Synthesis, X-ray Structure, Antimicrobial and Anticancer Activity of a Novel [Ag(ethyl-3-quinolate)2(citrate)] Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterizations
2.2. Synthesis of [Ag(Et3qu)2(citrate)]
2.3. Biological Studies
2.4. Crystal Structure Determination
3. Results and Discussion
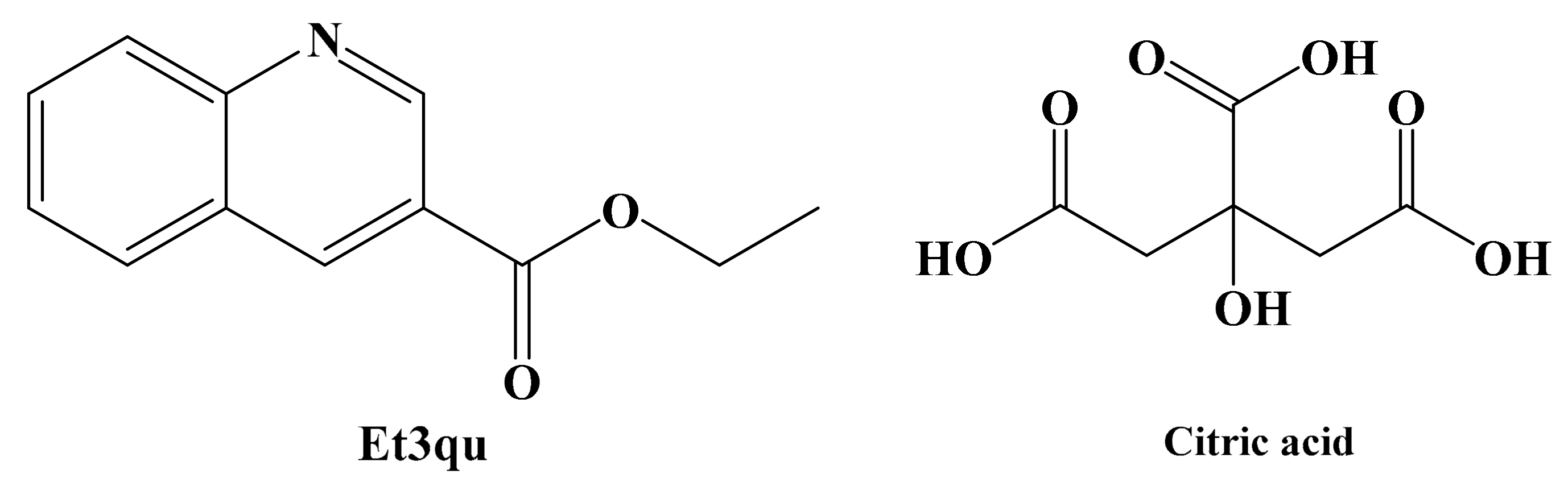
3.1. Crystal Structure Description
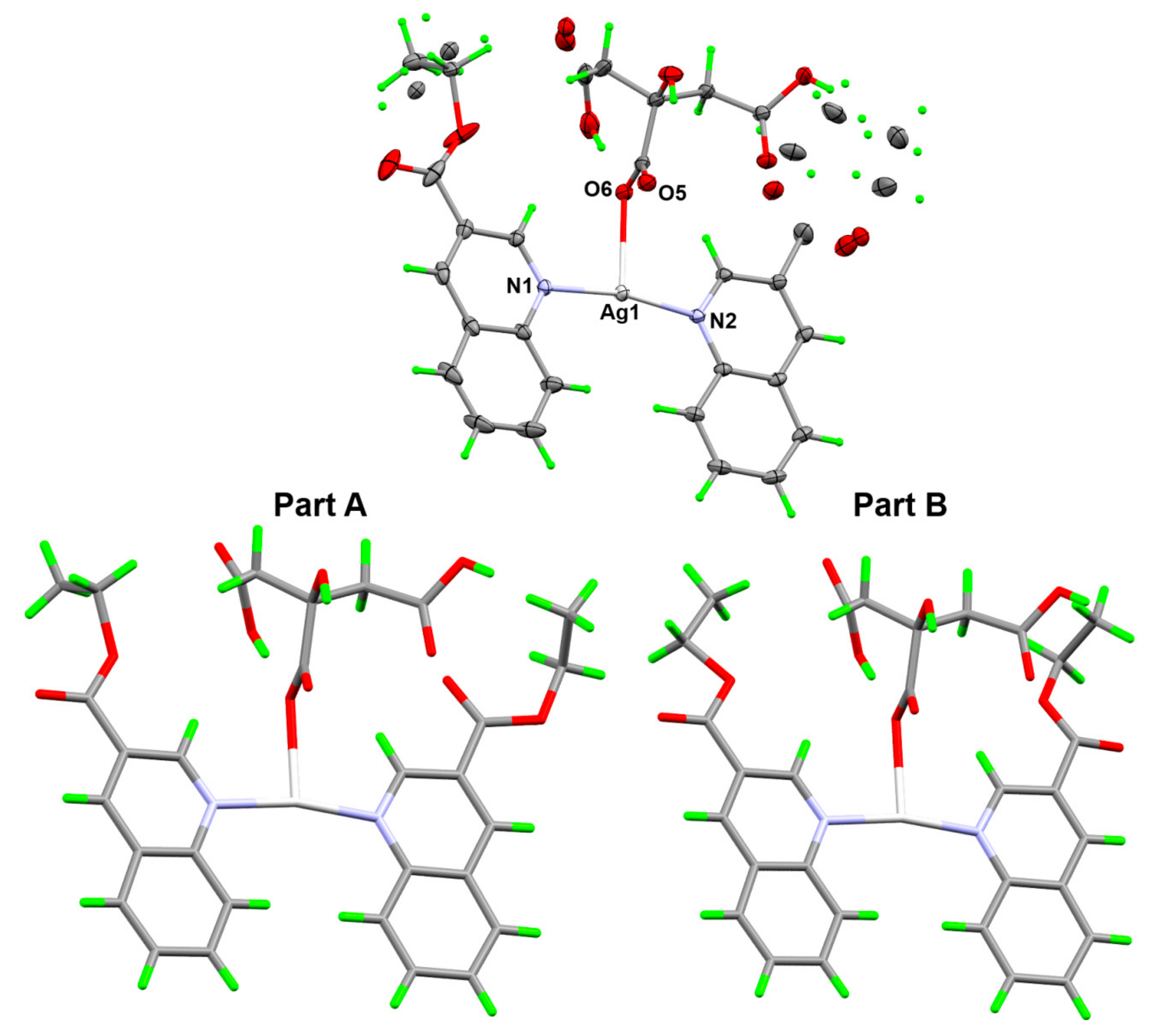
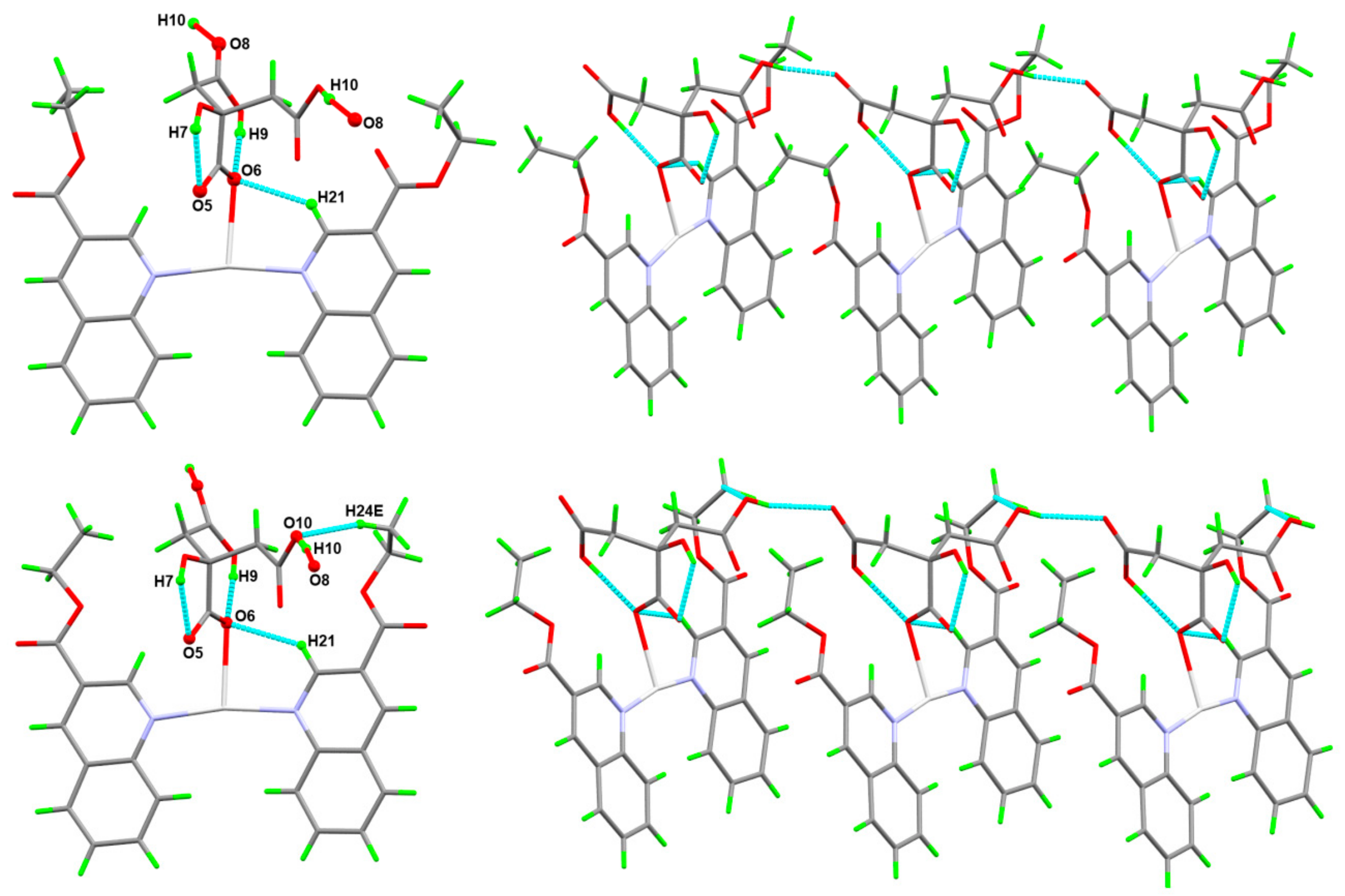
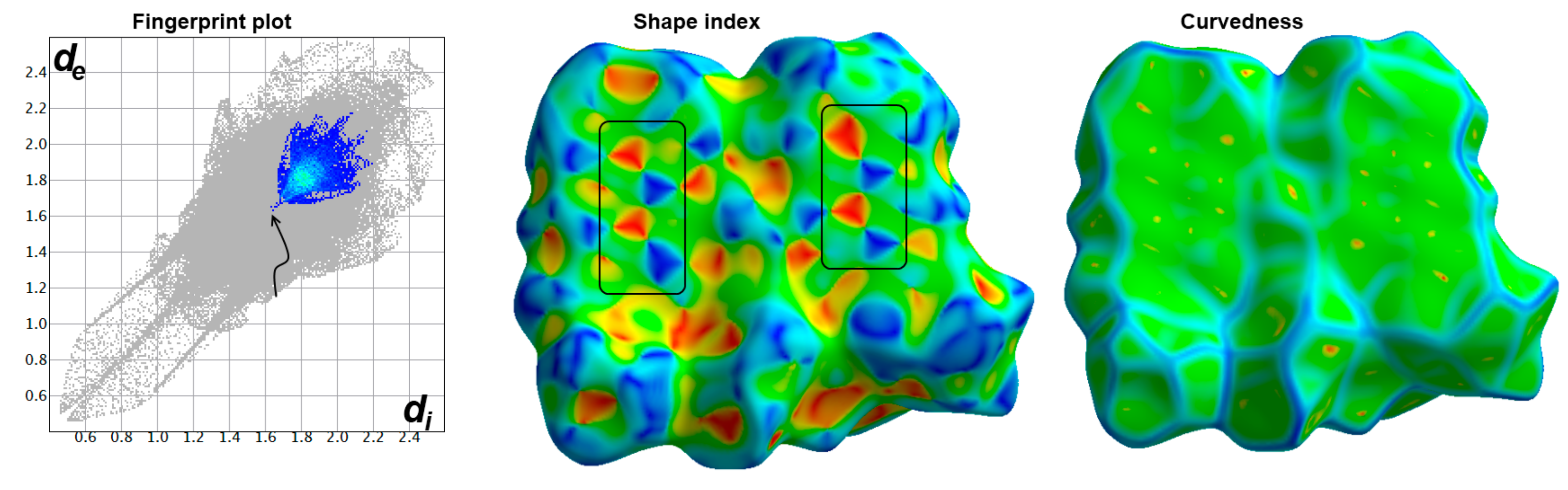
3.2. Analysis of Molecular Packing
3.3. FTIR Spectra
3.4. Antimicrobial Studies
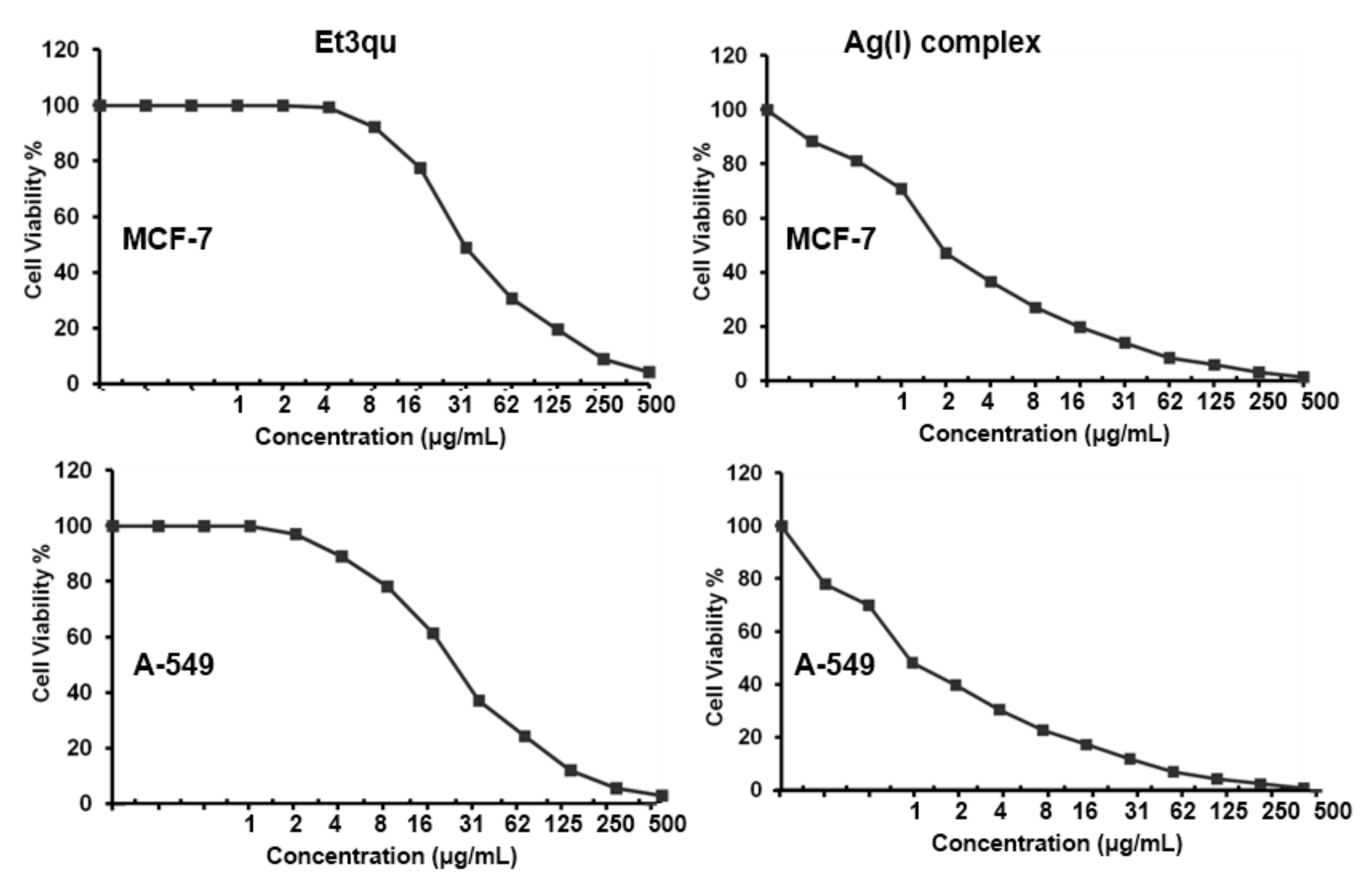
3.5. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nomiya, K.; Takahashi, S.; Noguchi, R.; Nemoto, S.; Takayama, T.; Oda, M. Synthesis and characterization of water-soluble silver(I) complexes with L-histidine (H2his) and (S)-(-)-2-pyrrolidone-5-carboxylic acid (H2pyrrld) showing a wide spectrum of effective antibacterial and antifungal activities. Crystal structures of chiral helical polymers [Ag(Hhis)]n and ([Ag(Hpyrrld)]2)n in the solid state. Inorg. Chem. 2000, 39, 3301–3311. [Google Scholar] [PubMed]
- Özdemir, I.; Demir, S.; Günal, S.; Özdemir, I.; Arici, C.; Ülkü, D. Synthesis, characterization and antimicrobial activity of new silver complexes with N-heterocyclic carbene ligands. Inorg. Chim. Acta 2010, 363, 3803–3808. [Google Scholar] [CrossRef]
- Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamontic, M.; Zanfardinoc, A.; Tommonarod, G.; Longoa, P. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Smoleński, P.; Jaros, S.W.; Pettinari, C.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Kochel, A.; Kirillov, A.M. New water-soluble polypyridine silver(I) derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA) with significant antimicrobial and antiproliferative activities. Dalt. Trans. 2013, 42, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Q.; Liang, X.; Yang, X.; Wang, N.; Wang, X.; Sun, H.; Lu, Y.; Guo, Z. Reactivity of platinum-based antitumor drugs towards a Met- and His-rich 20mer peptide corresponding to the N-terminal domain of human copper transporter 1. J. Biol. Inorg. Chem. 2009, 14, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lippard, S.J. Direct Cellular Responses to Platinum-Induced DNA Damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Hadjikakou, S.K. Anti-proliferative and anti-tumor activity of silver(I) compounds. Metallomics 2013, 5, 569–596. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Zaki, M.; Arjmand, F.; Tabassum, S. Current and future potential of metallo drugs: Revisiting DNA-binding of metal containing molecules and their diverse mechanism of action. Inorg. Chim. Acta. 2016, 444, 1–22. [Google Scholar] [CrossRef]
- Ortego, L.; Meireles, M.; Kasper, C.; Laguna, A.; Villacampa, M.D.; Gimeno, M.C. Group 11 complexes with amino acid derivatives: Synthesis and antitumoral studies. J. Inorg. Biochem. 2016, 156, 133–144. [Google Scholar] [CrossRef]
- Osman, S.A.; Mousa, H.A.; Abdallah, Y.H.A.; Hafez, T.S.; El-Sawy, A.A.; Abdallah, M.M.; Hassan, A.S. Synthesis, characterization and cytotoxicity of mixed ligand Mn(II), Co(II) and Ni(II) complexes. J. Serbian Chem. Soc. 2014, 79, 953–964. [Google Scholar] [CrossRef]
- Jayamani, A.; Bellam, R.; Gopu, G.; Ojwach, S.O.; Sengottuvelan, N. Copper(II) complexes of bidentate mixed ligands as artificial nucleases: Synthesis, crystal structure, characterization and evaluation of biological properties. Polyhedron 2018, 156, 138–149. [Google Scholar] [CrossRef]
- Azócar, M.I.; Gómez, G.; Levín, P.; Paez, M.; Muñoz, H.; Dinamarca, N. Review: Antibacterial behavior of carboxylate silver(I) complexes. J. Coord. Chem. 2014, 67, 3840–3853. [Google Scholar] [CrossRef]
- Vargová, Z.; Almáši, M.; Hudecová, D.; Titková, D.; Rostášová, I.; Zeleňák, V.; Györyová, K. New silver(I) pyridinecarboxylate complexes: Synthesis, characterization, and antimicrobial therapeutic potential. J. Coord. Chem. 2014, 67, 1002–1021. [Google Scholar] [CrossRef]
- Aldabaldetrecu, M.; Tamayo, L.; Alarcon, R.; Walter, M.; Salas-Huenuleo, E.; Kogan, M.J.; Guerrero, J.; Paez, M.; Azócar, M.I. Stability of Antibacterial Silver Carboxylate Complexes against Staphylococcus epidermidis and Their Cytotoxic Effects. Molecules 2018, 23, 1629. [Google Scholar] [CrossRef] [PubMed]
- Djokić, S. Synthesis and antimicrobial activity of silver citrate complexes. Bioinorg. Chem. Appl. 2008, 2008, 436458. [Google Scholar] [CrossRef]
- Cuin, A.; Massabni, A.C.; Leite, C.Q.F.; Sato, D.N.; Neves, A.; Szpoganicz, B.; Silva, M.S.; Bortoluzzi, A.J. Synthesis, X-ray structure and antimycobacterial activity of silver complexes with alpha-hydroxycarboxylic acids. J. Inorg. Biochem. 2007, 101, 291–296. [Google Scholar] [CrossRef]
- Nomiya, K.; Yokoyama, H. Syntheses, crystal structures and antimicrobial activities of polymeric silver(I) complexes with three amino-acids [aspartic acid (H2asp), glycine (Hgly) and asparagine (Hasn)]. J. Chem. Soc. Dalt. Trans. 2002, 2483–2490. [Google Scholar] [CrossRef]
- Batarseh, K.I. Anomaly and correlation of killing in the therapeutic properties of silver (I) chelation with glutamic and tartaric acids. J. Antimicrob. Chemother. 2004, 54, 546–548. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Twentieth Informational Supplement; M100-S22; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Santa Clara, CA, USA, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17. 2017. University of Western Australia. Available online: http://hirshfeldsurface.net (accessed on 18 February 2022).
- Massoud, A.A.; Langer, V.; Gohar, Y.M.; Abu-Youssef, M.A.M.; Janis, J.; Lindberg, G.; Hansson, K.; Ohrstrom, L. Effects of Different Substituents on the Crystal Structures and Antimicrobial Activities of Six Ag(I) Quinoline Compounds. Inorg. Chem. 2013, 52, 4046–4060. [Google Scholar] [CrossRef][Green Version]
- Kleinhans, G.; Chan, A.K.-W.; Leung, M.Y.; Liles, D.C.; Fernandes, M.A.; Yam, V.W.W.; Fernández, I.; Bezuidenhout, D.I. Synthesis and photophysical properties of T-shaped coinage metal complexes. Chem. A Eur. J. 2020, 26, 6993–6998. [Google Scholar] [CrossRef]
- Cabeza, J.A.; Fernandez, I.; Alvareza, P.G.; Gandaraa, C.J.L. A dipyrromethane-based diphosphane–germylene as precursor to tetrahedral copper(I) and T-shaped silver(I) and gold(I) PGeP pincer complexes. Dalton Trans. 2019, 48, 13273–13280. [Google Scholar] [CrossRef]
- Hamamci, S.; Yilmaz, V.T.; Harrison, W.T.A. Silver(I)-Saccharinato Complexes with 2-(Aminomethyl)pyridine and 2-(2-Aminoethyl)pyridine Ligands: [Ag(sac)(ampy)] and [Ag2(sac)2(μ-aepy)2]. Z. Nat. B 2005, 60, 978–983. [Google Scholar] [CrossRef]
- Soliman, S.M.; Elsilk, S.E. Synthesis, X-ray structure, DFT and antimicrobial studies of Ag(I) complexes with nicotinic acid derivatives. J. Photochem. Photobiol. B 2018, 187, 48–53. [Google Scholar] [CrossRef]
- Infrared Absorption Bands of Carboxylate. Available online: https://www.911metallurgist.com/ir-infrared-absorption-bands-carboxylate/ (accessed on 7 February 2022).
- Otero, V.; Sanches, D.; Montagner, C.; Vilarigues, M.; Carlyle, L.; Lopes, J.A.; Melo, M.J. Characterisation of metal carboxylates by Raman and infrared spectroscopy in works of art. J. Raman Spectrosc. 2014, 45, 1197–1206. [Google Scholar] [CrossRef]
- Masoud, M.S.; Ali, A.E.; Elasala, G.S.; Elwardany, R.E. Structural and thermal studies on some morpholine complexes. J. Mol. Struct. 2019, 1175, 648–662. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Blake, A.J.; Champness, N.R.; Lemenovskii, D.A.; Majouga, A.G.; Zyk, N.V.; Schröder, M. Supramolecular design of one-dimensional coordination polymers based on silver(I) complexes of aromatic nitrogen-donor ligands. Coord. Chem. Rev. 2001, 222, 155–192. [Google Scholar] [CrossRef]
- Nomiya, K.; Yoshizawa, A.; Tsukagoshi, K.; Kasuga, N.C.; Hirakawa, S.; Watanabe, J. Synthesis and structural characterization of silver(I), aluminium(III) and cobalt(II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver(I)-oxygen bonding complexes on the antimicrobial activities. J. Inorg. Biochem. 2004, 98, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Isab, A.A.; Ali, S.; Al–Arfaj, A.R. Perspectives in bioinorganic chemistry of some metal based therapeutic agents. Polyhedron 2006, 25, 1633–1645. [Google Scholar] [CrossRef]
- Al–Zamil, N.O.; Al–Sadham, K.A.; Isab, A.A.; Wazer, M.I.M.; Al–Arfaj, A.R.A. Silver(I) complexes of imidazolidine-2-thione and triphenylphosphines: Solid-state, solution NMR and antimicrobial activity studies. Spectroscopy 2007, 21, 61–67. [Google Scholar] [CrossRef][Green Version]
- Tuszkanow, M.P.; Grabowski, T.; Daszkiewicz, M.; Wietrzyk, J.; Filip, B.; MacIejewska, G.; Golonka, M.C. Silver(I) complexes with hydantoins and allantoin Synthesis, crystal and molecular structure, cytotoxicity and pharmacokinetics. J. Inorg. Biochem. 2011, 105, 17–22. [Google Scholar] [CrossRef]
- Tan, X.J.; Liu, H.Z.; Ye, C.Z.; Lou, J.F.; Liu, Y.; Xing, D.X.; Li, S.P.; Liu, S.L.; Song, L.Z. Synthesis, characterization and in vitro cytotoxic properties of new silver(I) complexes of two novel Schiff bases derived from thiazole and pyrazine. Polyhedron 2014, 71, 119. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Zhang, X.-M.; Liu, X.-Y.; Wang, X.-J.; Liu, G.-F.; Usman, A.; Fun, H.-K. Clear Ag–Ag bonds in three silver(I) carboxylate complexes with high cytotoxicity properties. Inorg. Chem. Commun. 2003, 6, 1113–1116. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, H.L. Strong silver-silver interactions in three silver(I) carboxylate complexes with high cytotoxicity properties. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2005, 35, 325–332. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Papini, G.; Morresi, B.; Galassi, R.; Ricci, S.; Tisato, F.; Porchia, M.; Rigobello, M.P.; Gandin, V.; et al. In vitro antitumour activity of water soluble Cu(I), Ag(I) and Au(I) complexes supported by hydrophilic alkyl phosphine ligands. J. Inorg. Biochem. 2011, 105, 232–240. [Google Scholar] [CrossRef]
- McKeage, M.J.; Papathanasiou, P.; Salem, G.; Sjaarda, A.; Swiegers, G.F.; Waring, P.; Wild, S.B. Antitumor activity of gold(I), silver(I) and copper(I) complexes containing chiral tertiary phosphines. Met. Based Drugs. 1998, 5, 217–223. [Google Scholar] [CrossRef]
- He, J.; Wang, Q.K.; Li, X.J.; Pu, S.P. Synthesis, Characterization and Antitumor Activities of Cis- chlorogenic acid[(1R,2R)-1,2-cyclohexanediamine] Platinum(II). Adv. Mater. Res. 2021, 884, 593–597. [Google Scholar] [CrossRef]
- Ali, K.A.; Abd-Elzaher, M.M.; Mahmoud, K. Synthesis and Anticancer Properties of Silver(I) Complexes Containing 2,6-Bis(substituted)pyridine Derivatives. Int. J. Med. Chem. 2013, 2013, 256836. [Google Scholar] [CrossRef] [PubMed]
| CCDC | 2151811 |
| empirical formula | C30H29AgN2O11 |
| fw | 701.42 |
| temp (K) | 120(2) K |
| λ (Å) | 1.54184 Å |
| crystal system | Triclinic |
| space group | P-1 |
| a (Å) | a = 8.6475(2) Å |
| b (Å) | b = 11.4426(3) Å |
| c (Å) | c = 15.2256(3) Å |
| α (deg) | 73.636(2)° |
| β (deg) | 79.692(2)° |
| γ (deg) | 86.832(2)° |
| V (Å3) | 1422.19(6) Å3 |
| Z | 2 |
| ρcalc (Mg/m3) | 1.638 Mg/m3 |
| μ (Mo Kα) (mm−1) | 6.273 mm−1 |
| No. reflns | 39671 |
| Unique reflns | 5968 |
| Completeness to θ = 67.684° | 99.9% |
| GOOF (F2) | 1.068 |
| Rint | 0.0295 |
| R1 a (I ≥ 2σ) | 0.0236 |
| wR2 b (I ≥ 2σ) | 0.0597 |
| Bond | Distance | Bonds | Angle |
|---|---|---|---|
| Ag(1)-N(2) | 2.1818(14) | N(2)-Ag(1)-N(1) | 169.29(5) |
| Ag(1)-N(1) | 2.1848(14) | N(2)-Ag(1)-O(6) | 90.53(5) |
| Ag(1)-O(6) | 2.5401(14) | N(1)-Ag(1)-O(6) | 99.90(5) |
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(D-H···A) |
|---|---|---|---|---|
| C(21)-H(21) ···O(6) | 0.95 | 2.39 | 3.151(2) | 137 |
| O(10)-H(10) ···O(8B) #1 | 0.75(3) | 2.10(4) | 2.798(18) | 155(3) |
| O(10)-H(10) ···O(8) #1 | 0.75(3) | 1.83(4) | 2.569(7) | 170(4) |
| C(24B)-H(24E) ···O(10) | 0.98 | 2.44 | 3.352(6) | 155.3 |
| O(7)-H(7) ···O(5) | 0.77(3) | 2.05(3) | 2.593(2) | 127(3) |
| O(9)-H(9)-O(6) | 0.78(4) | 1.72(4) | 2.499(2) | 176(4) |
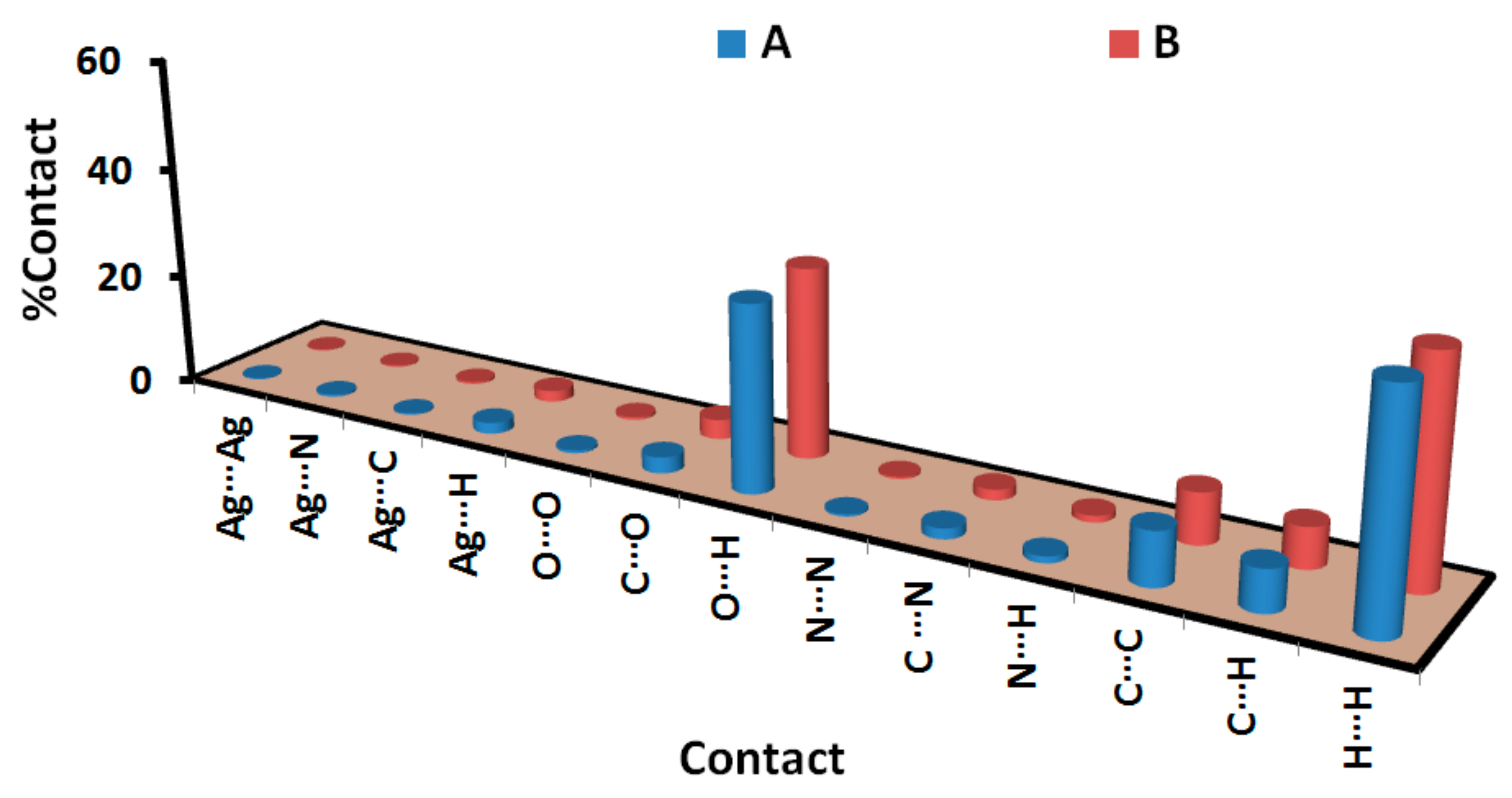
| Contact | A | B |
|---|---|---|
| Ag···Ag | 0.2 | 0.2 |
| Ag···N | 0.4 | 0.3 |
| Ag···C | 0.3 | 0.4 |
| Ag···H | 2.0 | 2.0 |
| O···O | 0.5 | 0.5 |
| C···O | 2.9 | 3.5 |
| O···H | 33.2 | 34.0 |
| N···N | 0.4 | 0.4 |
| C···N | 1.9 | 1.9 |
| N···H | 1.2 | 1.2 |
| C···C | 9.5 | 9.1 |
| C···H | 7.4 | 7.2 |
| H···H | 40.1 | 39.3 |
| Microbe | Et3qu | [Ag(Et3qu)2(citrate)] | Control |
|---|---|---|---|
| A. fumigatus | 30 (9.7) | 30 (4.8) | 17 (156.25) b |
| C. albicans | 19 (312.5) | 15 (312.5) | 20 (312.5) b |
| S. aureus | NA (NA) d | 13 (1250) | 24 (9.7) c |
| B. subtilis | NA(NA) d | 12 (625) | 26 (4.8) c |
| E. coli | 12 (2500) | 12 (1250) | 30 (4.8) c |
| P. vulgaris | 12 (2500) | 18 (1250) | 25 (4.8) c |
| Cell Line | Cisplatin [45] | Doxorubicin [46] | Et3qu | [Ag(Et3qu)2(citrate)] |
|---|---|---|---|---|
| Lung carcinoma (A-549) | 2.46 | 1.91 | 22.89 ± 1.48 | 0.95 ± 0.06 |
| Breast carcinoma (MCF-7) | 3.23 | 1.51 | 30.64 ± 1.98 | 1.87 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; El-Naggar, M.A.; Abu-Youssef, M.A.M.; Soliman, S.M.; Haukka, M.; Barakat, A.; Badr, A.M.A. Synthesis, X-ray Structure, Antimicrobial and Anticancer Activity of a Novel [Ag(ethyl-3-quinolate)2(citrate)] Complex. Crystals 2022, 12, 356. https://doi.org/10.3390/cryst12030356
Altowyan MS, El-Naggar MA, Abu-Youssef MAM, Soliman SM, Haukka M, Barakat A, Badr AMA. Synthesis, X-ray Structure, Antimicrobial and Anticancer Activity of a Novel [Ag(ethyl-3-quinolate)2(citrate)] Complex. Crystals. 2022; 12(3):356. https://doi.org/10.3390/cryst12030356
Chicago/Turabian StyleAltowyan, Mezna Saleh, Mostafa A. El-Naggar, Morsy A. M. Abu-Youssef, Saied M. Soliman, Matti Haukka, Assem Barakat, and Ahmed M. A. Badr. 2022. "Synthesis, X-ray Structure, Antimicrobial and Anticancer Activity of a Novel [Ag(ethyl-3-quinolate)2(citrate)] Complex" Crystals 12, no. 3: 356. https://doi.org/10.3390/cryst12030356
APA StyleAltowyan, M. S., El-Naggar, M. A., Abu-Youssef, M. A. M., Soliman, S. M., Haukka, M., Barakat, A., & Badr, A. M. A. (2022). Synthesis, X-ray Structure, Antimicrobial and Anticancer Activity of a Novel [Ag(ethyl-3-quinolate)2(citrate)] Complex. Crystals, 12(3), 356. https://doi.org/10.3390/cryst12030356










