Implanting MnO2 into Hexagonal Boron Nitride as Nanoadditives for Enhancing Tribological Performance
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials
2.2. Preparation of Material
2.3. Tribological Performance Tests
2.4. Performance Characterization
3. Results and Discussion
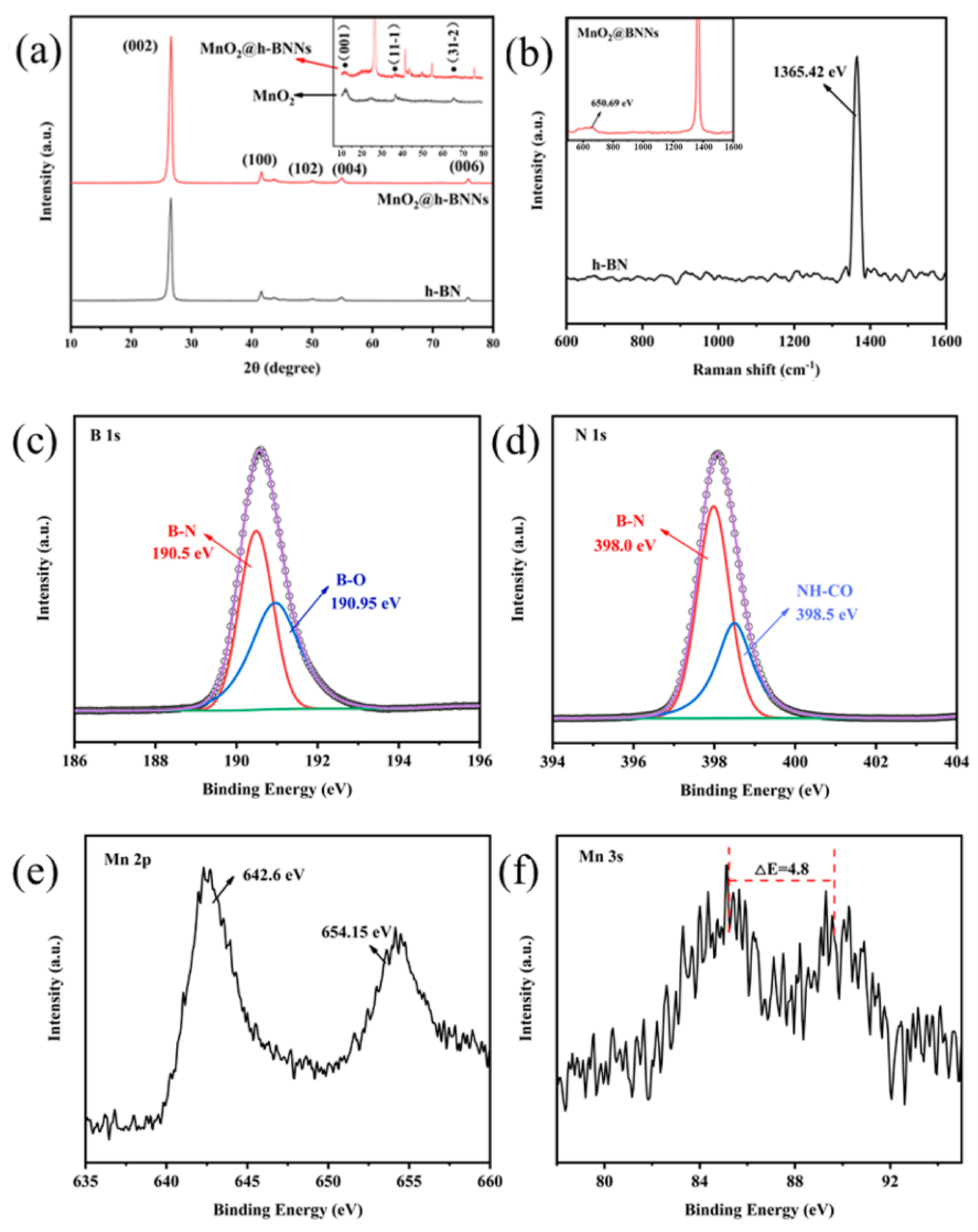
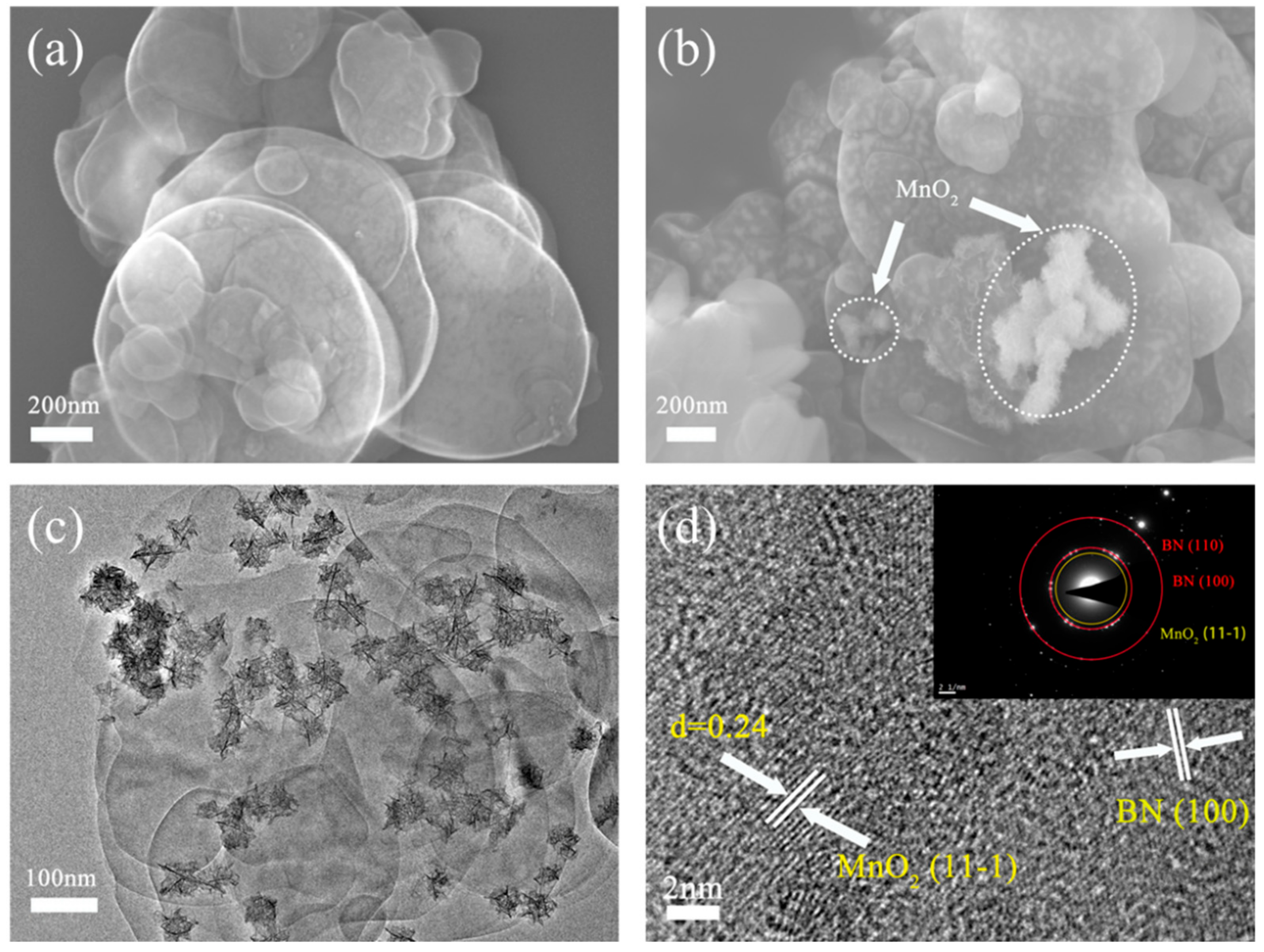
3.1. Characterization of MnO2@h-BNNs
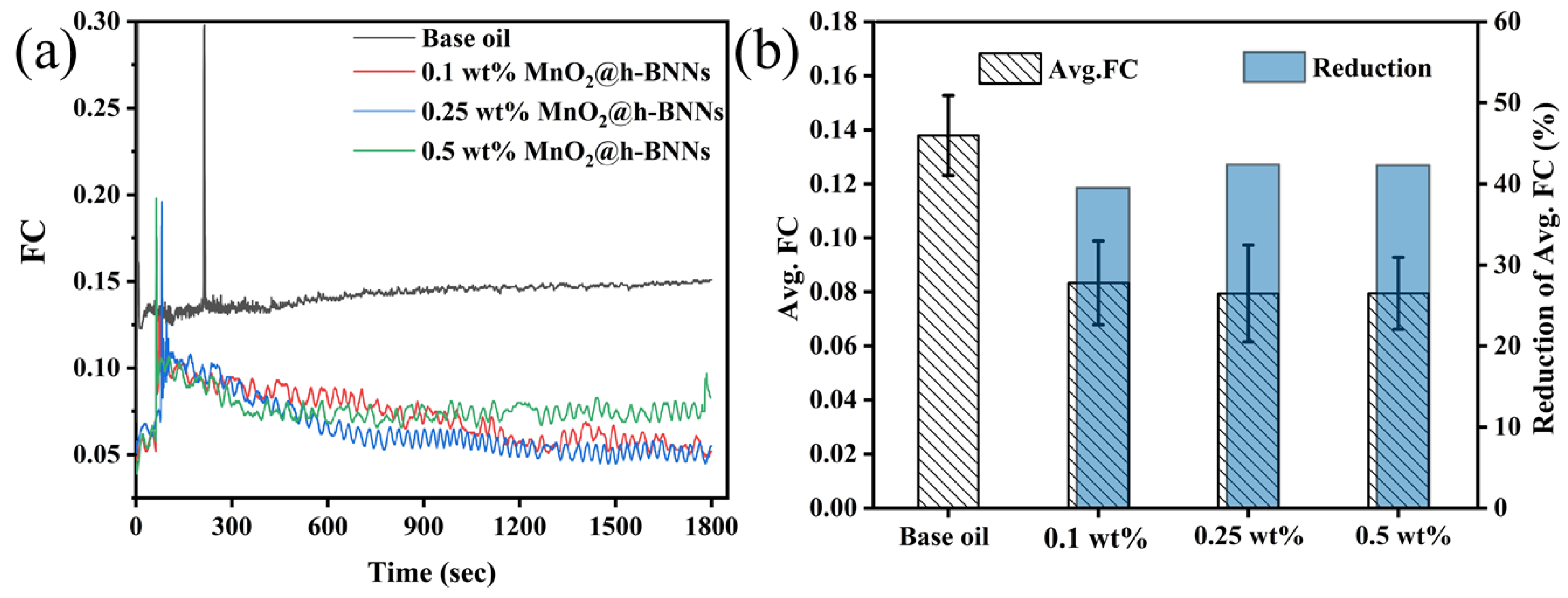
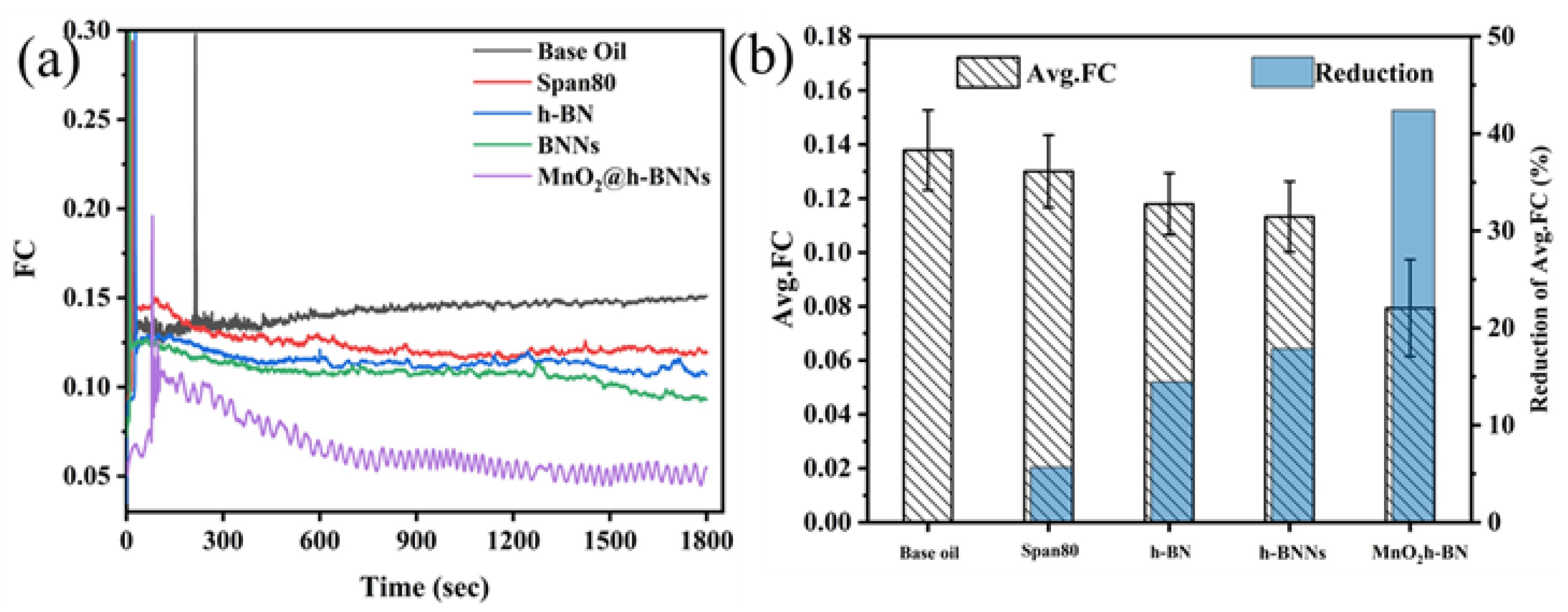
3.2. Tribological Properties
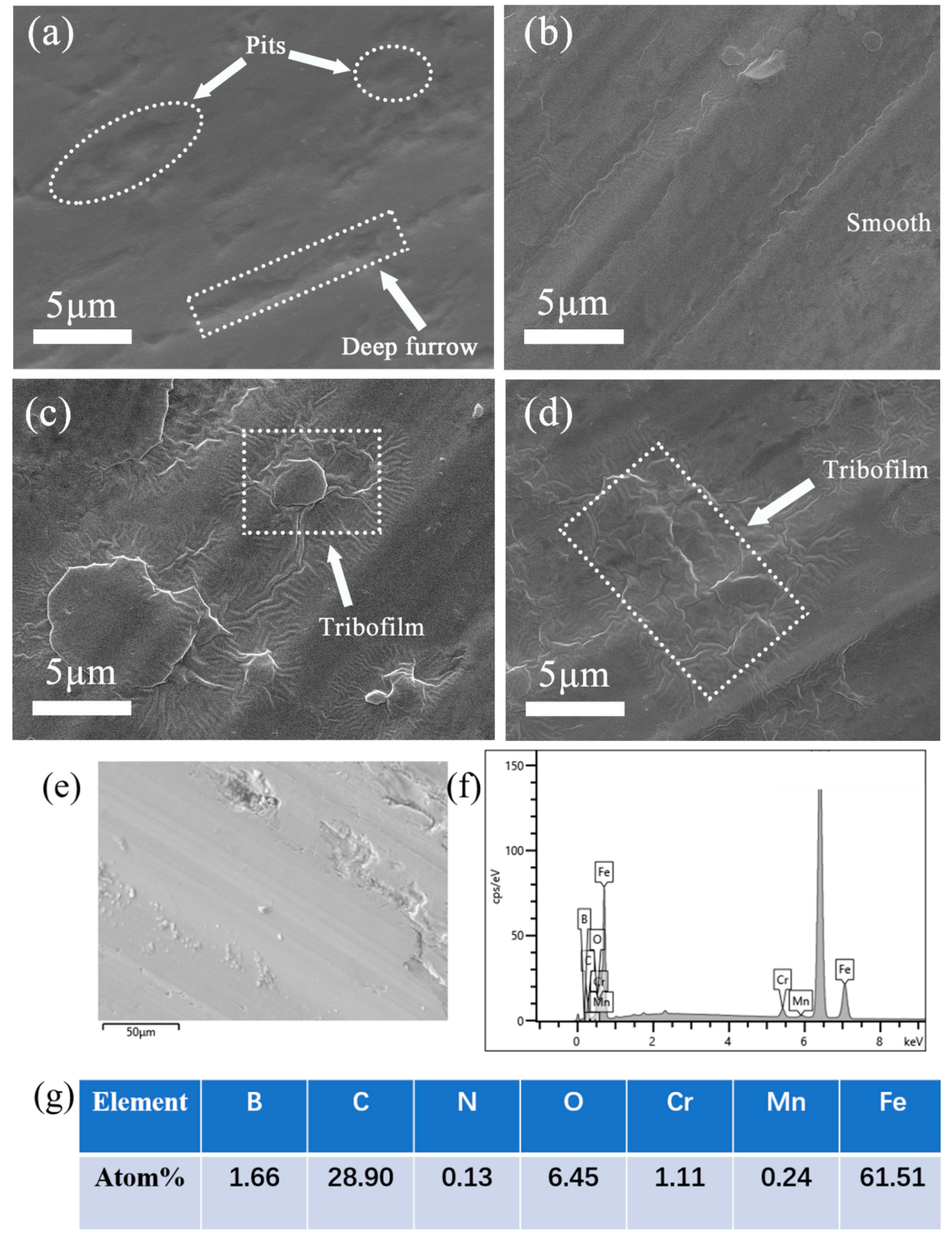
3.3. Analysis of Wear Interface
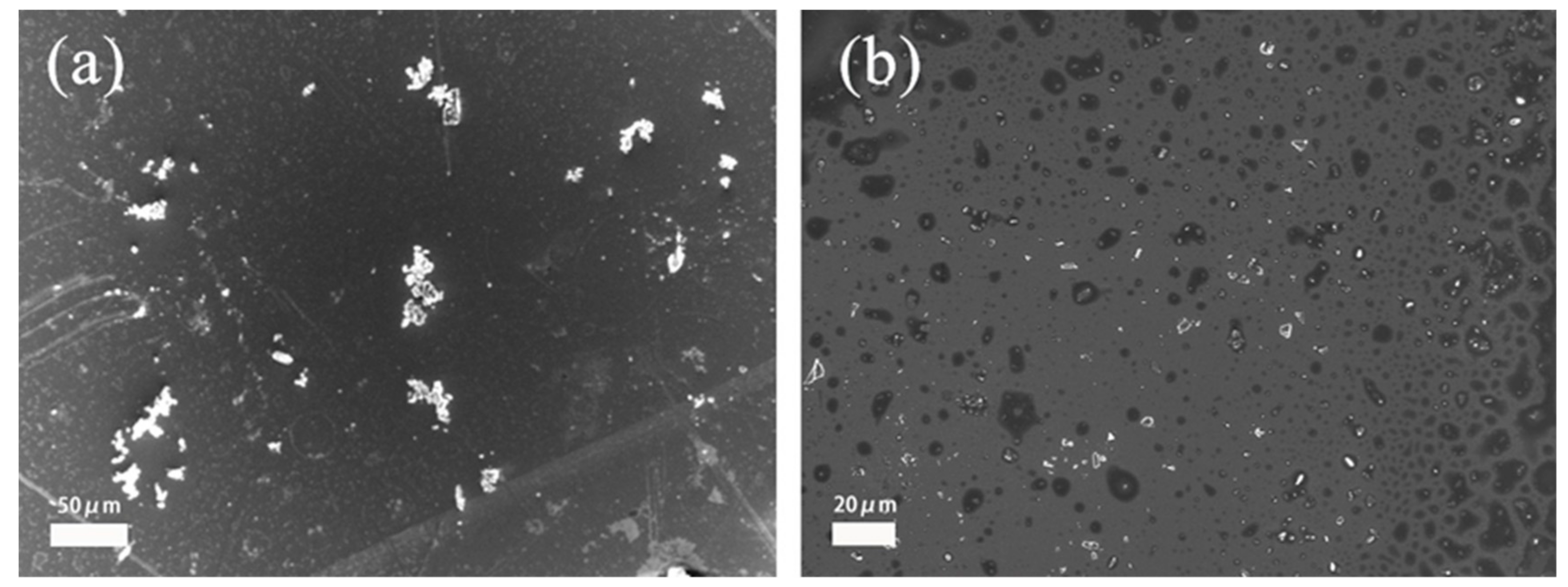
3.4. Dispersion Stability
3.5. Viscosity
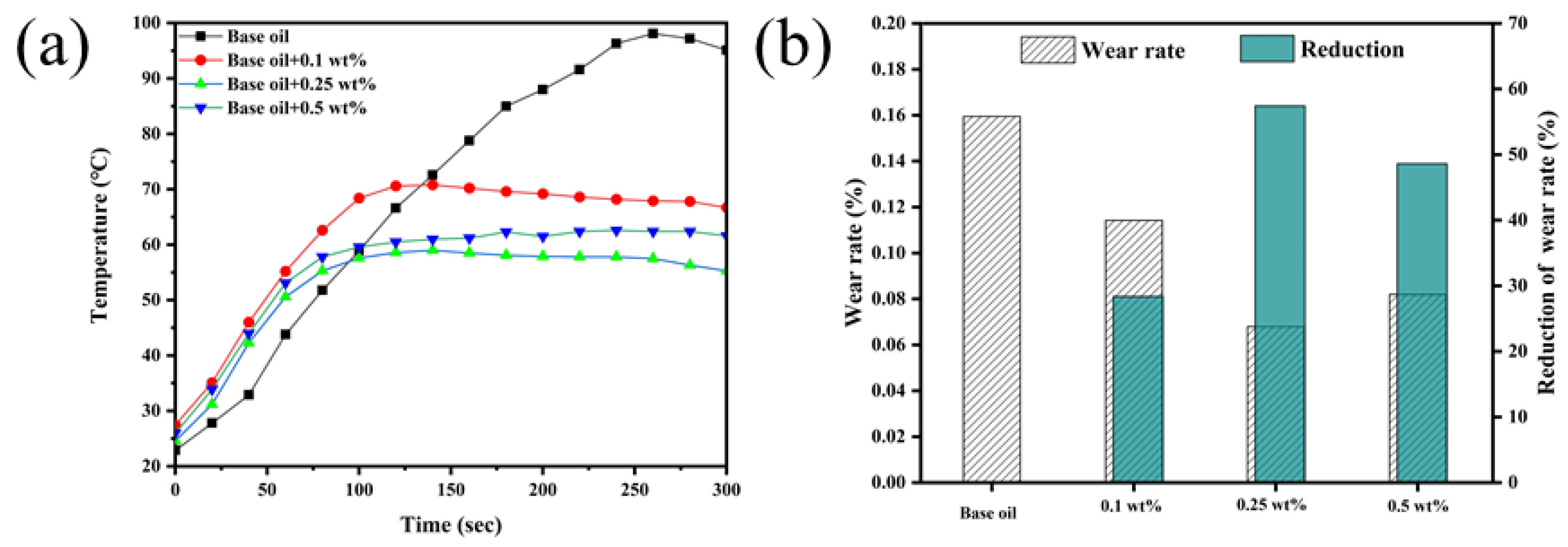
3.6. Variable-Temperature Test Analysis
3.7. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Andersson, P.; Nylund, N.-O.; Mäkelä, K.; Erdemir, A. Global energy consumption due to friction in trucks and buses. Tribol. Int. 2014, 78, 94–114. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Holmberg, K.; Kivikytö-Reponen, P.; Härkisaari, P.; Valtonen, K.; Erdemir, A. Global energy consumption due to friction and wear in the mining industry. Tribol. Int. 2017, 115, 116–139. [Google Scholar] [CrossRef]
- Sengupta, S.; Murmu, M.; Mandal, S.; Hirani, H.; Banerjee, P. Competitive corrosion inhibition performance of alkyl/acyl substituted 2-(2-hydroxybenzylideneamino)phenol protecting mild steel used in adverse acidic medium: A dual approach analysis using FMOs/molecular dynamics simulation corroborated experimental findings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126314. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X. Superlubricitive engineering—Future industry nearly getting rid of wear and frictional energy consumption. Friction 2020, 8, 643–665. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. 2D nanomaterials as lubricant additive: A review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Hu, H.; Khadem, M.; Penkov, O.V. Tribology of 2D Nanomaterials: A Review. Coatings 2020, 10, 897. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Green tribology: Principles, research areas and challenges. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 4677–4694. [Google Scholar] [CrossRef] [Green Version]
- Senatore, A.; D’Agostino, V.; Petrone, V.; Ciambelli, P.; Sarno, M. Graphene Oxide Nanosheets as Effective Friction Modifier for Oil Lubricant: Materials, Methods, and Tribological Results. ISRN Tribol. 2013, 2013, 425809. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Wu, Y.P.; Li, Z.Y.; Cai, Z.B. Tribological properties of WS2/graphene nanocomposites as lubricating oil additives. RSC Adv. 2017, 7, 14060–14068. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Gong, K.; Zhao, G.; Lou, W.; Wang, X.; Liu, W. Mechanical synthesis of chemically bonded phosphorus–graphene hybrid as high-temperature lubricating oil additive. RSC Adv. 2018, 8, 4595–4603. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, L.; Fleischer, N.; Tenne, R. Applications of WS2(MoS2) inorganic nanotubes and fullerene-like nanoparticles for solid lubrication and for structural nanocomposites. J. Mater. Chem. 2005, 15, 1782–1788. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Hu, K.; Xu, Y.; Hu, X. Formation of an adsorption film of MoS2 nanoparticles and dioctyl sebacate on a steel surface for alleviating friction and wear. Tribol. Int. 2015, 92, 172–183. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, H.; Ali, M.K.A.; Liu, H.; Su, D.; Tian, Z. Dispersion behavior assessment of the molybdenum disulfide nanomaterials dispersed into poly alpha olefin. J. Mol. Liq. 2020, 311, 113303. [Google Scholar] [CrossRef]
- Bondarev, A.V.; Fraile, A.; Polcar, T.; Shtansky, D.V. Mechanisms of friction and wear reduction by h-BN nanosheet and spherical W nanoparticle additives to base oil: Experimental study and molecular dynamics simulation. Tribol. Int. 2020, 151, 106493. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Wang, S.; Sun, Q.; Cheng, J.; Yu, Y.; Yang, J. Tribological properties of h-BN matrix solid-lubricating composites under elevated temperatures. Tribol. Int. 2020, 148, 106333. [Google Scholar] [CrossRef]
- Tang, G.; Su, F.; Xu, X.; Chu, P.K. 2D black phosphorus dotted with silver nanoparticles: An excellent lubricant additive for tribological applications. Chem. Eng. J. 2020, 392, 123631. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, T.; Wang, W.; Zhang, G.; Gao, Y.; Wang, K. Tribological behavior of black phosphorus nanosheets as water-based lubrication additives. Friction 2021, 10, 374–387. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.B.; Chen, F.; Xiao, P.; Li, Z.; Pang, L.; Li, Y. Effect of h-BN addition on friction and wear properties of C/C-SiC composites fabricated by LSI. Int. J. Appl. Ceram. Technol. 2021, 19, 108–118. [Google Scholar] [CrossRef]
- Wang, L.; Gong, P.; Li, W.; Luo, T.; Cao, B. Mono-dispersed Ag/Graphene nanocomposite as lubricant additive to reduce friction and wear. Tribol. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Lee, K.; Yoon, H.C.; Xu, Q.; Wang, D. Recent development in friction of 2D materials: From mechanisms to applications. Nanotechnology 2021, 32, 312002. [Google Scholar] [CrossRef]
- Gupta, M.K.; Bijwe, J.; Padhan, M. Role of size of hexagonal boron nitride particles on tribo-performance of nano and micro oils. Lubr. Sci. 2018, 30, 441–456. [Google Scholar] [CrossRef]
- Liu, C.; Tang, G.; Su, F.; Xu, X.; Li, Z. Functionalised h-BN as an effective lubricant additive in PAO oil for MoN coating sliding against Si3N4ball. Lubr. Sci. 2020, 33, 33–42. [Google Scholar] [CrossRef]
- Raina, A.; Irfan Ul Haq, M.; Anand, A.; Mohan, S.; Kumar, R.; Jayalakshmi, S.; Arvind Singh, R. Nanodiamond particles as secondary additive for polyalphaolefin oil lubrication of steel-aluminium contact. Nanomaterials 2021, 11, 1438. [Google Scholar] [CrossRef]
- Shukla, N.; Verma, D.K.; Singh, A.K.; Kumar, B.; Kavita; Rastogi, R.B. Ternary Composite of Methionine-Functionalized Graphene Oxide, Lanthanum-Doped Yttria Nanoparticles, and Molybdenum Disulfide Nanosheets for Thin-Film Lubrication. ACS Appl. Nano Mater. 2020, 3, 8012–8026. [Google Scholar] [CrossRef]
- Rajendhran, N.; Palanisamy, S.; Periyasamy, P.; Venkatachalam, R. Enhancing of the tribological characteristics of the lubricant oils using Ni-promoted MoS2 nanosheets as nano-additives. Tribol. Int. 2018, 118, 314–328. [Google Scholar] [CrossRef]
- Wu, H.; Yin, S.; Du, Y.; Wang, L.; Yang, Y.; Wang, H. Alkyl-Functionalized Boron Nitride Nanosheets as Lubricant Additives. ACS Appl. Nano Mater. 2020, 3, 9108–9116. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Z.; Hu, E.; Hu, K.; Hu, X. Preparation and tribological properties of WS2 and WS2/TiO2 nanoparticles. Tribol. Int. 2019, 130, 308–316. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Li, X.; Dong, Z.; Jia, Y.; Li, C. Tribological properties of epoxy-based self-lubricating composite coating enhanced by 2D/2D h-BN/MoS2 hybrid. Prog. Org. Coat. 2020, 147, 105767. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; He, Y.; Shi, Y. Nanolubricant additives: A review. Friction 2020, 9, 891–917. [Google Scholar] [CrossRef]
- Liñeira del Río, J.M.; López, E.R.; Fernández, J. Tribological properties of graphene nanoplatelets or boron nitride nanoparticles as additives of a polyalphaolefin base oil. J. Mol. Liq. 2021, 333, 115911. [Google Scholar] [CrossRef]
- Murmu, M.; Sengupta, S.; Pal, R.; Mandal, S.; Murmu, N.C.; Banerjee, P. Efficient tribological properties of azomethine-functionalized chitosan as a bio-lubricant additive in paraffin oil: Experimental and theoretical analysis. RSC Adv. 2020, 10, 33401–33416. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Wei, B. Nanostructured MnO2: Hydrothermal synthesis and electrochemical properties as a supercapacitor electrode material. J. Power Sources 2006, 159, 361–364. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, Y.; Jiang, P. Carbon@MnO2 core–shell nanospheres for flexible high-performance supercapacitor electrode materials. J. Power Sources 2014, 259, 219–226. [Google Scholar] [CrossRef]
- Du, M.; Wu, Y.; Hao, X. A facile chemical exfoliation method to obtain large size boron nitride nanosheets. CrystEngComm 2013, 15, 1782–1786. [Google Scholar] [CrossRef]
- Wang, L.; Bai, Y.; Ma, Z.; Ge, C.; Guan, H.; Zhang, X. Tribological performances of hexagonal boron nitride nanosheets via surface modification with silane coupling agent. SN Appl. Sci. 2021, 3, 368. [Google Scholar] [CrossRef]
- Mittal, N.; Kedawat, G.; Gupta, S.; Kumar Gupta, B. An Innovative Method for Larg-Scale Synthesis of Hexagonal Boron Nitride Nanosheets by Liquid Phase Exfoliation. ChemistrySelect 2020, 5, 12564–12569. [Google Scholar] [CrossRef]
- Azman, S.S.N.; Zulkifli, N.W.M.; Masjuki, H.; Gulzar, M.; Zahid, R. Study of tribological properties of lubricating oil blend added with graphene nanoplatelets. J. Mater. Res. 2016, 31, 1932–1938. [Google Scholar] [CrossRef]
- Moshkovith, A.; Perfiliev, V.; Lapsker, I.; Fleischer, N.; Tenne, R.; Rapoport, L. Friction of fullerene-like WS2 nanoparticles: Effect of agglomeration. Tribol. Lett. 2006, 24, 225–228. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Fullerene-like WS2 Nanoparticles: Superior Lubricants for Harsh Conditions. Adv. Mater. 2003, 15, 651–655. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 31246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Liu, D.M.; Guo, Y.; Liao, Z.M.; Luo, J.B.; Wen, S.Z. Thickness dependent friction on few-layer MoS2, WS2, and WSe2. Nanotechnology 2017, 28, 245703. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Li, Q.; Kalb, W.; Liu, X.Z.; Berger, H.; Carpick, R.W.; Hone, J. Frictional characteristics of atomically thin sheets. Science 2010, 328, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Song, W.; Yan, J.; Ji, H. Fabrication of GNS/MoS2 composite with different morphology and its tribological performance as a lubricant additive. Appl. Surf. Sci. 2019, 469, 226–235. [Google Scholar] [CrossRef]
- Wu, X.; Gong, K.; Zhao, G.; Lou, W.; Wang, X.; Liu, W. MoS2/WS2 Quantum Dots as High-Performance Lubricant Additive in Polyalkylene Glycol for Steel/Steel Contact at Elevated Temperature. Adv. Mater. Interfaces 2017, 5, 1700859. [Google Scholar] [CrossRef]

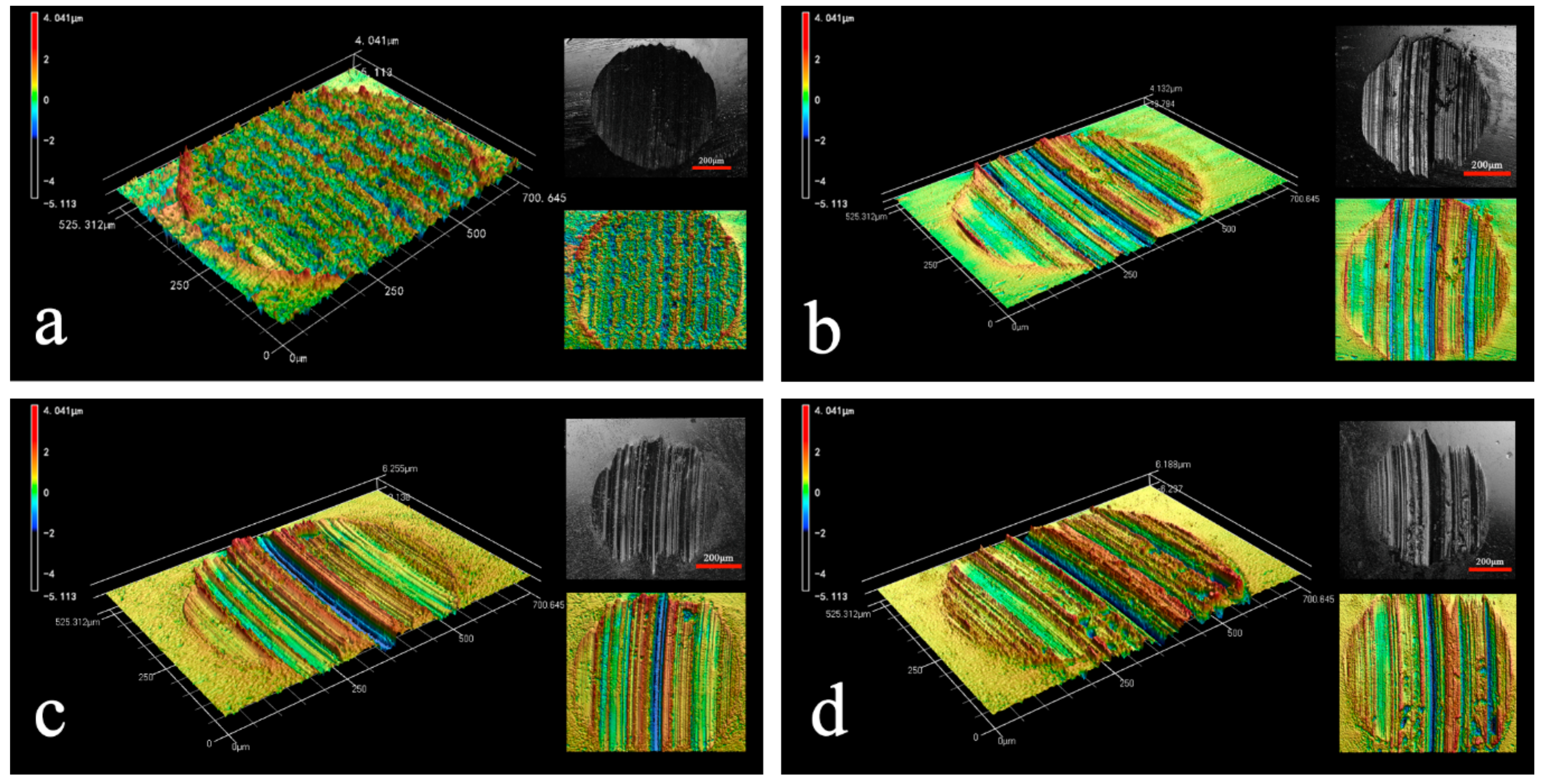
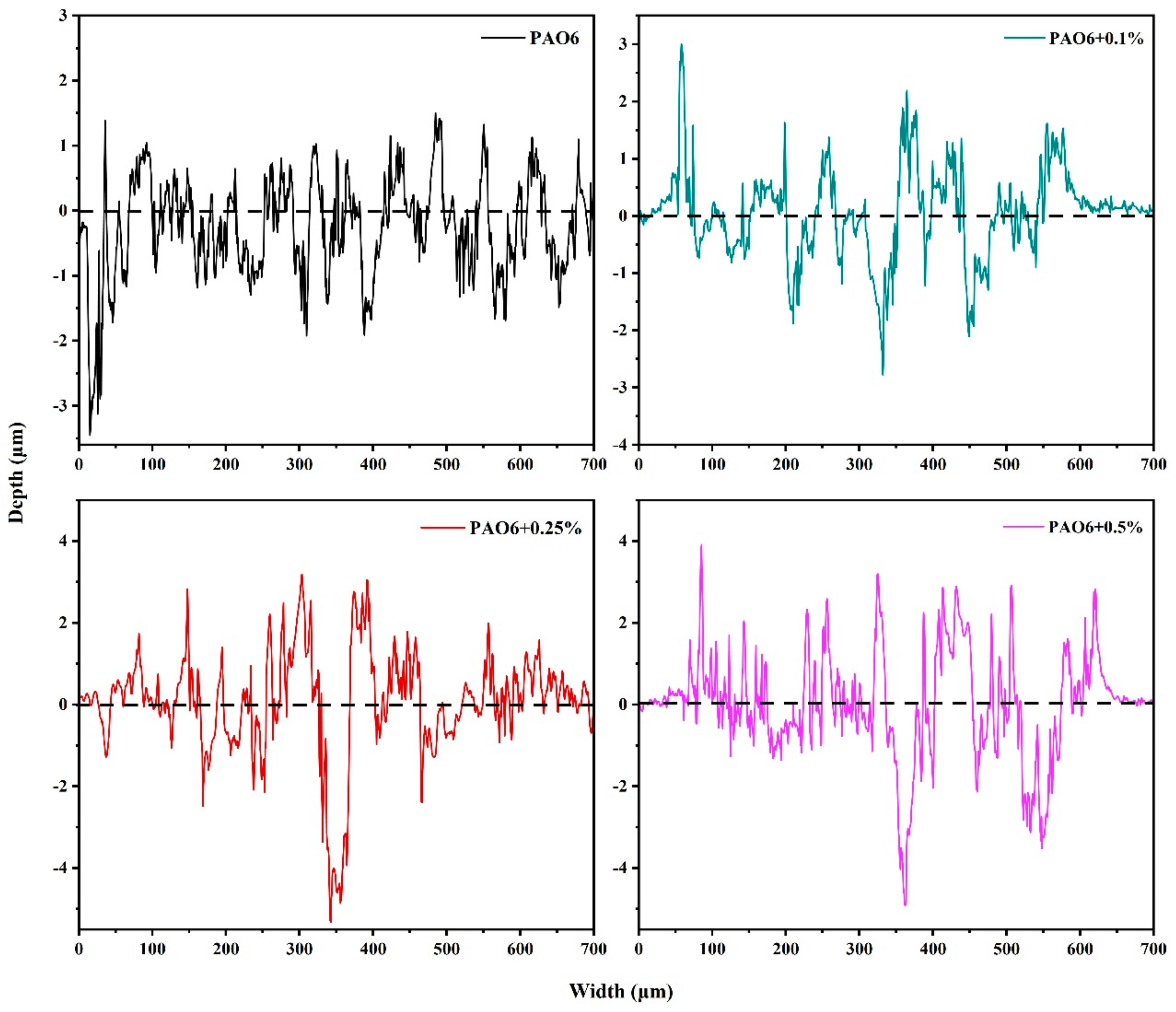



| Sample | Mean Wear Volume (μm3) |
|---|---|
| PAO6 | 28,763.738 |
| 0.1 wt% MnO2@BNNs | 11,004.551 |
| 0.25 wt% MnO2@BNNs | 20,887.380 |
| 0.5 wt% MnO2@BNNs | 24,573.150 |
| Sample | Viscosity (mPa·s) |
|---|---|
| PAO6 | 6.026 |
| Span80 | 26.14 |
| 3% Span80/0.1 wt% MnO2@h-BNNs/PAO6 | 6.371 |
| 3% Span80/0.25 wt% MnO2@h-BNNs/PAO6 | 6.567 |
| 3% Span80/0.5 wt% MnO2@h-BNNs/PAO6 | 6.620 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, W.; Zhang, L.; Zeng, M.; Yang, S.; Yang, Z.; Ola, O.; Thummavichai, K.; Wang, N.; Zhu, Y. Implanting MnO2 into Hexagonal Boron Nitride as Nanoadditives for Enhancing Tribological Performance. Crystals 2022, 12, 451. https://doi.org/10.3390/cryst12040451
Chen X, Chen W, Zhang L, Zeng M, Yang S, Yang Z, Ola O, Thummavichai K, Wang N, Zhu Y. Implanting MnO2 into Hexagonal Boron Nitride as Nanoadditives for Enhancing Tribological Performance. Crystals. 2022; 12(4):451. https://doi.org/10.3390/cryst12040451
Chicago/Turabian StyleChen, Xiaorong, Wenting Chen, Linyi Zhang, Minli Zeng, Shiming Yang, Zanhe Yang, Oluwafunmilola Ola, Kunyapat Thummavichai, Nannan Wang, and Yanqiu Zhu. 2022. "Implanting MnO2 into Hexagonal Boron Nitride as Nanoadditives for Enhancing Tribological Performance" Crystals 12, no. 4: 451. https://doi.org/10.3390/cryst12040451
APA StyleChen, X., Chen, W., Zhang, L., Zeng, M., Yang, S., Yang, Z., Ola, O., Thummavichai, K., Wang, N., & Zhu, Y. (2022). Implanting MnO2 into Hexagonal Boron Nitride as Nanoadditives for Enhancing Tribological Performance. Crystals, 12(4), 451. https://doi.org/10.3390/cryst12040451






