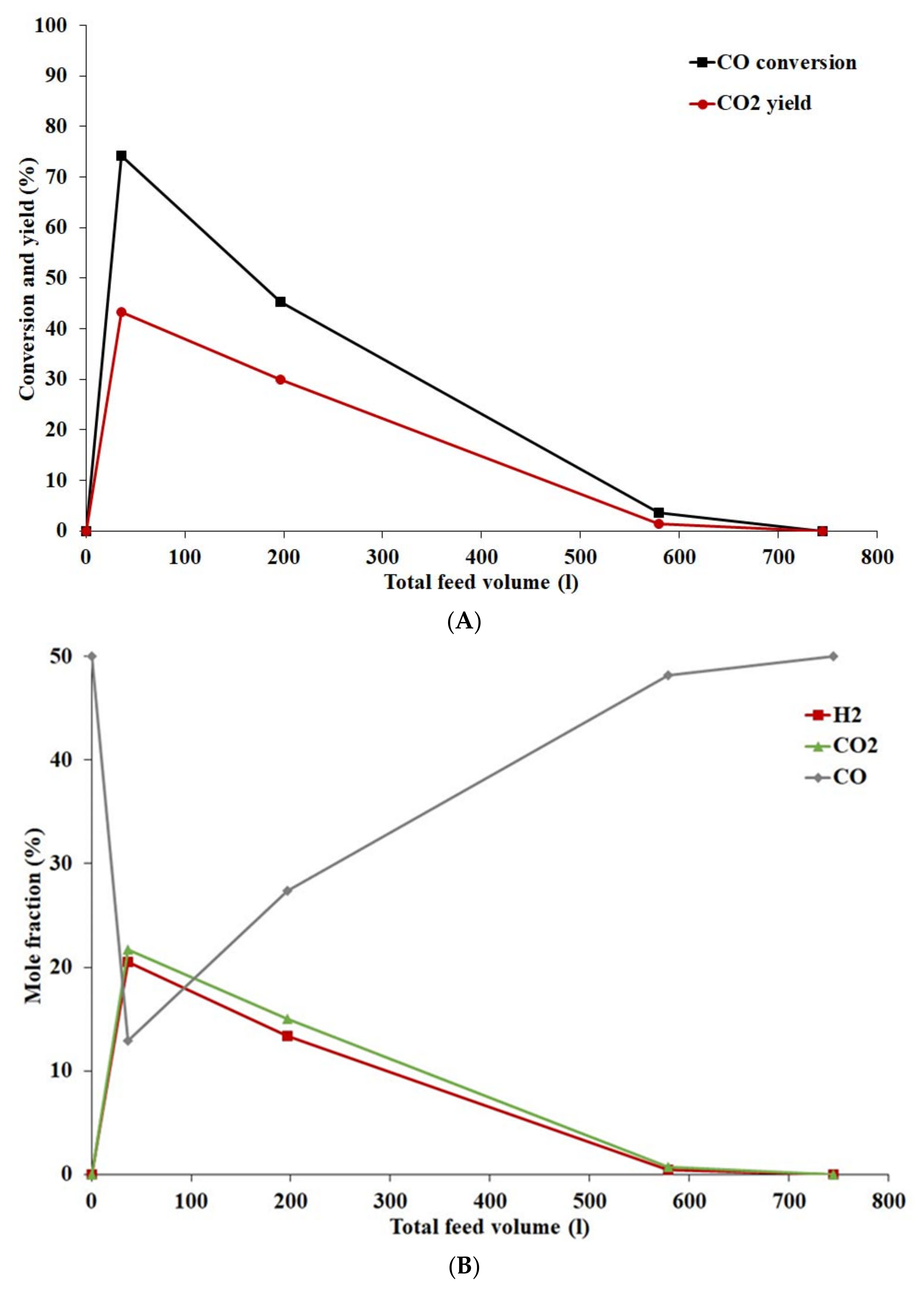
4.3.3. Effect of Hydrogen Sulphide on Catalyst Activity
4.3.4. Effect of Feed Composition
4.4. Comparison of Tested Catalyst
5. Conclusions
- −
- During WGSR catalytic tests at pressures 2, 4, and 6 MPa, the Ni-base catalyst behaved in a similar way. More than 95% carbon monoxide conversion was reached in the temperature range of 220–480 °C.
- -
- The use of calcined support (Al2O3) at 500 °C and activation in a hydrogen atmosphere had a positive effect on the performance of Cu-based catalysts prepared in the laboratory.
- −
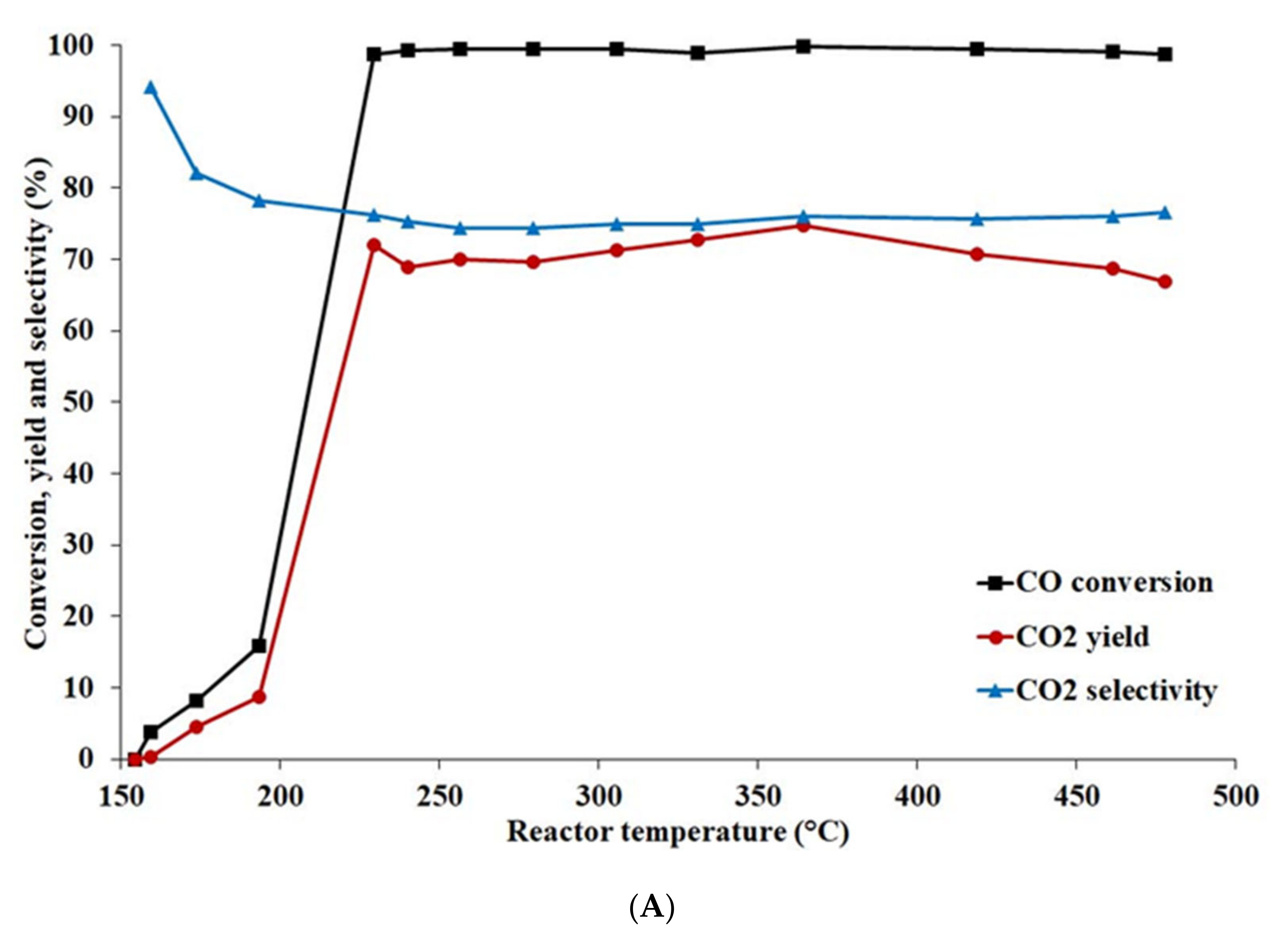
- The activated catalyst prepared with calcined support (Cu content 14.8 wt%) exhibited the best activity in the temperature range of 250–400 °C and pressure 0.5 MPa, achieving the highest CO conversion, CO2 yield, and H2 content.
- −
- A comparison of the catalytic activity of Cu-based catalysts was performed at pressures 0.5 and 6 MPa. The highest CO conversion and CO2 yield of 88.7 and 54.9%, respectively, were reached at pressure 6 MPa and temperature 284 °C.
- −
- The hydrogen content was the highest (26.6 mole%) at temperature 360 °C and pressure 0.5 MPa.
- −
- No hydrocarbons were present when carrying out the WGSR at pressure 0.5 MPa, while at pressure 6 MPa methane (up to 0.12% at 449 °C) and ethane were found at temperatures above 308 °C.
- −
- A lower flow rate positively influences catalytic activity since it increases the residence time of the reactant species, leading to better conversion.
- −
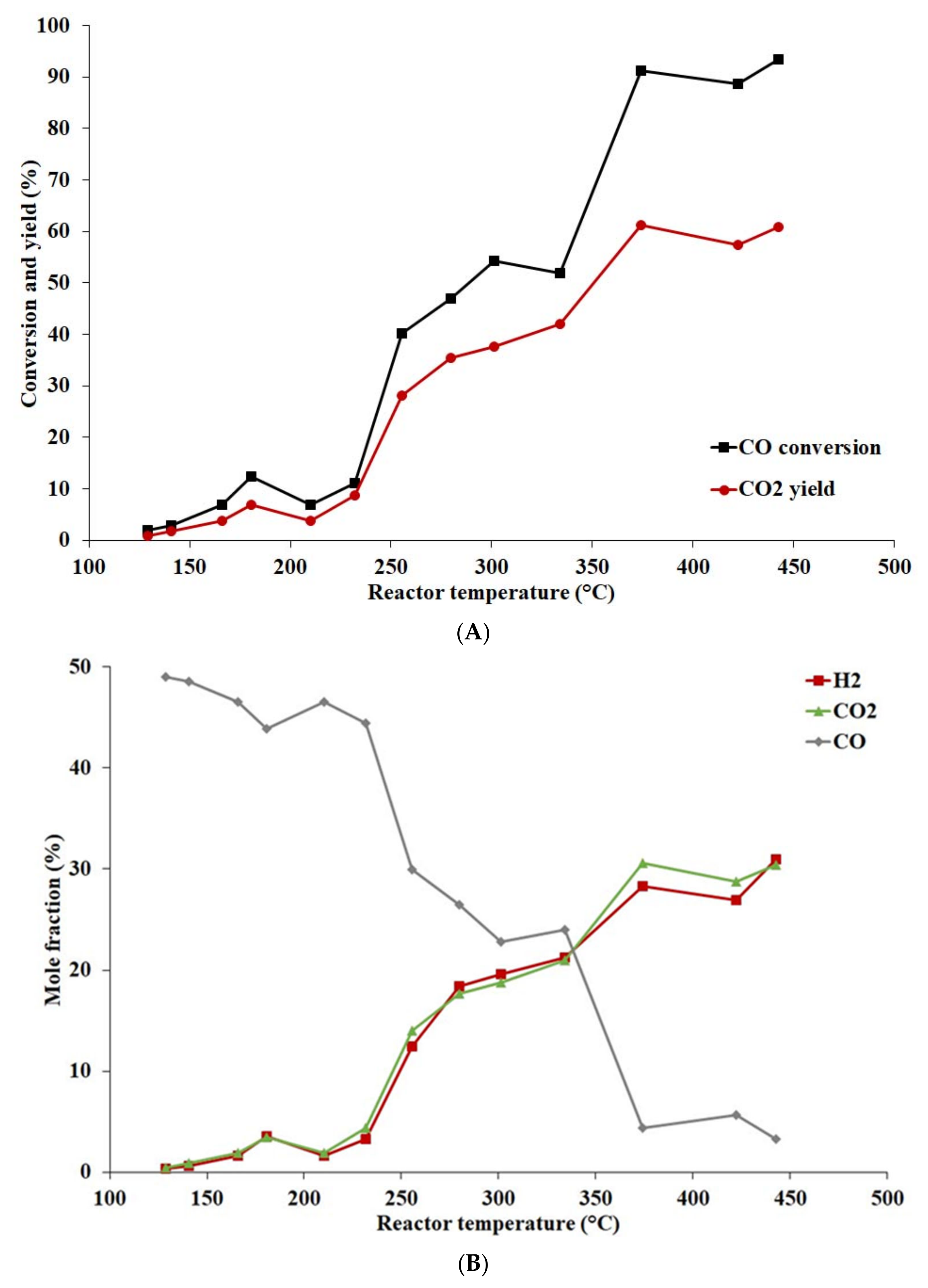
- The WGSR reaction over a laboratory-prepared Co-based catalyst (9.6 wt%) started to run at temperatures around 230 °C.
- −
- The catalyst exhibited a stable increase in activity in the temperature range of 200–450 °C.
- −
- At 450 °C, the highest CO conversion of 66.5% was reached. Due to construction limitations, the reaction could not be carried out at higher temperatures.
- −
- Selectivity towards CO2 formation was at its highest, over 99%, in the lower temperature range. With increasing temperature, the catalyst started to become more active, producing more CO2 but also forming methane, thus causing a decrease in selectivity of CO2.
- −
- At 450 °C, the highest hydrogen and methane content of 13.1 and 3.2 mole%, respectively, was achieved.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smirniotis, P.G.; Gunugunuri, K.R. Water Gas Shift Reaction: Research Developments and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Chen, E. History. In Fuel Cell Technology Handbook, 1st ed.; Hoogers, G., Ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Newsome, D.S. The Water-Gas Shift Reaction. Catal. Rev. 1980, 21, 275–318. [Google Scholar] [CrossRef]
- Mendes, D.; Mendes, A.; Madeira, L.M.; Iulianelli, A.; Sousa, J.M.; Basile, A. The water-gas shift reaction: From conventional catalytic systems to Pd-based membrane reactors—A review. Asia-Pac. J. Chem. Eng. 2010, 5, 111–137. [Google Scholar] [CrossRef]
- Alamolhoda, S.; Vitale, G.; Hassan, A.; Nassar, N.N.; Almao, P.P. Synergetic effects of cerium and nickel in Ce-Ni-MFI catalysts on low-temperature water-gas shift reaction. Fuel 2019, 237, 361–372. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Chen, W.H.; Jheng, J.G. Characterization of water gas shift reaction in association with carbon dioxide sequestration. J. Power Sources 2007, 172, 368–375. [Google Scholar] [CrossRef]
- Grenoble, D.C.; Estadt, M.M.; Ollis, D.F. The chemistry and catalysis of the water gas shift reaction: 1. The kinetics over supported metal catalysts. J. Catal. 1981, 67, 90–102. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotný, K.; Hlinčík, T. The water gas shift reaction: Catalysts and reaction mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Fuentes, E.M.; da Costa Faro Júnior, A.; de Freitas Silva, T.; Assaf, J.M.; do Carmo Rangel, M. A comparison between copper and nickel-based catalysts obtained from hydrotalcite-like precursors for WGSR. Catal. Today 2011, 171, 290–296. [Google Scholar] [CrossRef]
- Han, N.; Race, M.; Zhang, W.; Marotta, R.; Zhang, C.; Bokhari, A.; Klemeš, J.J. Perovskite and related oxide based electrodes for water splitting. J. Clean. Prod. 2021, 318, 128544. [Google Scholar] [CrossRef]
- Han, N.; Yao, Z.; Ye, H.; Zhang, C.; Liang, P.; Sun, H.; Wang, S.; Liu, S. Efficient removal of organic pollutants by ceramic hollow fibre supported composite catalyst. Sustain. Mater. Technol. 2019, 20, e00108. [Google Scholar] [CrossRef]
- Han, N.; Wang, S.; Yao, Z.; Zhang, W.; Zhang, X.; Zeng, L.; Chen, R. Superior three-dimensional perovskite catalyst for catalytic oxidation. EcoMat 2020, 2, e12044. [Google Scholar] [CrossRef]
- Mellor, J.R.; Copperthwaite, R.G.; Coville, N.J. The selective influence of sulfur on the performance of novel cobalt-based water-gas shift catalysts. Appl. Catal. A 1997, 164, 69–79. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Copperthwaitet, R.G.; Gottschalk, F.M.; Hunter, R.; Mellor, J.; Orchard, S.W.; Sangiorgio, T. A comparative evaluation of cobalt chromium oxide, cobalt manganese oxide, and copper manganese oxide as catalysts for the water-gas shift reaction. J. Catal. 1992, 137, 408–422. [Google Scholar] [CrossRef]
- Copperthwaite, R.G.; Gottschalk, F.M.; Sangiorgio, T.; Hutchings, G.J. Cobalt Chromium Oxide: A Novel Sulphur Tolerant Water-Gas Shift Catalyst. Appl. Catal. 1990, 63, L11–L16. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Sintering kinetics of supported metals: Perspectives from a generalized power law approach. Stud. Surf. Sci. Catal. 1994, 88, 1–18. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Hou, P.; Meeker, D.; Wise, H. Kinetic studies with a sulfur-tolerant water gas shift catalyst. J. Catal. 1983, 80, 280–285. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51(3), 325–440. [Google Scholar] [CrossRef]
- Maciel, C.G.; Silva, T.F.; Assaf, E.M.; Assaf, J.M. Hydrogen production and purification from the water–gas shift reaction on CuO/CeO2–TiO2 catalysts. Appl. Energy 2013, 112, 52–59. [Google Scholar] [CrossRef]
- Aliabadi, H.Z. Thermodynamic modeling of the high temperature shift converter reactor using minimization of Gibbs free energy. Int. J. Chem. Mol. Eng. 2009, 49, 189–193. [Google Scholar] [CrossRef]
- Babita, K.; Sridhar, S.; Raghavan, K.V. Membrane reactors for fuel cell quality hydrogen through WGSR—Review of their status, challenges and opportunities. Int. J. Hydrogen Energy 2011, 36, 6671–6688. [Google Scholar] [CrossRef]
- Popa, T.; Guoqing, X.; Barton, T.F.; Argyle, M.D. High temperature water gas shift catalysts with alumina. Appl. Catal. A 2010, 379, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Ginés, M.J.L.; Amadeo, N.; Laborde, M.; Apesteguia, C.R. Activity and structure-sensitivity of the water-gas shift reaction over CuZnAl mixed oxide catalysts. Appl. Catal. A 1995, 131, 283–296. [Google Scholar] [CrossRef]
- Twigg, M.V.; Spencer, M.S. Deactivation of supported copper metal catalysts for hydrogenation reactions. Appl. Catal. A 2001, 212, 161–174. [Google Scholar] [CrossRef]
- Batista, M.S.; Santos, R.K.; Assaf, E.M.; Assaf, J.M.; Ticianelli, E.A. High efficiency steam reforming of ethanol by cobalt-based catalysts. J. Power Sources 2004, 134, 27–32. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Fu, W.; Bao, Z.; Zhang, J.; Ding, W.; Chou, K.; Li, Q. The water-gas shift reaction for hydrogen production from coke oven gas over Cu/ZnO/Al2O3 catalyst. Catal. Today 2016, 263, 46–51. [Google Scholar] [CrossRef]
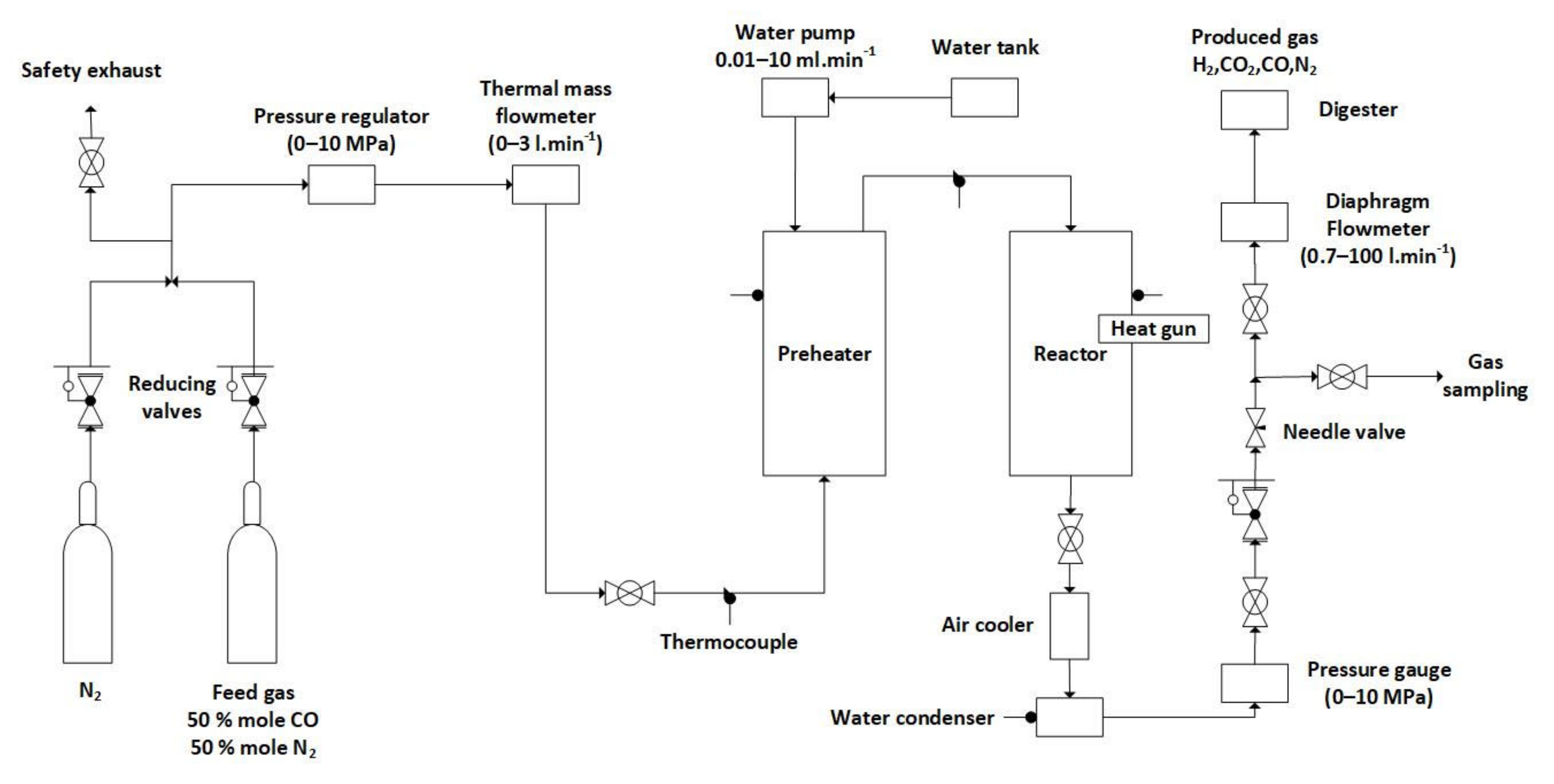



















| Compound | Model Gas Mixture 1 (mole%) | Model Gas Mixture 2 (mole%) | Model Gas Mixture 3 (mole%) |
|---|---|---|---|
| CO | 50.0 | 50.0 | 50.0 |
| CO2 | - | - | 8 |
| H2 | - | - | 34.0 |
| H2S | - | 0.4 | 0.4 |
| N2 | balance | balance | balance |
| Parameter | Value |
|---|---|
| Catalyst shape | Pellet |
| Catalyst dimensions [mm]: height × diameter | 3.8 × 4.0 |
| Ni content [wt%] | 72.17 |
| Si content [wt%] | 13.89 |
| Mg content [wt%] | 11.94 |
| BET surface area [m2·g−1] | 195.24 |
| Total pore volume [mL·g−1] | 0.326 |
| Pores below 6 nm [%] | 33.55 |
| Calcination (°C) | None (1) | 400 (2) | 500 (3) | 600 (4) | 700 (5) | 800 (6) |
|---|---|---|---|---|---|---|
| γ-Al2O3 (wt%) | 55 | 83 | 100 | 100 | 100 | 100 |
| AlO(OH) (wt%) | 45 | 17 | 0 | 0 | 0 | 0 |
| Parameter | Value |
|---|---|
| Catalyst shape | Cylinder |
| Catalyst dimensions [mm]: height × diameter; internal diameter | 6.2 × 4.9; 1.9 |
| Cu content [wt%] | 11.4 |
| Al content [wt%] | 47.5 |
| BET surface area [m2/g] | 215.4 |
| Total pore volume [mL/g] | 0.403 |
| Pores below 6 nm [%] | 64.88 |
| Parameter | Value |
|---|---|
| Catalyst shape | Cylinder |
| Catalyst dimensions [mm]: height × diameter; internal diameter | 6.2 × 4.9; 1.9 |
| Cu content [wt%] | 14.8 |
| Al content [wt%] | 49.0 |
| BET surface area [m2/g] | 198.07 |
| Total pore volume [mL/g] | 0.407 |
| Pores below 6 nm [%] | 48.62 |
| Parameter | Value |
|---|---|
| Catalyst shape | Cylinder |
| Catalyst dimensions [mm]: height × diameter; internal diameter | 6.2 × 4.9; 1.9 |
| Cu content [wt%] | 8.1 |
| Al content [wt%] | 47.8 |
| BET surface area [m2/g] | 213.6 |
| Total pore volume [mL/g] | 0.417 |
| Pores below 6 nm [%] | 55.13 |
| Catalyst | Metal Crystallite Size (nm, XRD) | Alumina Crystallite Size (nm, XRD) |
|---|---|---|
| Ni/γ-Al2O3 | 8 | 5 |
| Cu/γ-Al2O3 | 37 | 5 |
| Co/γ-Al2O3 | 20 | 5 |
| Parameter | Value |
|---|---|
| Catalyst shape | Cylinder |
| Catalyst dimensions [mm]: height × diameter; internal diameter | 6.2 × 4.9; 1.9 |
| Co content [wt%] | 9.6 |
| Al content [wt%] | 44.5 |
| BET surface area [m2/g] | 205.1 |
| Total pore volume [mL/g] | 0.403 |
| Pores below 6 nm [%] | 54.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraj, E.; Ciahotný, K.; Hlinčík, T. Advanced Catalysts for the Water Gas Shift Reaction. Crystals 2022, 12, 509. https://doi.org/10.3390/cryst12040509
Baraj E, Ciahotný K, Hlinčík T. Advanced Catalysts for the Water Gas Shift Reaction. Crystals. 2022; 12(4):509. https://doi.org/10.3390/cryst12040509
Chicago/Turabian StyleBaraj, Erlisa, Karel Ciahotný, and Tomáš Hlinčík. 2022. "Advanced Catalysts for the Water Gas Shift Reaction" Crystals 12, no. 4: 509. https://doi.org/10.3390/cryst12040509
APA StyleBaraj, E., Ciahotný, K., & Hlinčík, T. (2022). Advanced Catalysts for the Water Gas Shift Reaction. Crystals, 12(4), 509. https://doi.org/10.3390/cryst12040509






