Abstract
(1) Background: In time, stone monuments suffer a process of aging and loss of aesthetic and mechanical properties. In order to restore and stop the loss of their properties, various treatments are used, and in this context, a new class of discovered materials with interesting properties are layered double hydroxides, or LDHs. (2) Methods: The LDHs, prepared by a coprecipitation method, were characterized by the structure by X-ray diffraction, composition by FT-IR spectroscopy and X-ray fluorescence spectroscopy, size by diffuse light scattering, and porosity by N2 adsorption/desorption. Additionally, some microscopy techniques such as optical microscopy and SEM/EDAX were used for surface aspects and morphology, and finally, all these were checked with ImageJ software for representative roughness parameters of the treated surfaces by brushing or incorporation. (3) Results: The prepared materials show different degrees of crystallinity and textural properties, and the dispersion of the material presents good stability in time in water/ethanol mixtures. Treatment with the LDH dispersion applied by brushing led to improvements in the mechanical properties (about a 5% increase in compressive strength), to an increased surface stability (about 30%), and to an improvement in the resistance to freeze–thaw cycles. The textural properties of the specimens’ materials were not altered by these treatments. (4) Conclusions: The order of the consolidation efficacity was CaMgAl-LDH > MgAl-LDH > CaAl-LDH, better for application by brushing than by incorporation.
1. Introduction
During the last decades, the new materials in the conservation of cultural heritage have led to some important results, especially in the synthesis part of some consolidants and protective coatings achieved by them. The macro- and/or nanoparticles based on calcium/magnesium/barium in different alcoholic solvents have been used as consolidants, as has been previously published [1,2,3,4].
Prolonged exposure to natural elements leads to the aging and degradation of old stone constructions [5]. The stone supports many damaging processes, mainly due to the transport of water in pores, environmental pollutants (SOx, NOx, CO2), the crystallization of salts, freeze–thaw cycles, and biodeterioration. These processes cause irreversible defects such as erosion, flaking, encrustation, and granular disintegration, which finally lead to the loss of material and mechanical properties [6,7]. To preserve and restore these buildings, different treatments are applied to improve the stone cohesion and improve the resistance to further weathering. However, these treatments must be compatible with the building material, must have long time durability, and must not alter the original properties characteristic of the materials [8,9,10].
Masonry is made up of two components: masonry units and mortar. The structural stability of the masonry depends on a proper connection between the masonry unit and the mortar. Masonry walls are constructed using a variety of masonry pieces and mortars. Among the masonry components are burned clay bricks, concrete blocks, autoclaved bricks, and stones, among others.
Nowadays, a considerable number of consolidants are commercially available for the various types of stone. However, there are some areas (e.g., limestone consolidation) where novel consolidants are needed in order to overcome the limitations of those available: they can alter the stone properties and promote microbial growth (consolidants with organic nature such as acrylic, epoxy, and silicone resins), and can possess a low chemical compatibility with calcareous substrates. Meanwhile, the alkoxysilane compounds such as methyltrimethoxysilane (MTMOS) and tetraethyl orthosilicate (TEOS)) could be inefficient consolidants (limewater, lime milk) [11,12].
A group of materials with interesting properties are hydrotalcite-like anionic clays or LDHs. LDHs are known as synthetic or natural crystalline materials with positively charged two-dimensional sheets and water with exchangeable charge-compensating anions located in the interlayer region. Their general formula is: [M2+1−xM3+x (OH)2]x+[An−x/n] ·mH2O, where M2+ and M3+ represent divalent and trivalent cations, A is the interlayer charged anion, n represents the charge, x represents the trivalent cations (x is in the range of 0.20–0.50), and m is the number of water molecules of crystallization. The magnesium–aluminum hydroxycarbonate represents the mineral hydrotalcite, knowing that the class of hydrotalcites comprise different isostructural and polytype forms [13].
LDH minerals are known as hydrotalcite-like clay [14,15]. Hydrotalcite crystals [Mg6Al2(CO3) (OH)16 4(H2O)] are formed when Al3+ ions from Al2O3 combine with Mg2+ ions from MgO [16]. In the presence of water, the MgO-Al2O3 rehydrates and could combine with other different anions to form hydrotalcite [17,18]. The positively charged brucite-like layers alternate with anions from layers and water molecules in a hydrotalcite mineral. Some Mg2+ cations in octahedral sites of hydrotalcite are substituted by Al3+ cations in the crystallographic structure. CO32− anions in the interlayer sites could be involved, balancing the resultant positive charge [13,19,20].
LDH phases have been identified in ancient hydraulic mortars, or even in modern pozzolanic cements or dolomitic lime mortars [21,22,23,24]. However, the form and the size distribution of hydrotalcite and hydrocalumite in the lime have not been studied until now.
Depending on the precursors, preparation method, or thermal treatment, materials with flexible composition, uniform distribution of metallic cations, high chemical stability, controllable particle size, and varied functionality can be prepared [25]. Furthermore, literature studies showed that both pure LDH and LDH-like materials (materials in which organic compounds are fixed in the interlayer space of LDH) possess bacteriostatic properties [26,27,28,29,30] and an ability to absorb undesirable anions from a structure via anion exchange or memory effect, that allow the calcined LDH reconstruction by absorbing water and anions (chloride, sulphate, phosphate, etc.) from the environment [31,32,33]; all these anions are responsible for the building materials degradation process [34,35,36,37]. Their properties are of great interest in the conservation and restoration of cultural heritage monuments.
Regarding the use of LDHs in the field of building materials, the literature presents few examples. Mg-Al-LDH has been studied as an additive for concrete for the removal or stabilization of undesirable anions [20,38,39], or as hardening accelerators for concrete Ca-Al-LDH [36]. To our knowledge, no studies have been made regarding the influence of addition of treatments based on LDH for gypsum-based mortars.
In this work, a few layered double hydroxides with the following compositions: [Mg0.75Al0.25(OH)2](Cl)0.25, [Mg0.375Ca0.375Al0.25(OH)2](Cl)0.25, and [Ca0.70Al0.3(OH)2](Cl)0.30, were prepared, characterized, and used as dispersion or incorporated into the material slurry for the treatment of some specimens as models for the building materials.
Due to the materials’ decay and environmental influences, maintaining and consolidation masonry buildings has become necessary over time [40,41]. The proper use of traditional materials is required for proper maintenance practices, not only to ensure the performance of the re-built components, but also to protect the well-functioning parts. In this case, the masonry repair method should take into account the initial material’s likeness to the retrofitting material. The traditional manufacturing practices and traditional construction methods employing lime were disrupted, causing significant challenges in studying such materials. Furthermore, due to its fragility and low residual mechanical qualities, lime is a less desirable material than others.
The basic data for the manufacture of brick and lime mortar, as required for the renovation and reinforcement of cultural properties, have already been published [42,43], reporting the good properties of the hydrated lime for use in restoring traditional cultural heritage monuments and historic building preservation. Due to its increased longevity and strength, cement mortar has recently been adopted as a substitute for lime mortar in the modernization of masonry cultural heritage monuments [44,45,46,47]. The mechanical incompatibility of the existing binder and the new reinforcing material, according to Mosquera, M.J. [48], resulted in the concentration of stresses and cracks in the weaker area of the masonry construction. As a result, a restoration material compatible with the original substrate should be utilized to restore masonry structures, particularly those built using lime mortar as a binder material [49,50].
Because of its quick hardening process, natural hydraulic lime (NHL) is mostly employed to conserve cultural assets [51,52].
In this study, a traditional mortar based on traditional hydrated lime was used to compare and evaluate the properties. The findings of this research can be utilized as a starting point for the production of bricks and lime mortar, which are required for the rehabilitation and strengthening of cultural properties.
2. Materials and Methods
2.1. Consolidant Preparation
The preparation of the LDH with following structures: [Mg0.75Al0.25(OH)2](Cl)0.25, [Mg0.375Ca0.375Al0.25(OH)2](Cl)0.25, and [Ca0.70Al0.3(OH)2](Cl)0.30 was achieved by the coprecipitation of the corresponding metallic (chloride) salts, with aqueous solution NaOH 2M at a constant pH (pH = 10), (pH = 12 for Ca-Mg-Al-LDH). The method used was the coprecipitation at low supersaturation and constant pH. The exact quantities of metallic salts were determined so that the ratio between the total number of bivalent and trivalent metallic cations was equal to 3 in the final materials (CaMgAl-LDH and MgAl-LDH); exception was the case of CaAl-LDH where a ratio of 2.3 was used. For the preparation of CaAl-LDH: 17.94 g CaCl2 anhydrous and 16.69 g AlCl3 * 6 H2O (metallic cations salts were dissolved together in 200 mL distilled water). The solution of the metallic salts was added dropwise in a 2000 mL beaker simultaneously with 300 mL NaOH 2M solution at room temperature under vigorous stirring and the pH was adjusted so that the pH in the precipitation beaker was 12. For the preparation of MgAl-LDH, the following quantities of reactants were used: 35.42 g MgCl2 ∙ 6 H2O and 14.01 g AlCl3 ∙ 6 H2O (metallic cation salts were dissolved together in 200 mL distilled water). After that, the solution of the metallic salts was added dropwise in a 2000 mL beaker simultaneously with 300 mL NaOH 2M solution at room temperature under vigorous stirring and keeping the pH at 10. For the preparation of MgCaAl-LDH: 15.61 g MgCl2 ∙ 6 H2O; 8.53 g CaCl2 anh.; 12.38 g AlCl3 ∙ 6 H2O (metallic cation salts were dissolved together in 200 mL distilled water) and finally, the solution of the metallic salts was added dropwise in a 2000 mL beaker simultaneously with 275 mL NaOH 2M solution at room temperature under vigorous stirring and pH 10. For all the synthesis, the entire volume of metallic salts (200 mL) was added dropwise in the beaker together with the solution of NaOH 2M, the rate at which we dropped the NaOH solution was adjusted so that the pH in the precipitation beaker was maintained as close to 10 as possible (12 in the case of CaAl-LDH) for the whole duration of the process.
In all cases, the formed precipitates were left overnight in their mother liquor at 80 °C with stirring, then cooled to room temperature, separated from their suspensions by filtration and finally washed with distilled water at a neutral pH. Finally, the materials obtained were dried in air at 80 °C overnight.
2.2. Specimens Preparation
Cubic specimens (4 × 4 × 4 cm) were prepared in the laboratory using a silicone resin matrix. The samples were prepared from river sand (granulation 0–2 mm), which is a silica sand, with a broad granulometric variation and diversity of shapes and colors of its components (Table 1). The particle size of fine river sand was measured using a laser analyzer (Malvern Mastersizer 3000 laser diffractometer equipped with a He-Ne—632.8 nm wavelength and LED blue light—470 nm wavelength, adequate for particle sizes from 0.01 to 3500 μm (Malvern Instruments, Malvern, UK)). For the specimen preparation, silica sand, commercial gypsum, and water were mixed in a ratio of 2:1:0.75 g/g, after literature reports [53]. After mixing, the sand was poured in the silicone resin matrix and kept at dryness under controlled conditions (T = 20 °C, 65% RH) for 1 week [54].

Table 1.
The size distribution of the sand particles.
2.3. Application of the LDH Derivatives
These prepared specimens above were treated with the newly prepared LDHs. The LDH consolidants at a concentration of 0.5 g/L were dispersed in distilled water, ultrasonicated or vigorously stirred for one hour at 40 °C and were applied for three times by brushing (at 6 h intervals), this method of application being adopted in the practice of preservation. The treated samples were then stored at 65% RH, T = 20 °C, air velocity < 0.1 m/s for over 1 week, this treatment being necessary for a good efficacity of LDH nanoparticles. This technique was selected for a good penetration of the consolidant into the stone specimens. In parallel, the consolidants were incorporated into samples at concentrations of 5%, and the specimens’ behavior was compared with the results obtained when applying LDH consolidants by brushing.
2.4. Methods
Structural X-ray diffraction (XRD) investigations of the prepared materials were recorded with a Rigaku X-ray diffractometer with Cu-Ka radiation wavelength (λ = 0.15406 nm) operating at 40 kV and 30 mA. XRD measurements were recorded in the angular range 5–80° 2θ at a scan rate of 2° min−1. Silicon powder (NIST SRM 640) was used as an external standard and the experimental XRD patterns were analyzed using whole powder pattern fitting method (WPPF) on the PDXL software 1.8 (Rigaku) connected to the ICDD PDF-2 database. The Rietveld method for the whole powder pattern fitting (WPPF) was used to refine crystal structure parameters (atom coordinates, temperature factors, etc.) based on the diffraction pattern.
For elemental analysis of samples, wavelength dispersive X-ray fluorescence spectroscopy (WDXRF) was used, with a Rigaku ZSX Primus II spectrometer equipped with an X-ray tube with Rh anode, power 4.0 kW, with front window Be (30 μm thick). The samples were pelleted under vacuum.
Fourier-transform infrared (FT-IR) spectra of the investigated samples were obtained using a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) measuring 400 and 4000 cm−1. The spectra were recorded using the KBr pellet technique, at a resolution of 4 cm−1.
Particle size was determined by Dynamic Light Diffusion (DLS) using Zetasizer Nano ZS, Malvern Instruments Ltd., Malvern, UK, by using colloidal dispersions of LDH samples in isopropyl alcohol and the results being performed following the intensity distribution. All measurements were performed in triplicate.
For a kinetic stability of the consolidants’ dispersions of LDHs in different solvents, a turbidimetric analysis with a UV-VIS spectrophotometer (T60, PG Instruments Ltd., Lutterworth, UK) was used. The absorbance change measurements in time, over 300 min, were achieved at λ = 630 nm at 25 °C. Glass cuvettes with an optical path of 1 cm were used. The relative kinetic stability (KS) of the suspension was calculated by using Formula (1) [55]:
where A0 represents the absorbance at 0 min, At is the absorbance at time t. The consolidation effect for the prepared materials was assessed in two ways:
KS% = 1 − [(A0 − At)/A0] × 100
- (a)
- by applying the dispersion on the specimen samples by brushing, 3 times on every side.
- (b)
- by incorporating 5% (w/w) of every consolidant into specimen samples.
For all the samples, the color, mechanical strength, and porosity versus the control samples were studied.
Konica Minolta-Chroma Meter CR-410 colorimeter (Tokyo, Japan.) was used for measuring the chromatic parameters of the treated sample. The total color differences ΔEx, the difference in lightness (ΔLx = Ltreated stone – Lcontrol), the chromatic deviation of the a coordinates (red and green color) (Δax = atreated stone – acontrol), and the chromatic deviation of the b coordinates (yellow and blue color) (Δbx = btreated stone – bcontrol) were measured and the correct calculus was evaluated [56].
∆Ex = (∆Lx*2 + ∆ax*2 + ∆bx*2)1/2
A positive ∆Lx* value indicates that the sample is whiter than the control, while a negative ∆L* value indicates that the sample is darker [57].
For the mechanical properties (compressive strength) a Proceq-SilverSchmidt Concrete Test Hammer Type L (Zurich, Switzerland) as portable device was used. The uniaxial compressive strength (UCS) was obtained through the rebound number, according to J. Brozovsky [58] and EN 1015-10 [59], considering for the stone density a value of 1.504 g/m3.
The peeling test was used to evaluate the consolidant cohesion on the specimens’ surface, after the Drdácký et al. method [60], with additional steps mentioned in Scrivano et al. [61], by using Scotch Cristal tape (3M) with 10 repetitions on the same location. After application with constant pressure (2 kgf/cm2), the tape was removed at a rate of about 10 mm/s and at an angle of 90°. The samples were weighed. The percentage of consolidation (%C) was calculated according to Equation (3):
where: TRMuntreated represents the total amount of material removed by peeling in the untreated sample, g and TRMtreated are the total amount of material removed in the treated sample, g.
Water absorption capacity and the apparent porosity were determined after STAS 6200/12-73 and STAS 6200/12-80. For the water absorption test, the samples supported alternative treatment cycles, as: dried in the oven at 40 °C for 8 h, cooled at room temperature and then weighed (W1), immersed in distilled water for 24 h and removed from distilled water and weighed (W2). The water absorption content was calculated with Equation (4).
The total specific area, total pore volume, and pore size distribution curves of the substrates were evaluated by means of BET (Brunauer–Emmet–Teller), t-plot analyses, and the BJH (Barret–Joyner–Halenda) methods.
Freeze thaw cycles were performed according to EN 12371:2010 [62]. The treated and the blank specimens, dried to constant mass at 105 ± 5 degrees (M1), were introduced in distilled water, wiped with a cloth and weighed (M2) and exposed to 30 cycles of freezing–thawing. After a while, the samples were introduced in the freezer at −20 °C for 3 h, at a distance of 10–20 cm from each other, after which the cycle repeated. The thawed samples were weighed after the last cycle (M3). The following formula was used (5):
where: μg is the freezing coefficient.
%μg = (M2 − M3/M1) × 100
For optical microscopy, a Novex trinocular microscope (EUROMEX Microscopen B.V. Arnhem, Netherlands) (at different magnifications: 40×, 100×, 400×, 1000×), in transmitted light, was used. The microscope was equipped with a digital video camera (ZEISS, AxioCam 105, Göttingen, Germany) and the images were processed using the Java 1.6, ImageJ 1.50a (free software).
The scanning electron microscopy (SEM) was achieved with a Philips XL 30 ESEM TMP microscope (Hillsboro, OR, USA) coupled with an EDAX Sapphire spectrometer, necessary for the energy X-ray dispersive spectroscopy (EDS) measurements. The main used parameters were: acceleration voltage of 25 kV and working distance of 10 mm for five randomly chosen areas. ImageJ software (National Institutes of Health, Bethesda, Rockville, MD, USA) served for processing the obtained SEM micrographs.
3. Results and Discussion
3.1. Crystal Structure
The XRD analysis of the prepared hydrotalcites (CaAl-LDH, MgAl-LDH, and CaMgAl-LDH) shows some common characteristics of layered materials (narrow, strong, symmetrical lines at low values of 2 theta and weaker, less symmetrical lines at high values of 2 theta) [63,64]. From the analysis of Figure 1 it can be seen that the XRD diagram lines (003) and (006) are significant, and lines (110) and (113) can be distinguished between 60° and 63°. Line (003) is characterized by high intensities and wide line shapes that indicate that hydrotalcites have a good crystallinity, but low crystallites, as shown in Table 2, which also shows the network parameters.
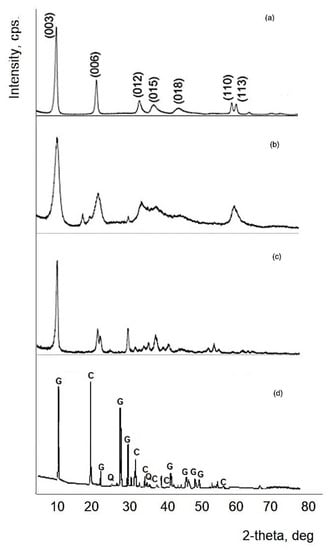
Figure 1.
XRD pattern for the prepared materials (C = calcite, Q = quartz, G = gypsum) (a) CMgAl-LDH; (b) CaMgAl-LDH; (c) CaAl-LDH; (d) specimen.

Table 2.
The XRD parameters for the LDHs.
The diffraction maxima are similar to those previously reported in the literature and correspond to a structure similar to well-crystallized hydrotalcites [65]. The lattice parameters and the crystallite size have been calculated according to Williamson–Hall method.
The XRD patterns of CaAl-LDH present sharp and symmetrical reflections at 11.2°, 22.5°, 23.2°, 31.1°, 39.0°, and 42.7° attributed to diffraction planes (002), (004), (112), (020), (316), and (208). The structure of CaAl-LDH (JCPDS 31-0245) was well crystallized with monoclinic symmetry and chemical formula Ca4Al2O6Cl2·10H2O. The broadening of the (110) reflection around 60° indicated some disorder in the structure, which was directly related to a reduced aggregation of the particles during sonication [66].
The XRD pattern of the MgAl-LDH, corresponds to a pure LDH structure (JCPDS 37-0630) with strong and narrow diffraction peaks at 2θ values, 11.1° and 22.3°, corresponding to the (003) and (006) planes. Additionally, wide asymmetric peaks are seen at higher values of 2θ (34.3°, 38.1°, 45.2°, 60.3°, and 61.5° corresponding to (012), (015), (018), (110) and (113) diffraction planes).
The XRD pattern of each prepared sample was analyzed by the Rietveld method. A less crystallized structure was observed for the CaMgAl-LDH; the additional lines observed at 18.00° and 20.4° correspond to a segregated Al (OH)3 gibbsite phase (JCPDS74-1775). Notably, the reflection peak at around 31° is characteristic of hydrocalumite CaAl-HC (JCPDS 31-0245), most likely all the other peaks are overlapped over those of CaMgAl-LDH.
The influence of calcium or magnesium substitution on the lattice parameters was also studied by analysis the 2θ value, the inter-planar distance (d), and the fullwidth at half-maximum of the peak (β), and the crystallite size (L) of the CaAl-LDH, MgAl-LDH, and CaMdAl-LDH samples was calculated from lines (003) and (006) using Scherrer’s Equation (6). These values are presented in Table 2.
where: L = crystallite size; K = Scherrer constant (0.9); λ = X-ray wavelength (1.54 Å); β = peak width at half maximum intensity (in radians), from the apparatus programme; θ = Bragg angle, from the apparatus programme.
L = K · λ/β · cosθ
The degree of crystallinity was evaluated as the ratio of the intensity of crystalline lines to the sum of the crystalline and amorphous intensities. The mean crystallite size (D) was calculated using Scherrer’s Equation (6) and Williamson–Hall (W-H) method (7), Table 2 and Table 3.

Table 3.
Crystallite size from Scherrer method. FWHM: full width at half maximum.
Williamson–Hall method does not follow a 1/cos θ dependency as in the Scherrer equation but instead varies with tan θ [67].
where D is the mean crystalline size, λ is the X-ray wavelength, β is the full width at half maximum (FWHM), ε is the microstrain and θ is the Bragg angle, K is the shape factor equal to 0.9.
β · cosθ = K · λ/D + 4 · ε · sinθ
By analyzing the specimen XRD diagram by comparison with the hydrotalcites, it could be observed no significant bands of the specimens are present in the hydrotalcites bands, this being proof that hydrotalcites completely cover the specimens’ surface.
FT-IR data of the synthesized LDH, Figure 2, are quite similar to those in the literature [20,21] for layered double hydroxides. The broadband located at 3400–3700 cm−1 correspond to OH stretching vibrations. The presence of water molecules found in the interlamellar region is identified at ~1630 cm−1, as bands attributed to the bending vibration of water (v H-O-H); the small band at 1384 cm−1 is attributed to the antisymmetric stretching mode of the carbonate anion of the interlamellar layer. The presence of this band suggests that part of the compensating anions consist of carbonate—due to the exposure to CO2 from air, this band is slightly more pronounced in the case of MgAl-LDH. The bands present at wavenumbers lower than 1000 cm−1 are assigned to the vibration mode of O-Me-O (~790 cm−1). The spectrum contains the following bands, too: 720 sh (Al-OH), 660 (Mg-OH), 556 (M-O, M-O-M, and O-M-O) [68,69,70,71].
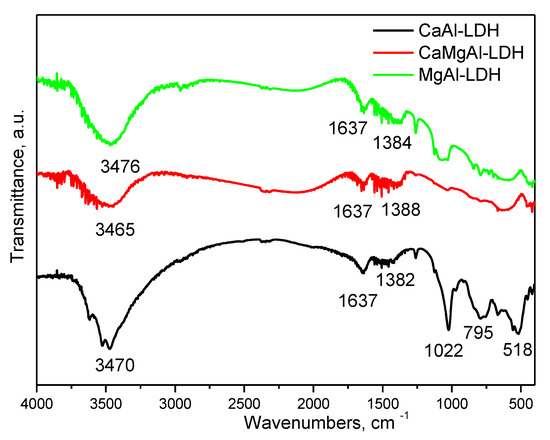
Figure 2.
Fourier-transformed infrared spectroscopy of the prepared samples.
The data obtained from X-ray fluorescence analysis shows that other than metallic cations and oxygen, the sample contains carbon, chlorine, and minor amounts of impurities: sulphur, silica, sodium, and iron. The mass percentage of different elements present in samples and metallic cations molar ratio is represented in Table 4. The amount of carbon in the samples shows that in the presence of chloride, important quantities of CO2 are present in the materials as compensating anions.

Table 4.
XRF analysis of the materials.
Notably, in the case of CaMgAl-LDH, a fraction of the Ca2+ cations were lost, mostly due to re-solubilization of Ca2+ as soluble compounds during preparation.
DLS analysis showed that the particle diameters of the prepared materials are broad, and their average diameter varies between 150 nm for MgAl-LDH to 200 nm for CaMgAl-LDH and 262 nm in the case of CaAl-LHD.
3.2. Kinetic Stability of LDH Dispersions
The kinetic studies on the consolidant stability in water/ethanol mixtures showed the best results for all the consolidants at intermediate ratios of water/ethanol, between 8/2 and 6/4 water: ethanol (vol%), a stability much higher than in pure water or ethanol. The best stability was achieved in the case of CaAl-LDH and it is over 40% after 300 min. However, it is noteworthy that in most cases, after 100–150 min the dispersion stability does not undergo major changes (Figure 3 and Figure 4).
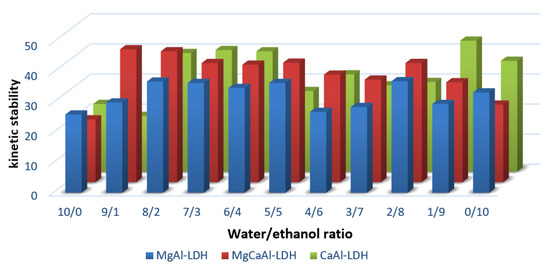
Figure 3.
Kinetic stabilities of the LDH dispersed in solvents after 300 min.
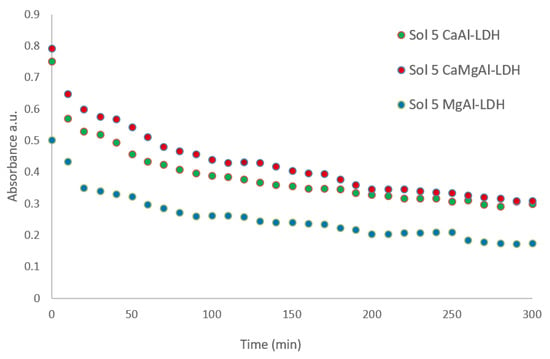
Figure 4.
The kinetic stability of consolidants at water/ethanol ratio of 6/4 (vol).
3.3. Consolidant Testing
3.3.1. Brushed Samples
The results of the treatment with the consolidants’ dispersions are showed in Table 5.

Table 5.
The effect of consolidants applied by brushing over the brick samples’ properties.
The treated specimens showed small (ΔE* < 3) differences in color versus the control specimens, with the highest changes in color (ΔE* = 2.2) being observed for the specimens treated with CaAl-LDH dispersion.
In terms of mechanical properties (compressive strength), the treated samples performed slightly better than the test specimen, with the highest improvement of about 4.7% compared to the untreated sample being achieved after treatment with MgAl-LDH.
The peeling tests showed that the treated specimens’ surface is significantly higher than 30% for MgAl-LDH, which is more stable after treatment with the consolidant dispersion. This, along with the previous data, shows that the treatments have the property to slightly improve the cohesion of the brick material, with the improvement being more pronounced on the surface.
Water absorption capacity and the porosity of the treated specimens showed small differences compared to the control specimens.
It is noteworthy that while applying the dispersion of CaAl-LDH on the brick samples, the apparent porosity decreased, most probably due to the coverage of micropore and micro fissures in the material at the surface. However, exposed to freeze–thaw cycles, the consolidation procedure showed a non-significant effect on the material resistance.
The treatment with CaMgAl-LDH and MgAl-LDH, however, had little impact on apparent porosity and water absorption. Most likely due to the improvement in material cohesion at the surface, the resistance to freeze–thaw cycles for the treated samples improved considerably.
3.3.2. Samples with Incorporated 5% LDH
The incorporation of LDH in the slurry showed a small effect on the final color (ΔE* < 3). It is noteworthy that the brick material and the consolidant powders were both white, with a small difference mostly in terms of the ΔL* coefficient (the consolidant powder was brighter).
The mechanical properties of the brick samples with 5% consolidants decreased compared to the test sample. This decrease in compressive strength could be due to the impeded growth of CaSO4 crystals (gypsum) caused by the presence of LDH in the slurry, thus decreasing the total cohesion of the final material. Referring to the mechanical properties, it could be observed that all the treated samples presented higher compressive strength values for the samples treated by brushing than those treated by incorporation [72]. The addition of the hydrotalcites on the specimen surface enhanced the stone durability in comparison with the non-treated samples, most probably due to the stone reinforcing, through their interaction with the stone grains. The rebound number measurements for those untreated and treated with LDHs applied by brushing or being incorporated into the specimens led to the conclusion.
However, all the other properties are certainly better for brushing applications, so for a proper consolidation, a proper evaluation of the consolidant should be carried out.
The apparent porosity and water absorption capacity of the test specimens increased. The exception are samples containing CaAl-LDH where only a slight difference in pore volume can be observed (Table 6).

Table 6.
Results of adding 5% consolidant powder into the brick composition.
Finally, the consolidants and the samples which were characterized by nitrogen adsorption, the specific surface, average pore size and pore volume are shown in Table 7.

Table 7.
Specific surface, pore volume, and pore diameter for consolidants and treated samples.
The LDH consolidants present type IV adsorption/desorption isotherms, in good agreement with IUPAC, with hysteresis loops characteristic of mesoporous materials (Figure 5a). Their specific surface is moderate and varies between 16.9 m2/g in the case of Ca Al-LDH to 49.7 m2/g in the case of MgAl-LDH, which also presents the highest pore volume in the series of about 0.35 cm3/g.
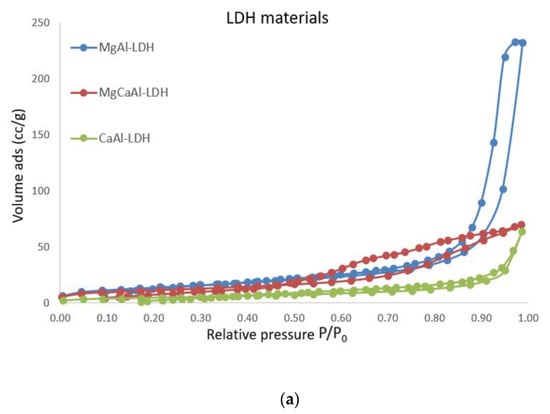
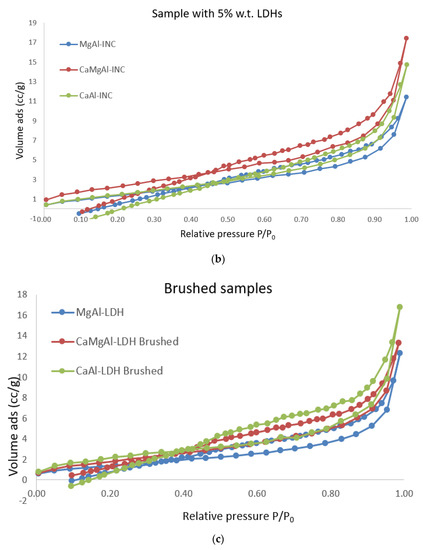
Figure 5.
Nitrogen adsorption isotherms for LDH consolidants and treated materials (a) LDH materials; (b) samples with 5% wt LDH; (c) brushed samples.
The materials of the treated specimens (brushed and with 5% consolidants) also present type IV isotherms according to IUPAC/H4 hysteresis loops characteristic of material particles with internal voids of irregular shape and broad size distribution, Figure 5b,c. Their specific surfaces and pore volumes are relatively small compared to the consolidants. The addition of LDH into the slurry did not significantly increase the pore volumes or pore diameters for the brick material but it clearly altered its textural properties, as can be seen in Table 7.
From the above data it can be observed that the layer obtained by brushing LDHs on model samples led to the following conclusions: the pore volume is quite similar (0.008–0.01 cc/g), and the surface area and pore diameter are different and are strongly dependent on the consolidant type. The application type contributes to the layer homogeneity applied on the stone surface. For the control specimens, these values are not different at all.
3.4. Microscopy Investigations/ImageJ Processing
For these investigations, the control specimens were analyzed first. The microstructural surface features of these samples are suggestively presented in Figure 6, in which the valleys marked with dark blue indicate the pores’ location within the material, and the peaks marked with the white area indicate higher roughness of the material. A higher roughness of the material is observed.
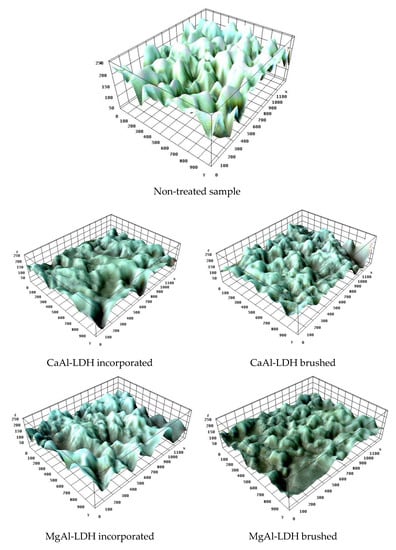
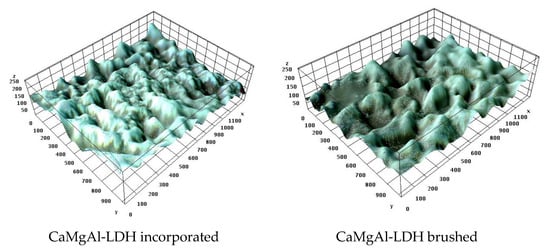
Figure 6.
The images resulted from roughness processing ImageJ software.
The values of the most representative roughness parameters recorded for the model stone samples are presented in Table 6. From the measurements, R-values on the whole surface give roughness values according to the ISO 4287/2000 standard [73]. The main parameters that had to be analyzed were: Ra: Arithmetical mean deviation; Rq: Root mean square deviation; Rku: Kurtosis of the assessed profile; Rsk: Skewness of the assessed profile; all these are given in calibrated units [74]. Their values are shown in Table 8 for all types of samples: control, brushed, and incorporated ones.

Table 8.
Roughness parameters and pore surface area for the treated and control model samples.
The microporous character of the specimen surface and the structure of the internal channels are supported by the values of the roughness parameters, as shown in Table 8, and especially by the parameters Rsk (asymmetry) and Rv (valley depth) which represent peak–valley sequences [75], and are more pronounced for the surface treated with CaMgAl-LDH. However, the Rku (Kurtosis) parameter shows a slightly lower trend associated with smoother peaks and irregular symmetry. Additionally, the stochastic parameters (Rt, Rp) indicate lower profile heights and amplitudes (Ra, Rz) for the samples covered by brushing, which confirms the results of morphological mapping. The arithmetic mean value of the roughness (Ra) is around 0.1 μm, higher for the samples treated by incorporation than by brushing. The values of these parameters do not differ significantly, with the highest values being obtained for the samples treated by brushing with CaMgAl-LDH. In conclusion, the texture generated after treatment is changed, being randomly distributed inside the internal channels and provides a larger active surface, which could be associated with a mechanical consolidation of the treated surface. So, the specimens treated by brushing are highly efficiently consolidated [76].
The analysis of the average surface roughness of the treated samples shows that Ra is practically identical for each type of specimen (embedded and brushed) but decreases significantly, due to the deposition of the hydrotalcite layer on the surface (Table 8), materialized by reducing pore diameters. The consolidants have the capacity to fill the pores and the contacts between the intercrystalline grains become more intimate, suitable for the consolidation of the stone. The analyses reveal that the roughness parameters adequately interpret the contact with the surface and serve to interpret the physical and water properties of the stones.
From the SEM micrograms, it was possible to observe the lamellar particles with a rounded shape, specific to hydrotalcite-type materials, as proof of the fine crystalline morphology, more visible at a smaller magnitude, Figure 7. The elemental composition registered from EDS technique is shown in Table 9.
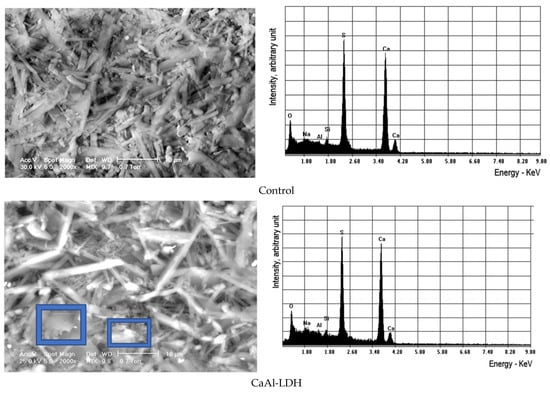
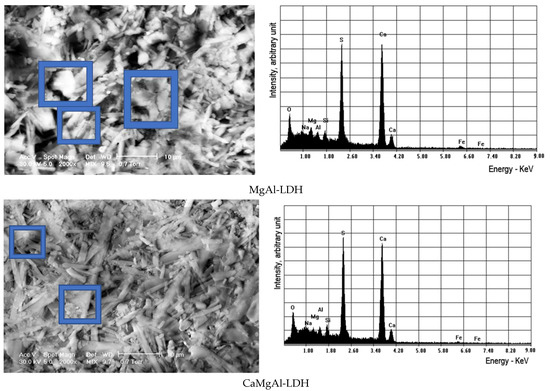
Figure 7.
The SEM images for different magnifications of the samples treated with LDHs.

Table 9.
The EDS results of the investigated samples (C = control sample or specimen).
4. Conclusions
The proposed treatment with LDH compounds showed a good mechanical, structural, and aesthetical compatibility and did not generate a film on the stone surface. Their structure was confirmed by powder X-ray diffraction and their properties were characterized by various techniques. Mechanical properties of the treated sample showed the most notable results (about a 5% increase in compressive strength) after treatment with MgAl-LDH, with the effect being more pronounced at the specimens’ surface, where the peeling tests also showed a notable consolidation effect of 30%. Water absorption and porosity of the test bricks were slightly improved by these treatments; the most notable effects were obtained after treatment with CaAl-LDH, where slight decreases in water absorption can be observed. The freeze–thaw cycles showed that the treatments with MgAl-LDH-containing consolidants yielded the best results, most likely due to the improvement in material cohesion at the surface and in the structure.
By adding the LDH powder into the suspension, slight changes in color were observed. The decrease in material mechanical properties and the increase in water absorption suggest that the addition of the consolidants in the material structure can lead to an increase in the micro-fissures and a decrease in material cohesion, at least for the gypsum mortar specimens. In conclusion, the best consolidant properties were observed for CaMgAl-LDH, followed by MgAl-LDH then CaAl-LDH.
Author Contributions
Conceptualization, R.-M.I. and C.E.R.; methodology, R.-M.I.; software, R.-M.I.; validation, R.-M.I. and C.E.R.; formal analysis, C.E.R.; investigation, R.-M.I., D.A.V., L.P., A.R., I.A., G.V. and F.M.; resources, R.-M.I.; data curation, R.-M.I.; writing—original draft preparation, R.-M.I.; writing—review and editing, R.-M.I.; visualization, R.-M.I.; supervision, R.-M.I.; project administration, R.-M.I.; funding acquisition, R.-M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This paper was supported by the Romanian Ministry of Research, Project 51PCCDI/2018 within PNIII.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baglioni, P.; Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2006, 2, 293–303. [Google Scholar] [CrossRef]
- Salvadori, B.; Dei, L. Synthesis of Ca(OH)2 nanoparticles from diols. Langmuir 2001, 17, 2371–2374. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G.; Quaresima, R. The nanolimes in cultural heritage conservation: Characterization and analysis of the carbonation process. J. Cult. Herit. 2008, 9, 294–301. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage. In A Compedium of Materials and Techniques, 1st ed.; Springer: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ciofini, D.; Mencaglia, A.A.; Siano, S. A photoacoustic pulse-echo probe for monitoring surface stone mechanical properties: Validation tests in consolidation of Carrara marble. Constr. Build. Mater. 2018, 187, 610–619. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Ortiz, R.; Karapanagiotis, I.; Ortiz, P. Comparison of the performance of a novel nanolime doped with ZnO quantum dots with common consolidants for historical carbonate stone buildings. Appl. Clay Sci. 2020, 195, 105732. [Google Scholar] [CrossRef]
- Vasanelli, E.; Calia, A.; Masieri, M.; Baldi, G. Stone consolidation with SiO2 nanoparticles: Effects on a high porosity limestone. Constr. Build. Mater. 2019, 219, 154–163. [Google Scholar] [CrossRef]
- Da Fonseca, B.S.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.F. Artificial aging route for assessing the potential efficacy of consolidation treatments applied to porous carbonate stones. Mater. Des. 2017, 120, 10–21. [Google Scholar] [CrossRef]
- Barriuso, B.C. Conservation of calcareous stone monuments: Screening different diammonium phosphate based formulations for countering phototrophic colonization. J. Cult. Herit. 2017, 27, 97–106. [Google Scholar] [CrossRef]
- Raneri, S.; Barone, G.; Mazzoleni, P.; Alfieri, I.; Bergamonti, L.; De Kock, T.; Cnudde, V.; Lottici, P.P.; Lorenzi, A.; Predieri, A.G.; et al. Multi-scale laboratory routine in the efficacy assessment of conservative products for natural stones. MethodsX 2018, 5, 1095–1101. [Google Scholar] [CrossRef]
- Badreddine, D.; Beck, K.; Brunetaud, X.; Chaaba, A.; Al-Mukhtar, M. Nanolime consolidation of the main building stone of the archaeological site of Volubilis (Morocco). J. Cult. Herit. 2020, 43, 98–107. [Google Scholar] [CrossRef]
- Becerra, J.; Ortiz, P.; Martín, J.M.; Zaderenko, A.P. Nanolimes doped with quantum dots for stone consolidation assessment. Constr. Build. Mater. 2019, 199, 581–593. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite type anionic clays, preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Chapter 13.1 layered double hydroxides. Dev. Clay Sci. 2006, 1, 1021–1095. [Google Scholar]
- Guo, Q.; Reardon, E.J. Calcined dolomite: Alternative to lime for minimizing undesirable element leachability from fly ash. Ind. Eng. Chem. Res. 2012, 51, 9106–9116. [Google Scholar] [CrossRef]
- Miyata, S.; Okada, A. Synthesis of hydrotalcite-like compounds and their physico-chemical properties—The systems Mg2+-Al3+-SO42- and Mg2+-Al3+-CrO42. Clays Clay Miner. 1977, 25, 14–18. [Google Scholar] [CrossRef]
- Miyata, S. Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ion. 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Artioli, G.; Secco, M.; Addis, A.; Bellotto, M. 5 Role of hydrotalcite-type layered double hydroxides in delayed pozzolanic reactions and their bearing on mortar dating. In Cementitious Materials: Composition, Properties, Application; Walter de Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Siedel, H.; Michalski, S.; Ullrich, B. Characterisation of dolomitic lime mortars from the Benedictine monastery in Riesa, Saxony (Germany). In Historic Mortars; Springer: Dordrecht, The Netherlands, 2012; pp. 115–124. [Google Scholar]
- Brandon, C.J.; Hohlfelder, R.L.; Jackson, M.D.; Oleson, J.P. Building for Eternity: The History and Technology of Roman Concrete Engineering in the Sea; Oxbow Books: Oxford, UK; Philadelphia, PA, USA, 2014. [Google Scholar]
- Massazza, F. Pozzolana and pozzolanic cements. In Lea’s Chemistry of Cement and Concrete, 4th ed.; Hewlett, P., Ed.; Elsevier-Butterworth-Heinemann: Oxford, UK, 1998; pp. 471–635. [Google Scholar]
- Zümreoglu-Karan, B.; Ay, A. Layered double hydroxides—Multifunctional nanomaterials. Chem. Pap. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Moaty, S.A.A.; Farghali, A.A.; Khaled, R. Preparation, characterization and antimicrobial applications of Zn-Fe LDH against MRSA’. Mater. Sci. Eng. C 2016, 68, 184–193. [Google Scholar] [CrossRef]
- Wang, X.-R.; Cheng, H.-M.; Gao, X.-W.; Zhou, W.; Li, S.-J.; Cao, X.-L.; Yan, D. Intercalation assembly of kojic acid into Zn-Ti layered double hydroxide with antibacterial and whitening performances. Chin. Chem. Lett. 2019, 30, 919–923. [Google Scholar] [CrossRef]
- Lobo-Sánchez, M.; Nájera-Meléndez, G.; Luna, G.; Segura-Pérez, V.; Rivera, J.A.; Fetter, G. ZnAl layered double hydroxides impregnated with eucalyptus oil as efficient hybrid materials against multi-resistant bacteria. Appl. Clay Sci. 2018, 153, 61–69. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, Q.; Chi, H.; Li, M.; Lv, Y.; Feng, S.; Zhu, R.; Li, K. Methyl blue adsorption properties and bacteriostatic activities of Mg-Al layer oxides via a facile preparation method. Appl. Clay Sci. 2018, 163, 119–128. [Google Scholar] [CrossRef]
- Bugatti, V.; Esposito, L.; Franzetti, L.; Tammaro, L.; Vittoria, V. Influence of the powder dimensions on the antimicrobial properties of modified layered double hydroxide. Appl. Clay Sci. 2013, 75–76, 46–51. [Google Scholar] [CrossRef]
- Qu, Z.Y.; Yu, Q.L.; Brouwers, H.J.H. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem. Concr. Res. 2018, 105, 81–90. [Google Scholar] [CrossRef]
- Iftekhar, S.; Küçük, M.E.; Srivastava, V.; Repo, E.; Sillanpää, M. Application of zinc-aluminium layered double hydroxides for adsorptive removal of phosphate and sulfate: Equilibrium, kinetic and thermodynamic. Chemosphere 2018, 209, 470–479. [Google Scholar] [CrossRef]
- Varga, G.; Kónya, Z.; Kukovecz, Á.; Sipos, V.; Pálinkó, I. Co(II)-amino acid-CaAl-layered double hydroxide composites—Construction and characterization. J. Mol. Struct. 1179, 2019, 263–268. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Yu, Q.; Fan, J.; Brouwers, V. Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends. Constr. Build. Mater. 2020, 250, 118865. [Google Scholar] [CrossRef]
- Shui, Z.H.; Yu, R.; Chen, Y.X.; Duan, P.; Ma, J.T.; Wang, X.P. Improvement of concrete carbonation resistance based on a structure modified Layered Double Hydroxides (LDHs): Experiments and mechanism analysis. Constr. Build. Mater. 2018, 176, 228–240. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Duan, P.; Zhang, Z. Improving sulfate attack resistance of concrete by using calcined Mg-Al-CO3 LDHs: Adsorption behavior and mechanism. Constr. Build. Mater. 2020, 232, 117256. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, R.; Wang, X.; Chen, J.; Shui, Z. Evaluation and optimization of Ultra-High Performance Concrete (UHPC) subjected to harsh ocean environment: Towards an application of Layered Double Hydroxides (LDHs). Constr. Build. Mater. 2018, 177, 51–62. [Google Scholar] [CrossRef]
- Wang, J.; Huang, B.; Mao, Z.; Wang, Y. Study on Adsorption Properties of Calcined Mg-Al Hydrotalcite for Sulfate Ion and Chloride Ion in Cement Paste. Materials 2021, 14, 994. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, Z.; Zhang, B.; Yu, J.; Zhang, F.; Evans, D.G. Facile preparation of pure CaAl-layered double hydroxides and their application as a hardening accelerator in concrete. Chem. Eng. J. 2009, 155, 881–885. [Google Scholar] [CrossRef]
- Messali, F.; Metelli, G.; Plizzari, G. Experimental Results on the Retrofitting of Hollow Brick Masonry Walls with Reinforced High-Performance Mortar Coatings. Constr. Build. Mater. 2017, 141, 619–630. [Google Scholar] [CrossRef]
- Gattesco, N.; Macorini, L. In-Plane Stiffening Techniques with Nail Plates or CFRP Strips for Timber Floors in Historical Masonry Buildings. Constr. Build. Mater. 2014, 58, 64–76. [Google Scholar] [CrossRef]
- BS EN 459-1:2010; Building Lime-Part 1: Definition, Specifications and Conformity Criteria; BSI: London, UK, 2010.
- Faria, P.; Henriques, F.; Rato, V. Comparative Evaluation of Lime Mortars for Architectural Conservation. J. Cult. Herit. 2008, 9, 338–346. [Google Scholar] [CrossRef]
- Lawrence, R.M.H. A Study of Carbonation in Non-Hydraulic Lime Mortars; University of Bath: Bath, UK, 2006. [Google Scholar]
- Kang, S.H.; Lee, S.O.; Hong, S.G.; Kwon, Y.H. Historical and Scientific Investigations into the Use of Hydraulic Lime in Korea and Preventive Conservation of Historic Masonry Structures. Sustainability 2019, 11, 5169. [Google Scholar] [CrossRef] [Green Version]
- Ince, C.; Derogar, S.; Tiryakiolu, N.Y.; Toklu, Y.C. The Influence of Zeolite and Powdered Bayburt Stones on the Water Transport Kinetics and Mechanical Properties of Hydrated Lime Mortars. Constr. Build. Mater. 2015, 98, 345–352. [Google Scholar] [CrossRef]
- Van Hees, R.P.; Binda, L.; Papayianni, I.; Toumbakari, E. Damage Analysis as a Step Towards Compatible Repair Mortars. Rilem Rep. 2015, 28, 107–152. [Google Scholar]
- Mosquera, M.J.; Silva, B.; Prieto, B.; Ruiz-Herrera, E. Addition of Cement to Lime-Based Mortars: Effect on Pore Structure and Vapor Transport. Cem. Concr. Res. 2006, 36, 1635–1642. [Google Scholar] [CrossRef]
- Sala, E.; Zanotti, C.; Passoni, C.; Marini, A. Lightweight Natural Lime Composites for Rehabilitation of Historical Heritage. Constr. Build. Mater. 2016, 125, 81–93. [Google Scholar] [CrossRef]
- Collepardi, M. Degradation and Restoration of Masonry Walls of Historical Buildings. Mater. Struct. 1990, 23, 81–102. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Wang, D.; Xu, C.; Zhai, M.; Ma, X. Comparative Study on the Properties of Three Hydraulic Lime Mortar Systems: Natural Hydraulic Lime Mortar, Cement-Aerial Lime-Based Mortar and Slag-Aerial Lime-Based Mortar. Constr. Build. Mater. 2018, 186, 45–52. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Bakolas, A.; Karatasios, I.; Kilikoglou, V. Hydraulic Lime Mortars for the Restoration of Historic Masonry in Crete. Cem. Concr. Res. 2005, 35, 1577–1586. [Google Scholar] [CrossRef]
- Teutonico, J.M. Laboratory Manual for Architectural Conservators; ICCROM: Rome, Italy, 1988. [Google Scholar]
- Salavessa, E.; Jalali, S.; Sousa, L.M.O.; Fernandes, L.; Duarte, A.M. Historical plasterwork techniques inspire new formulations. Constr. Build. Mater. 2013, 48, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Taglieri, G.; Otero, G.; Danielea, V.; Gioia, G.; Macera, L.; Starinieri, V.; Charola, A.E. The biocalcarenite stone of Agrigento (Italy): Preliminary investigations of compatible nanolime treatments. J. Cult. Herit. 2018, 30, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Wiszecki, G.; Stiles, W.S. Colour Science, Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of uniform color spaces developed after the adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar]
- Brozovsky, J. Implementation of Non-Destructive Impact Hammer Testing Methods in Determination of Brick Strength. Appl. Mech. Mater. 2012, 174–177, 280–285. [Google Scholar] [CrossRef]
- EN 1015-10:1999/A1:2006; Determination of Dry Bulk Density of Hardened Mortar; CEN: Bruxelles, Belgium, 2006.
- Dracky, M.; Lesak, J.; Rescic, S.; Slızkova, Z.; Tiano, P.; Valach, J. Standardization of peeling tests for assessing the cohesion and consolidation characteristics of historic stone surfaces. Mater. Struct. 2012, 45, 505–520. [Google Scholar] [CrossRef] [Green Version]
- Scrivano, S.; Gaggero, L.; Gonzalez, A.Y.; Aguilar, J.G. Assessing surface weathering by revision and implementation of the peeling-test: In situ sampling and integrated analyses. J. Cult. Herit. 2017, 27, 88–96. [Google Scholar] [CrossRef]
- EN 12371:2010; 24.05.2019; Natural Stone Test Methods—Determination of Frost Resistance; CEN: Bruxelles, Belgium, 2019.
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catal. 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Influence of the preparation method on the structural and surface properties of various magnesium oxides and their catalytic activity in the Meerwein-Ponndorf-Verley reaction. Appl. Catal. A Gen. 2003, 244, 207–215. [Google Scholar] [CrossRef]
- Bookin, A.S.; Cherkashin, V.I.; Drits, V.A. Polytype Diversity of the Hydrotalcite-Like Minerals II. Determination of the Polytypes of Experimentally Studied Varieties. Clays Clay Miner. 1993, 41, 558–564. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, J.-A.; Choi, S.-J.; Oh, J.-M. Polymer Coated CaAl-Layered Double Hydroxide Nanomaterials for Potential Calcium Supplement. Int. J. Mol. Sci. 2014, 15, 22563–22579. [Google Scholar] [CrossRef] [Green Version]
- Zak, A.K.; Abd Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Hernandez-Moreno, M.J.; Ulibarri, M.A.; Rendon, J.L.; Serna, C.J. IR characteristics of hydrotalcite-like compounds. Phys. Chem. Miner. 1985, 12, 34–38. [Google Scholar]
- Moroz, T.N.; Arkhipenko, D.K. The crystal chemical study of natural hydrotalcites. Sov. Geol. Geophys. 1991, 2, 52–58. [Google Scholar]
- Kloprogge, J.T.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32−, NO3, SO42− and ClO4− in Mg/Al-hydrotalcite. Am. Mineral. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Frost, R.L.; Spratt, H.J.; Palmer, S.L. Infrared and near-infrared spectroscopic study of synthetic hydrotalcites with variable divalent/trivalent cationic ratios. Spectrochim. Acta A 2009, 72, 984–988. [Google Scholar] [CrossRef]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, R.E.; Radu, G.-I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.-L.; et al. Ion-Substituted Carbonated Hydroxyapatite Coatings for Model Stone Samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- SR CEN/TS 772-22:2009; Metode de Încercare a Elementelor Pentru Zidărie. Partea 22: Determinarea Rezistenţei la Îngheţ/Dezgheţ a Elementelor Pentru Zidărie de Argilă; CEN: Bucuresti, Romania, 2009.
- SR EN 14617-5:2012; Piatră Aglomerată. Metode de Încercare. Partea 5: Determinarea Rezistenţei la Îngheţ şi Dezgheţ; CEN: Bucuresti, Romania, 2012.
- Thomas, T.R. Characterization of surface roughness. Precis. Eng. 1981, 3, 97–104. [Google Scholar] [CrossRef]
- ISO:4287; Geometrical Product Specifications (GPS). Surface Texture: Profile Method. Terms, Definitions and Surface Texture Parameters; International Organization for Standardization: Geneva, Switzerland, 1997.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).