Exploring Nucleation Pathways in Distinct Physicochemical Environments Unveiling Novel Options to Modulate and Optimize Protein Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polarized and Depolarized Dynamic Light Scattering (DLS/DDLS) Experiments
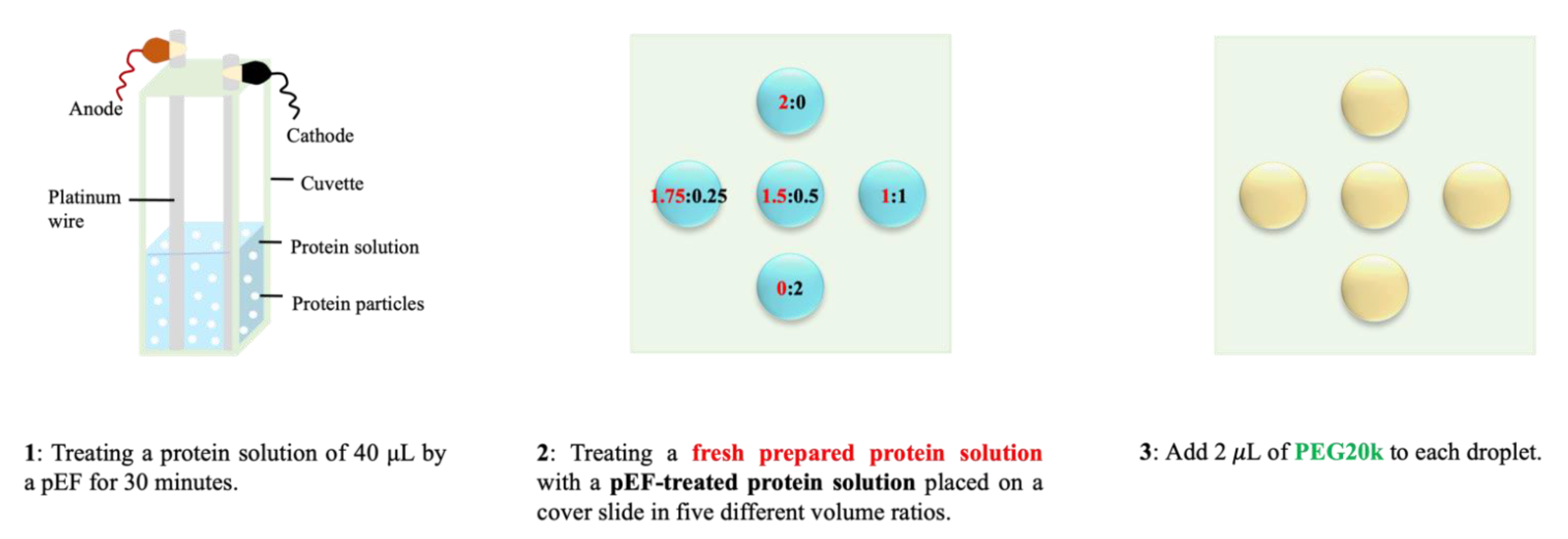
2.1.1. DDLS Instrument and Setup for Applying Pulsed Electric Field
2.1.2. Sample Preparation and Experimental Methods for DLS/DDLS Measurements
2.2. Measuring Diffusion Interaction Parameter—KD
2.3. Hanging-Drop Crystallization of Protein with Mesoscopic Ordered Clusters (MOCs) Induced by pEF
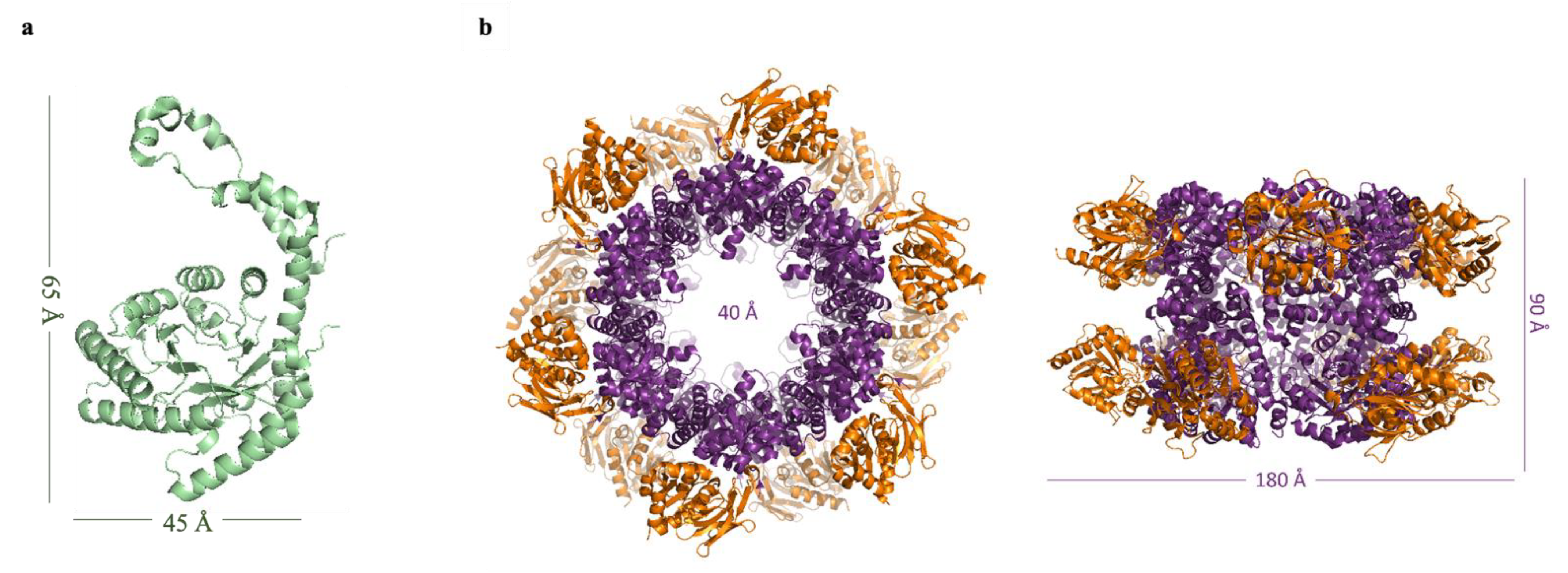
2.3.1. Glucose Isomerase
2.3.2. Pdx Complex
3. Results
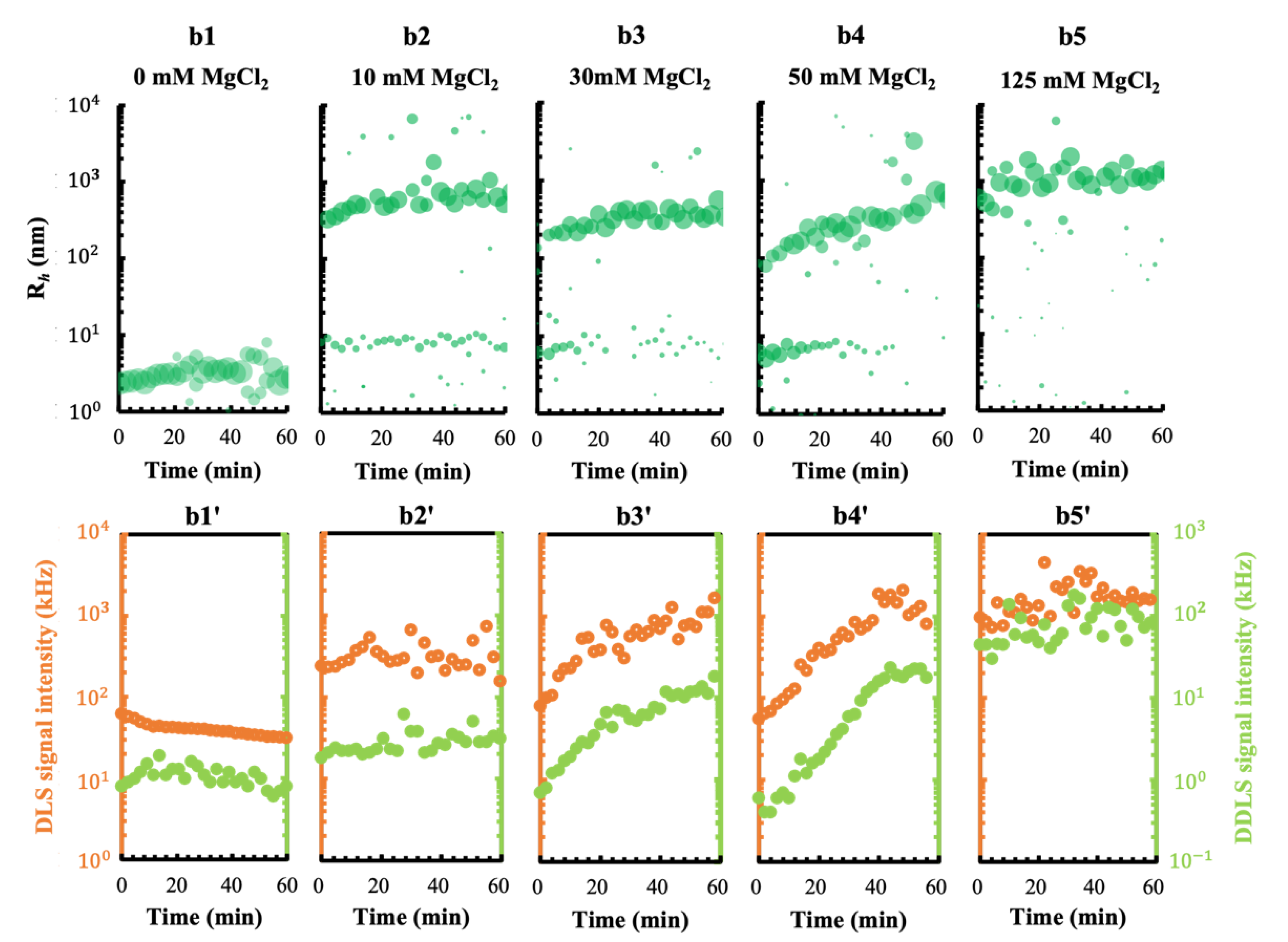
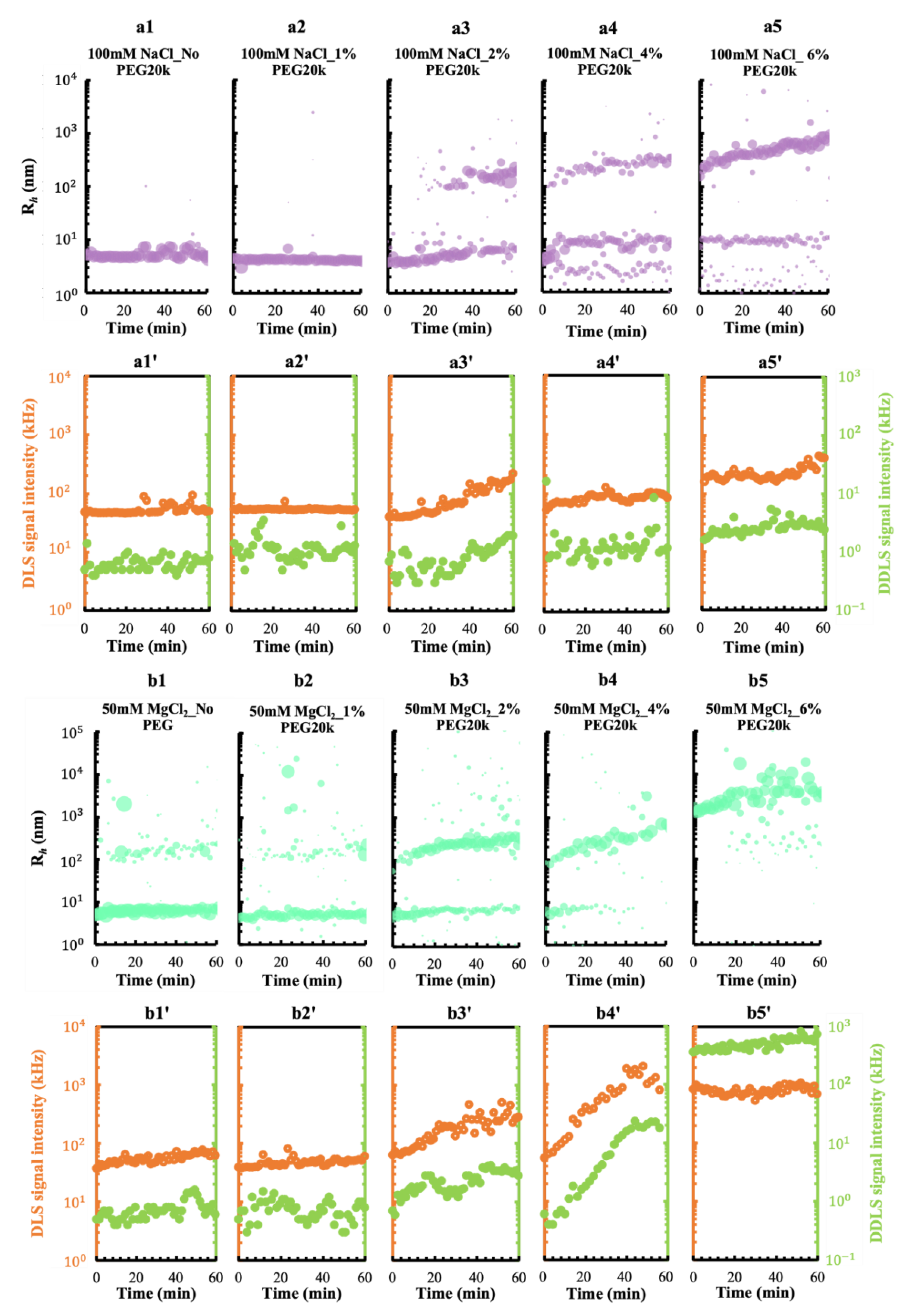
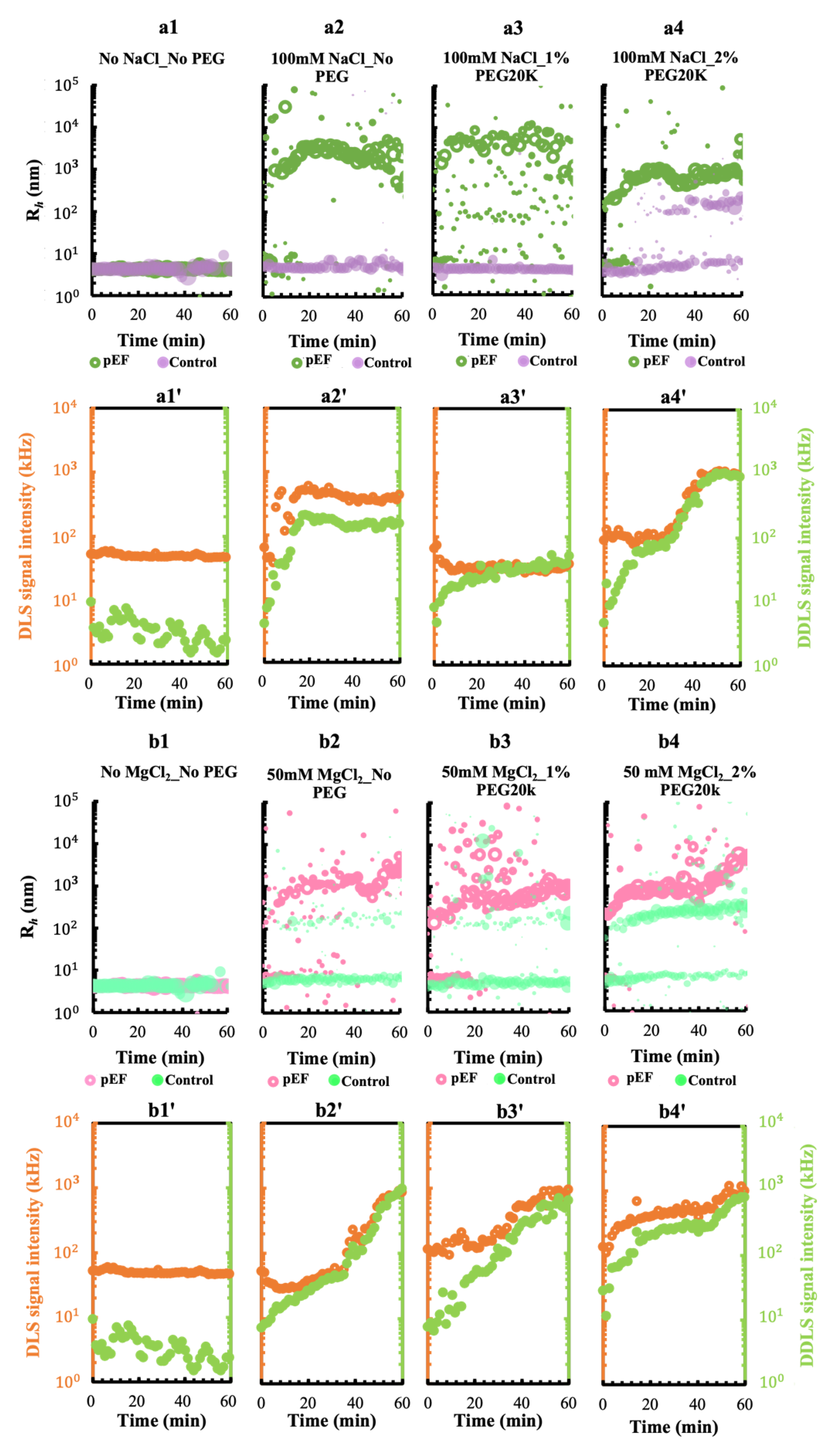
3.1. Effect of the Metal Cations Na+ and Mg2+ on Early Stage Condensing and Prenucleation Ordering of GI
3.2. Effect of a Crowding Agent on Condensing and Prenucleation Ordering of GI
3.3. Effect of a pEF on the Crystallization Process of GI under Different Ionic and Crowding Conditions
3.3.1. A pEF Modulate the Early Stage of Condensing and Prenucleation Ordering of GI
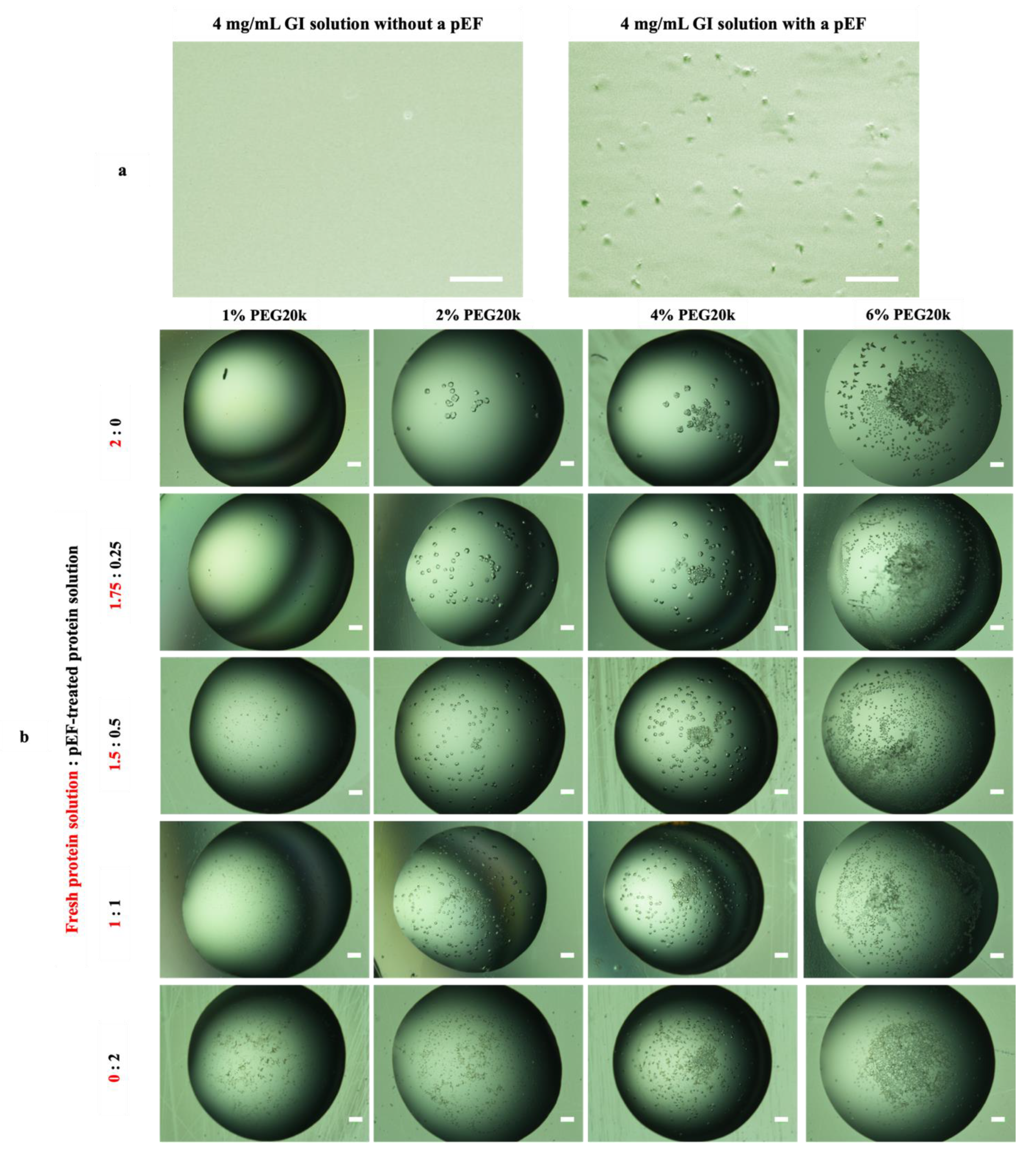
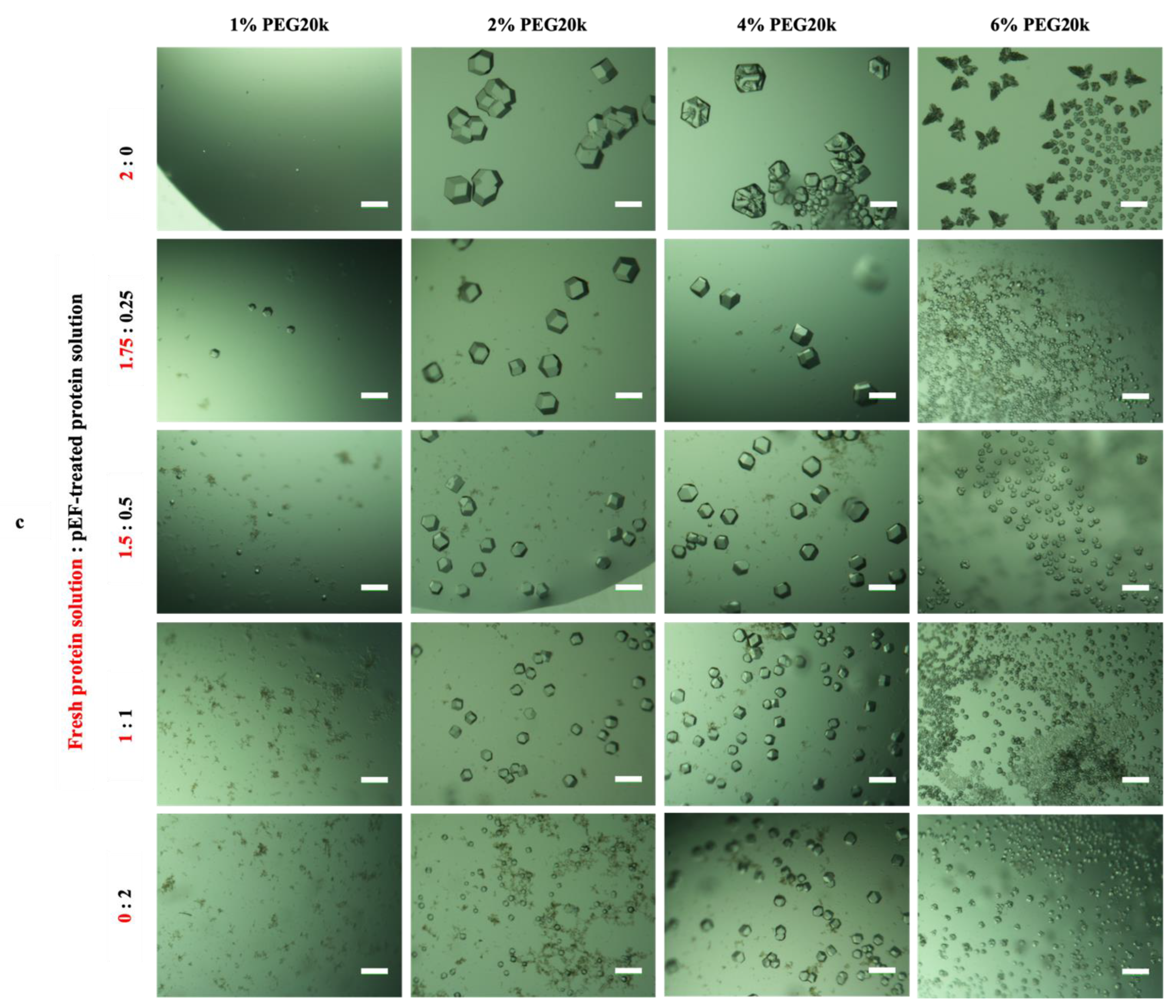
3.3.2. Effect of Mesoscopic Ordered Clusters (MOCs) Induced by a pEF on GI Crystallization
3.4. Effect of a pEF on Crystallizing the Pdx Complex from S. aureus
3.4.1. pEFs Promote the Early-Stage Ordering of Pdx Complex Nucleation
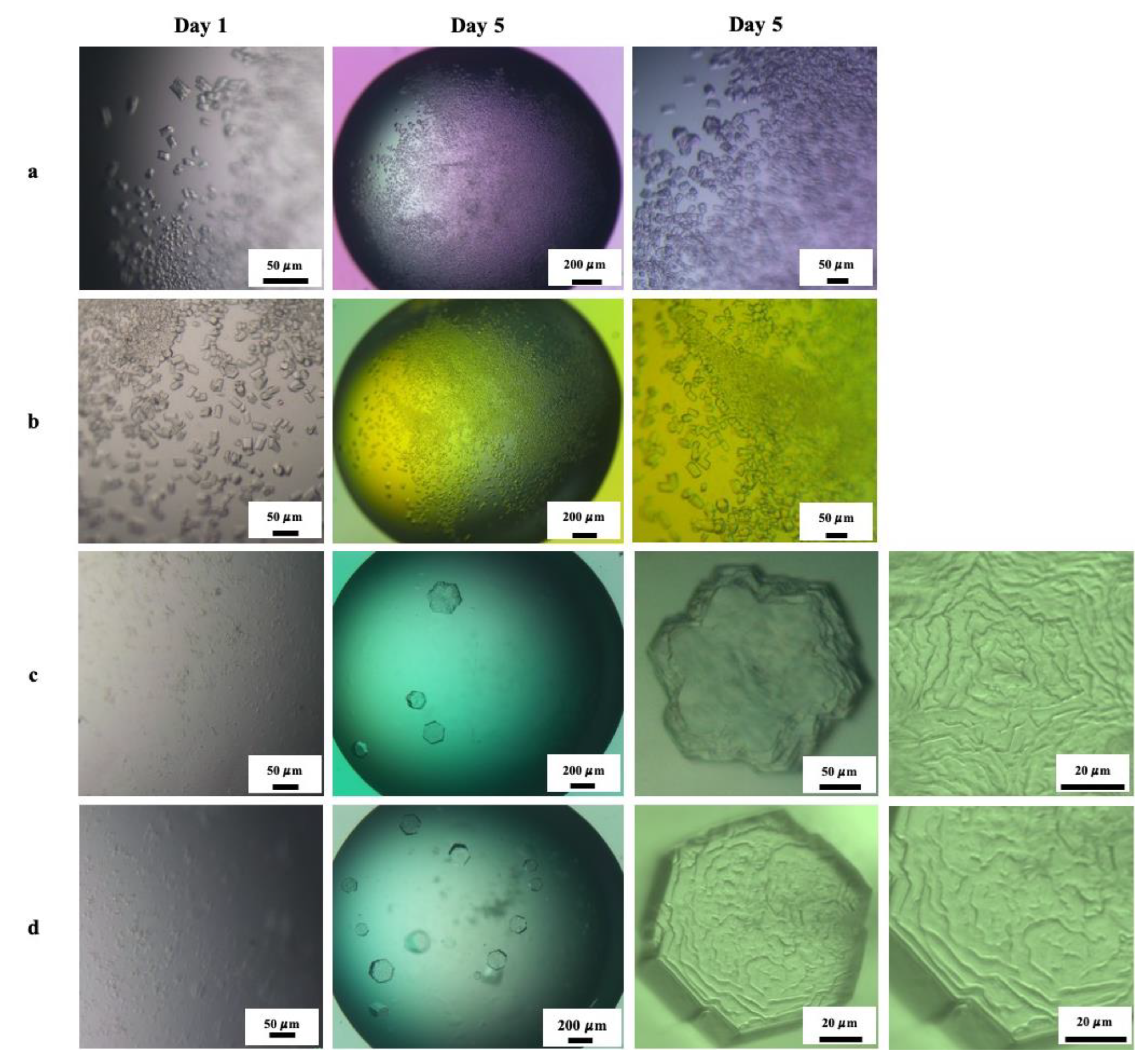
3.4.2. Effect of Mesoscopic Ordered Clusters (MOCs) Induced by a pEF on Pdx Crystallization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Volmer, M.; Weber, A. Keimbildung in übersättigten Gebilden. Z. Für Phys. Chem. 1926, 119, 277–301. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Goonetilleke, E.C.; Huang, X. Temperature-dependent kinetic pathways of heterogeneous ice nucleation competing between classical and non-classical nucleation. Nat. Commun. 2021, 12, 4954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gavira, J.A.; Lee, G.W.; Zahn, D. Nonclassical Nucleation—Role of Metastable Intermediate Phase in Crystal Nucleation: An Editorial Prefix. Crystals 2021, 11, 174. [Google Scholar] [CrossRef]
- Kashchiev, D. Classical nucleation theory approach to two-step nucleation of crystals. J. Cryst. Growth 2020, 530, 125300. [Google Scholar] [CrossRef]
- Jin, B.; Liu, Z.; Tang, R. Recent experimental explorations of non-classical nucleation. CrystEngComm 2020, 22, 4057–4073. [Google Scholar] [CrossRef]
- Brognaro, H.; Falke, S.; Mudogo, C.N.; Betzel, C. Multi-Step Concanavalin A Phase Separation and Early-Stage Nucleation Monitored Via Dynamic and Depolarized Light Scattering. Crystals 2019, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Van Driessche, A.E.S.; Van Gerven, N.; Bomans, P.H.H.; Joosten, R.R.M.; Friedrich, H.; Gil-Carton, D.; Sommerdijk, N.A.J.M.; Sleutel, M. Molecular nucleation mechanisms and control strategies for crystal polymorph selection. Nature 2018, 556, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Sleutel, M.; Van Driessche, A.E.S. Nucleation of protein crystals–a nanoscopic perspective. Nanoscale 2018, 10, 12256–12267. [Google Scholar] [CrossRef]
- Zhang, F. Nonclassical nucleation pathways in protein crystallization. J. Physics Condens. Matter 2017, 29, 443002. [Google Scholar] [CrossRef]
- Sauter, A.; Roosen-Runge, F.; Zhang, F.; Lotze, G.; Jacobs, R.M.J.; Schreiber, F. Real-Time Observation of Nonclassical Protein Crystallization Kinetics. J. Am. Chem. Soc. 2015, 137, 1485–1491. [Google Scholar] [CrossRef]
- Prestipino, S.; Laio, A.; Tosatti, E. Systematic Improvement of Classical Nucleation Theory. Phys. Rev. Lett. 2012, 108, 225701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Yang, J.; Kwon, S.G.; Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 2016, 1, 16034. [Google Scholar] [CrossRef]
- Ten Wolde, P.R.; Frenkel, D. Enhancement of Protein Crystal Nucleation by Critical Censity Fluctuations. Science 1997, 277, 1975–1978. [Google Scholar] [CrossRef] [Green Version]
- Galkin, O.; Vekilov, P.G. Control of protein crystal nucleation around the metastable liquid–liquid phase boundary. Proc. Natl. Acad. Sci. USA 2000, 97, 6277–6281. [Google Scholar] [CrossRef] [Green Version]
- Vekilov, P.G. Dense Liquid Precursor for the Nucleation of Ordered Solid Phases from Solution. Cryst. Growth Des. 2004, 4, 671–685. [Google Scholar] [CrossRef]
- Dumetz, A.C.; Chockla, A.M.; Kaler, E.W.; Lenhoff, A.M. Comparative Effects of Salt, Organic, and Polymer Precipitants on Protein Phase Behavior and Implications for Vapor Diffusion. Cryst. Growth Des. 2009, 9, 682–691. [Google Scholar] [CrossRef]
- Savage, J.R.; Dinsmore, A.D. Experimental Evidence for Two-Step Nucleation in Colloidal Crystallization. Phys. Rev. Lett. 2009, 102, 198302. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Dai, L.; Douglas, E.P.; Gower, L.B. Compositional analysis of a polymer-induced liquid-precursor (PILP) amorphous CaCO3 phase. J. Non-Cryst. Solids 2008, 354, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, L.; Cui, M.; Ma, L.; Cao, F. A Cationic Polyelectrolyte-Controlled Liquid Mineral Precursor Process in the BaCO 3 System. Eur. J. Inorg. Chem. 2015, 2015, 1819–1826. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cui, M.; Wang, J.; Zha, J. Crystallization of the polymer induced liquid mineral precursor in the constraint nanopores. J. Cryst. Growth 2018, 507, 362–369. [Google Scholar] [CrossRef]
- Ha, T.; Kang, S. Crystallization Mechanism and Photoluminescence Properties of CaF2–Al2O3–SiO2: Eu Glass-Ceramics Based on Nano-Scale Phase Separation Phenomenon. J. Nanosci. Nanotechnol. 2020, 20, 6609–6615. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Meyer, A.; Baitan, D.; Dierks, K.; Perbandt, M.; Betzel, C. Real-Time Observation of Protein Dense Liquid Cluster Evolution during Nucleation in Protein Crystallization. Cryst. Growth Des. 2017, 17, 954–958. [Google Scholar] [CrossRef] [Green Version]
- Mosses, J.; Turton, D.A.; Lue, L.; Sefcik, J.; Wynne, K. Crystal templating through liquid–liquid phase separation. Chem. Commun. 2015, 51, 1139–1142. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Chen, L.; Zhang, B.; Si, G.; Liu, Y.J. Optically isotropic, electrically tunable liquid crystal droplet arrays formed by photopolymerization-induced phase separation. Opt. Lett. 2015, 40, 2723–2726. [Google Scholar] [CrossRef]
- Trilisky, E.; Gillespie, R.; Osslund, T.D.; Vunnum, S. Crystallization and liquid-liquid phase separation of monoclonal antibodies and fc-fusion proteins: Screening results. Biotechnol. Prog. 2011, 27, 1054–1067. [Google Scholar] [CrossRef]
- Mudogo, C.N.; Falke, S.; Brognaro, H.; Duszenko, M.; Betzel, C. Protein phase separation and determinants of in cell crystallization. Traffic 2020, 21, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Hasecke, F.; Miti, T.; Perez, C.; Barton, J.; Schölzel, D.; Gremer, L.; Grüning, C.S.R.; Matthews, G.; Meisl, G.; Knowles, T.P.J.; et al. Origin of metastable oligomers and their effects on amyloid fibril self-assembly. Chem. Sci. 2018, 9, 5937–5948. [Google Scholar] [CrossRef] [Green Version]
- Hardenberg, M.C.; Sinnige, T.; Casford, S.; Dada, S.T.; Poudel, C.; Robinson, E.A.; Fuxreiter, M.; Kaminksi, C.F.; Kaminski-Schierle, G.S.; Nollen, E.A.A.; et al. Observation of an α-synuclein liquid droplet state and its maturation into Lewy body-like assemblies. J. Mol. Cell Biol. 2021, 13, 282–294. [Google Scholar] [CrossRef]
- Sawner, A.S.; Ray, S.; Yadav, P.; Mukherjee, S.; Panigrahi, R.; Poudyal, M.; Patel, K.; Ghosh, D.; Kummerant, E.; Kumar, A.; et al. Modulating α-Synuclein Liquid–Liquid Phase Separation. Biochemistry 2021, 60, 3676–3696. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; Vekilov, P.G.; Kolomeisky, A.B. Kinetics of two-step nucleation of crystals. J. Chem. Phys. 2005, 122, 244706. [Google Scholar] [CrossRef] [PubMed]
- Vekilov, P.G. The two-step mechanism of nucleation of crystals in solution. Nanoscale 2010, 2, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Vekilov, P.G. Nucleation. Cryst. Growth Des. 2010, 10, 5007–5019. [Google Scholar] [CrossRef] [PubMed]
- Sleutel, M.; Van Driessche, A.E.S. Role of clusters in nonclassical nucleation and growth of protein crystals. Proc. Natl. Acad. Sci. USA 2014, 111, E546–E553. [Google Scholar] [CrossRef] [Green Version]
- Houben, L.; Weissman, H.; Wolf, S.G.; Rybtchinski, B. A mechanism of ferritin crystallization revealed by cryo-STEM tomography. Nature 2020, 579, 540–543. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Van Gerven, N.; Joosten, R.R.M.; Ling, W.L.; Bacia, M.; Sommerdijk, N.; Sleutel, M. Nucleation of protein mesocrystals via oriented attachment. Nat. Commun. 2021, 12, 3902. [Google Scholar] [CrossRef]
- Holloway, L.; Roche, A.; Marzouk, S.; Uddin, S.; Ke, P.; Ekizoglou, S.; Curtis, R. Determination of Protein-Protein Interactions at High Co-Solvent Concentrations Using Static and Dynamic Light Scattering. J. Pharm. Sci. 2020, 109, 2699–2709. [Google Scholar] [CrossRef]
- Da Vela, S.; Begam, N.; Dyachok, D.; Schäufele, R.S.; Matsarskaia, O.; Braun, M.K.; Girelli, A.; Ragulskaya, A.; Mariani, A.; Zhang, F.; et al. Interplay between Glass Formation and Liquid–Liquid Phase Separation Revealed by the Scattering Invariant. J. Phys. Chem. Lett. 2020, 11, 7273–7278. [Google Scholar] [CrossRef]
- Begam, N.; Matsarskaia, O.; Sztucki, M.; Zhang, F.; Schreiber, F. Unification of lower and upper critical solution temperature phase behavior of globular protein solutions in the presence of multivalent cations. Soft Matter 2020, 16, 2128–2134. [Google Scholar] [CrossRef]
- Braun, M.K.; Sauter, A.; Matsarskaia, O.; Wolf, M.; Roosen-Runge, F.; Sztucki, M.; Roth, R.; Zhang, F.; Schreiber, F. Reentrant Phase Behavior in Protein Solutions Induced by Multivalent Salts: Strong Effect of Anions Cl− Versus NO3−. J. Phys. Chem. B 2018, 122, 11978–11985. [Google Scholar] [CrossRef] [PubMed]
- Salje, E.K.H. Multi-scaling and mesoscopic structures. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2010, 368, 1163–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, A.L.C.; Dantas, L.D.O.C.; Morão, L.G.; Gutierrez, R.F.; Polikarpov, I.; Wrenger, C.; Nascimento, A.S. Essential Metabolic Routes as a Way to ESKAPE From Antibiotic Resistance. Front. Public Health 2020, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Strohmeier, M.; Raschle, T.; Mazurkiewicz, J.; Rippe, K.; Sinning, I.; Fitzpatrick, T.B.; Tews, I. Structure of a bacterial pyridoxal 5′-phosphate synthase complex. Proc. Natl. Acad. Sci. USA 2006, 103, 19284–19289. [Google Scholar] [CrossRef] [Green Version]
- Lobley, C.M.C.; Sandy, J.; Sanchez-Weatherby, J.; Mazzorana, M.; Krojer, T.; Nowak, R.P.; Sørensen, T.L.-M. A generic protocol for protein crystal dehydration using the HC1b humidity controller. Acta Crystallogr. Sect. D Struct. Biol. 2016, 72, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Bolisetty, S.; Harnau, L.; Jung, J.-M.; Mezzenga, R. Gelation, Phase Behavior, and Dynamics of β-Lactoglobulin Amyloid Fibrils at Varying Concentrations and Ionic Strengths. Biomacromolecules 2012, 13, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Meyer, A.; Dierks, K.; Kapis, S.; Reimer, R.; Einspahr, H.; Perbandt, M.; Betzel, C. Reliably distinguishing protein nanocrystals from amorphous precipitate by means of depolarized dynamic light scattering. J. Appl. Crystallogr. 2015, 48, 1476–1484. [Google Scholar] [CrossRef]
- Balog, S.; Rodriguez-Lorenzo, L.; Monnier, C.A.; Obiols-Rabasa, M.; Rothen-Rutishauser, B.; Schurtenberger, P.; Petri-Fink, A. Characterizing nanoparticles in complex biological media and physiological fluids with depolarized dynamic light scattering. Nanoscale 2015, 7, 5991–5997. [Google Scholar] [CrossRef] [Green Version]
- Chayen, N.; Dieckmann, M.; Dierks, K.; Fromme, P. Size and Shape Determination of Proteins in Solution by a Noninvasive Depolarized Dynamic Light Scattering Instrument. Ann. N. Y. Acad. Sci. 2004, 1027, 20–27. [Google Scholar] [CrossRef]
- Vailati, A.; Asnaghi, D.; Giglio, M.; Piazza, R. Depolarized dynamic light scattering from optically anisotropic reaction-limited aggregates. Phys. Rev. E 1993, 48, R2358–R2361. [Google Scholar] [CrossRef]
- Ullah, N.; Andaleeb, H.; Mudogo, C.N.; Falke, S.; Betzel, C.; Wrenger, C. Solution Structures and Dynamic Assembly of the 24-Meric Plasmodial Pdx1–Pdx2 Complex. Int. J. Mol. Sci. 2020, 21, 5971. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Falke, S.; Schubert, R.; Lorenzen, K.; Cheng, Q.-D.; Exner, C.; Brognaro, H.; Mudogo, C.N.; Betzel, C. Pulsed electric fields induce modulation of protein liquid–liquid phase separation. Soft Matter 2020, 16, 8547–8553. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R. Preparation and Scoring of Protein Nano- and Microcrystals for Synchrotron and Free-Electron Laser X-ray Radiation Sources. Ph.D. Thesis, University Hamburg, Hamburg, Germany, 2016; p. 157. [Google Scholar]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Einstein, A. Theory of the Brownian movements. Ann. Phys. 1905, 17, 549. [Google Scholar] [CrossRef] [Green Version]
- Debye, P. Polar Molecules; The Chemical Catalog Company, Inc.: Dover, NY, USA, 1929. [Google Scholar]
- Kenrick, S.; Some, D. The Diffusion Interaction Parameter (kD) as an Indicator of Colloidal and Thermal Stability; Wyatt Technology Corporation: Santa Barbara, CA, USA, 2014; pp. 1–6. [Google Scholar]
- Roberts, D.; Keeling, R.; Tracka, M.; van der Walle, C.F.; Uddin, S.; Warwicker, J.; Curtis, R. The Role of Electrostatics in Protein–Protein Interactions of a Monoclonal Antibody. Mol. Pharm. 2014, 11, 2475–2489. [Google Scholar] [CrossRef]
- Kozak, M. Glucose isomerase from Streptomyces rubiginosus–potential molecular weight standard for small-angle X-ray scattering. J. Appl. Crystallogr. 2005, 38, 555–558. [Google Scholar] [CrossRef]
- Falke, S.; Brognaro, H.; Martirosyan, A.; Dierks, K.; Betzel, C. A multi-channel in situ light scattering instrument utilized for monitoring protein aggregation and liquid dense cluster formation. Heliyon 2019, 5, e03016. [Google Scholar] [CrossRef] [Green Version]
- Oberthuer, D.; Melero-García, E.; Dierks, K.; Meyer, A.; Betzel, C.; Garcia-Caballero, A.; Gavira, J.A. Monitoring and Scoring Counter-Diffusion Protein Crystallization Experiments in Capillaries by in situ Dynamic Light Scattering. PLoS ONE 2012, 7, e33545. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Miklos, A.C.; Li, C.; Sharaf, N.G.; Pielak, G.J. Volume Exclusion and Soft Interaction Effects on Protein Stability under Crowded Conditions. Biochemistry 2010, 49, 6984–6991. [Google Scholar] [CrossRef] [Green Version]
- Zitserman, V.Y.; Stojilkovich, K.S.; Berezhkovskii, A.M.; Bezrukov, S.M. Electrical conductivity of aqueous solutions of polyethylene glycol. Russ. J. Phys. Chem. A 2005, 79, 1083–1089. [Google Scholar]
- McPherson, A.; Kuznetsov, Y.G. Mechanisms, kinetics, impurities and defects: Consequences in macromolecular crystallization. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 384–403. [Google Scholar] [CrossRef] [PubMed]
- Bye, J.W.; Curtis, R.A. Controlling Phase Separation of Lysozyme with Polyvalent Anions. J. Phys. Chem. B 2018, 123, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.-E.; Hwang, K.Y.; Nam, K.H. Structural analysis of substrate recognition by glucose isomerase in Mn2+ binding mode at M2 site in S. rubiginosus. Biochem. Biophys. Res. Commun. 2018, 503, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Roosen-Runge, F.; Zhang, F.; Schreiber, F.; Roth, R. Ion-activated attractive patches as a mechanism for controlled protein interactions. Sci. Rep. 2014, 4, 7016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staneva, I.; Frenkel, D. The role of non-specific interactions in a patchy model of protein crystallization. J. Chem. Phys. 2015, 143, 194511. [Google Scholar] [CrossRef] [Green Version]
- Hedges, L.O.; Whitelam, S. Limit of validity of Ostwald’s rule of stages in a statistical mechanical model of crystallization. J. Chem. Phys. 2011, 135, 164902. [Google Scholar] [CrossRef] [Green Version]
- Whitelam, S. Control of Pathways and Yields of Protein Crystallization through the Interplay of Nonspecific and Specific Attractions. Phys. Rev. Lett. 2010, 105, 88102. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Barra, A.L.C.; Brognaro, H.; Betzel, C. Exploring Nucleation Pathways in Distinct Physicochemical Environments Unveiling Novel Options to Modulate and Optimize Protein Crystallization. Crystals 2022, 12, 437. https://doi.org/10.3390/cryst12030437
Wang M, Barra ALC, Brognaro H, Betzel C. Exploring Nucleation Pathways in Distinct Physicochemical Environments Unveiling Novel Options to Modulate and Optimize Protein Crystallization. Crystals. 2022; 12(3):437. https://doi.org/10.3390/cryst12030437
Chicago/Turabian StyleWang, Mengying, Angélica Luana C. Barra, Hévila Brognaro, and Christian Betzel. 2022. "Exploring Nucleation Pathways in Distinct Physicochemical Environments Unveiling Novel Options to Modulate and Optimize Protein Crystallization" Crystals 12, no. 3: 437. https://doi.org/10.3390/cryst12030437
APA StyleWang, M., Barra, A. L. C., Brognaro, H., & Betzel, C. (2022). Exploring Nucleation Pathways in Distinct Physicochemical Environments Unveiling Novel Options to Modulate and Optimize Protein Crystallization. Crystals, 12(3), 437. https://doi.org/10.3390/cryst12030437






