Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
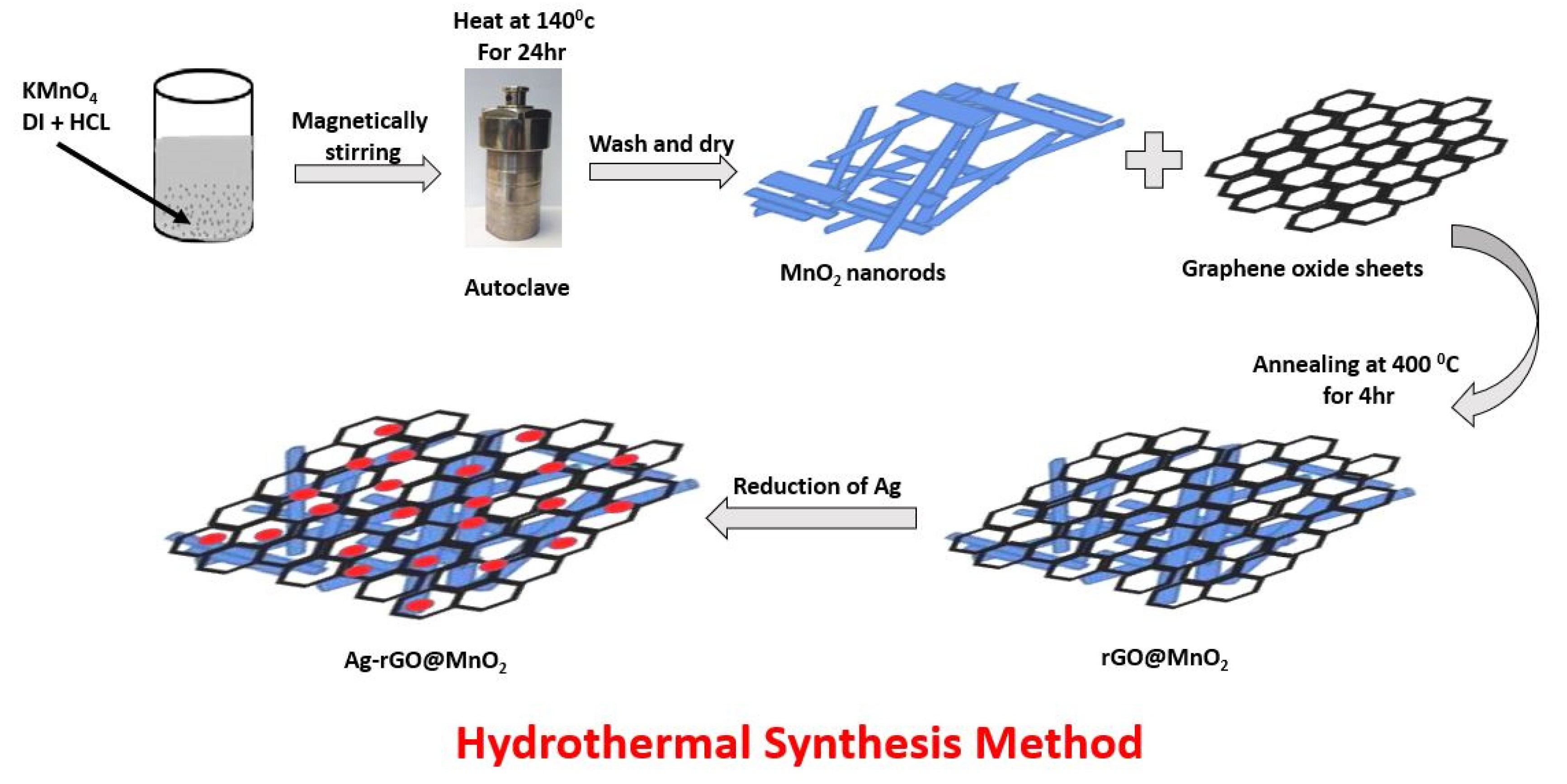
2.2. Synthesis of Electrode Materials
2.3. Characterization Technique
2.4. Development of Electrodes and Electrochemical Measurements
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. FESEM and EDX Analysis
3.3. TEM Analysis
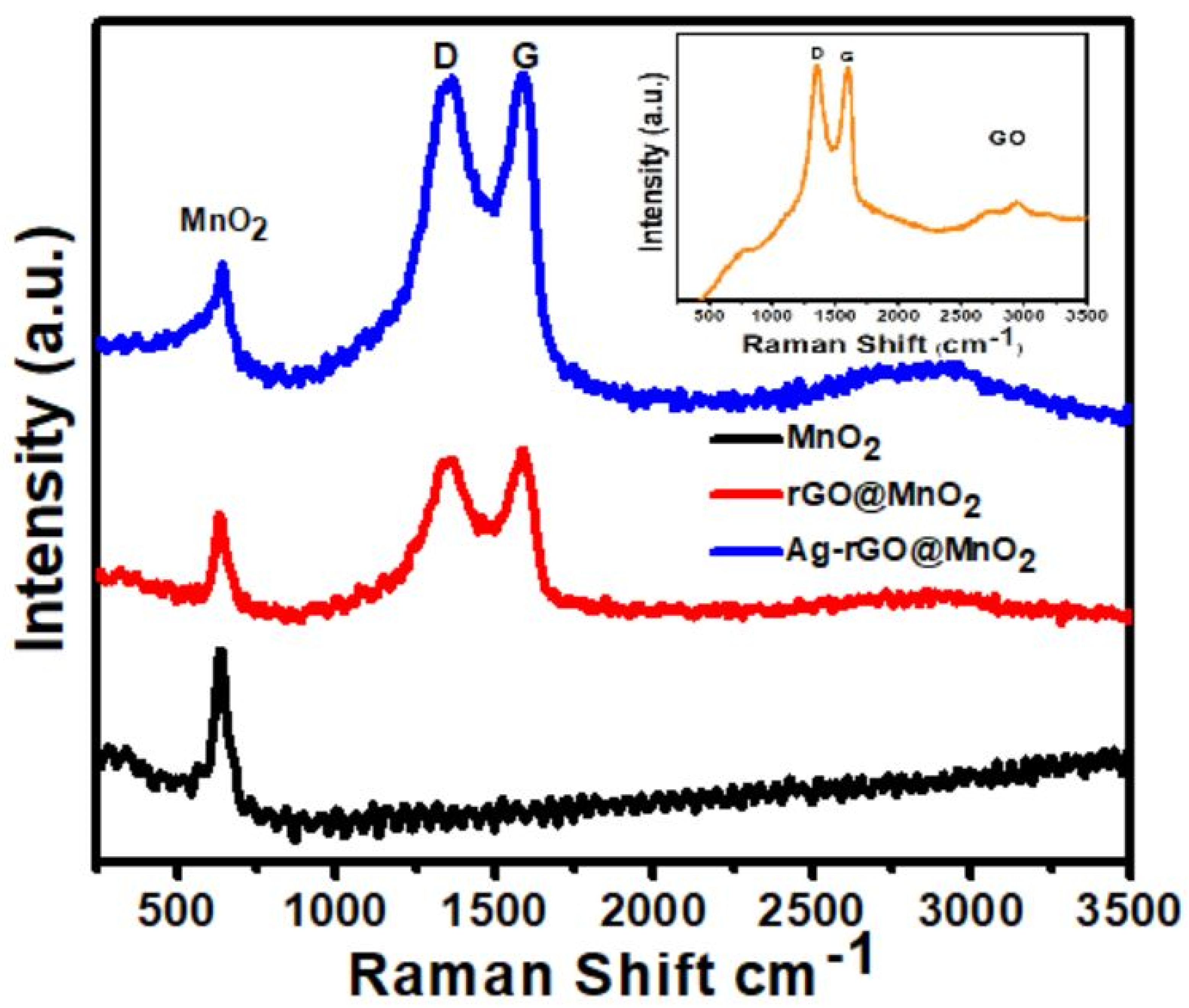
3.4. Raman Analysis
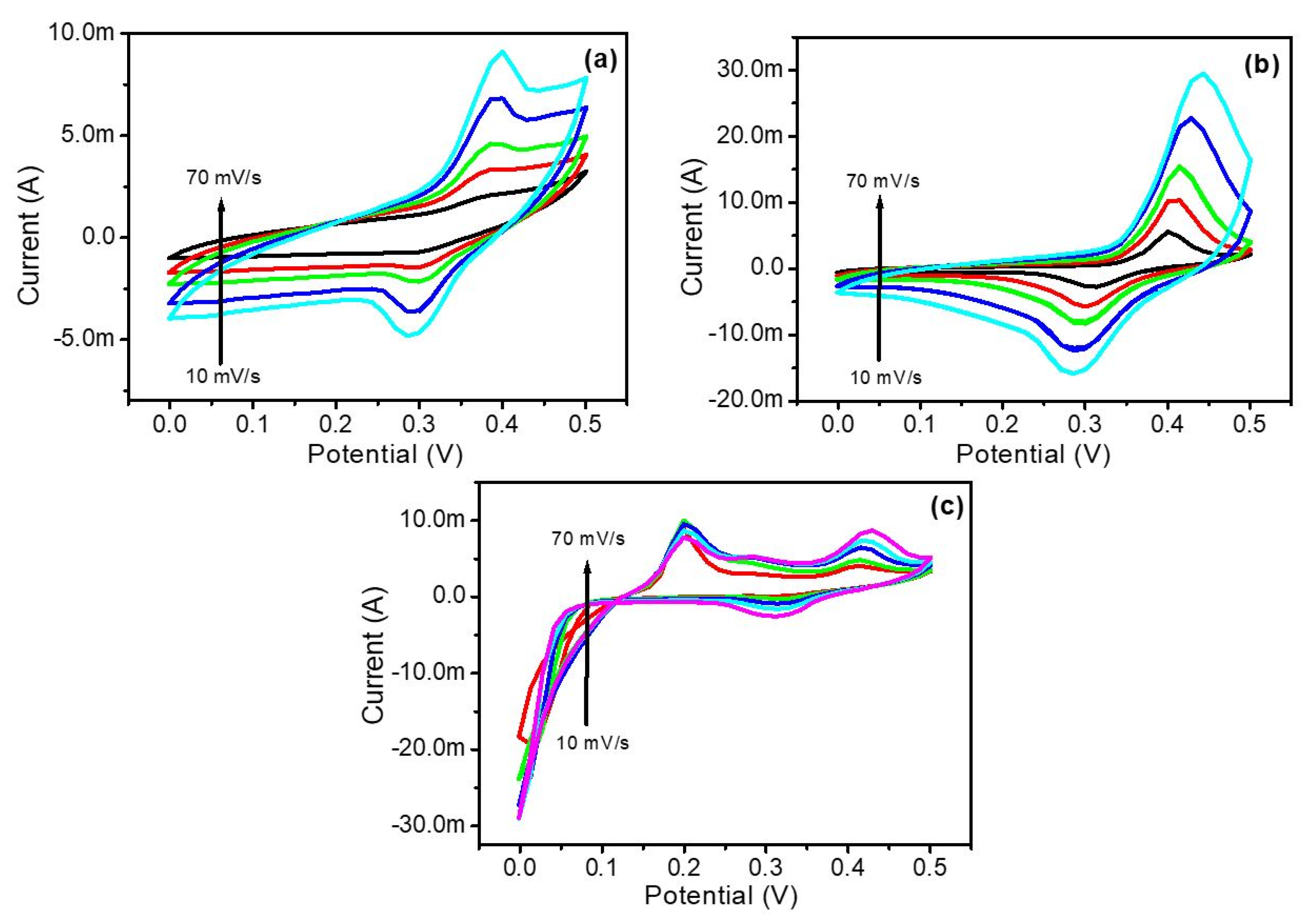
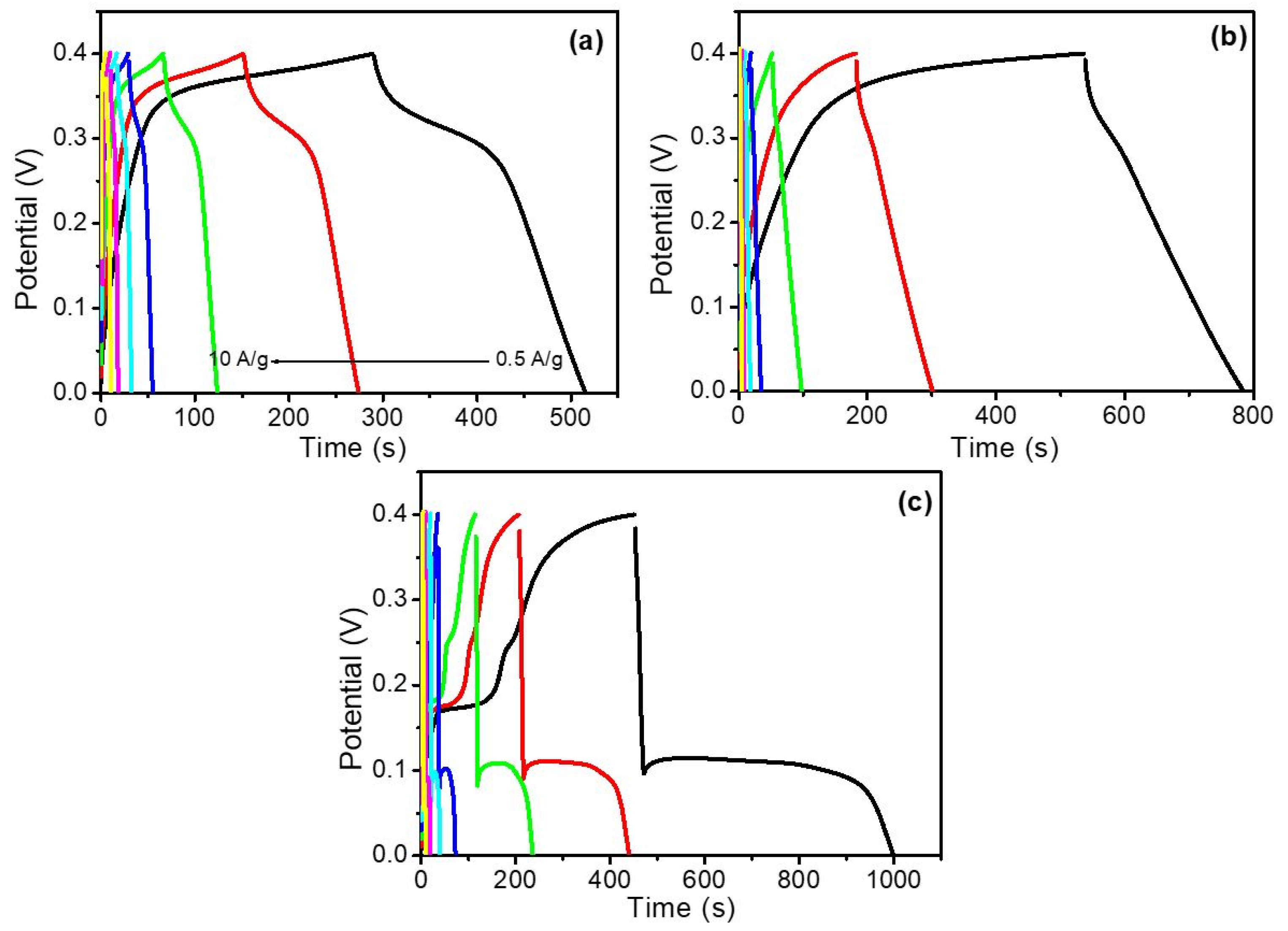
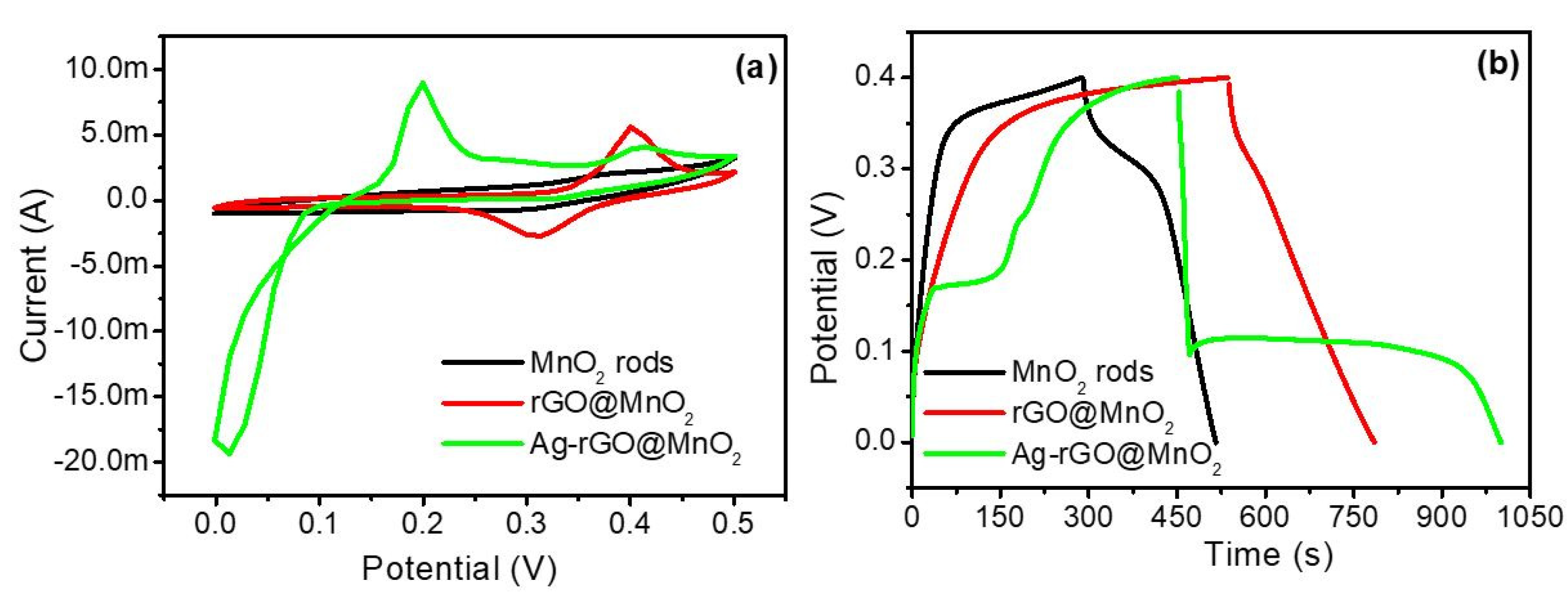
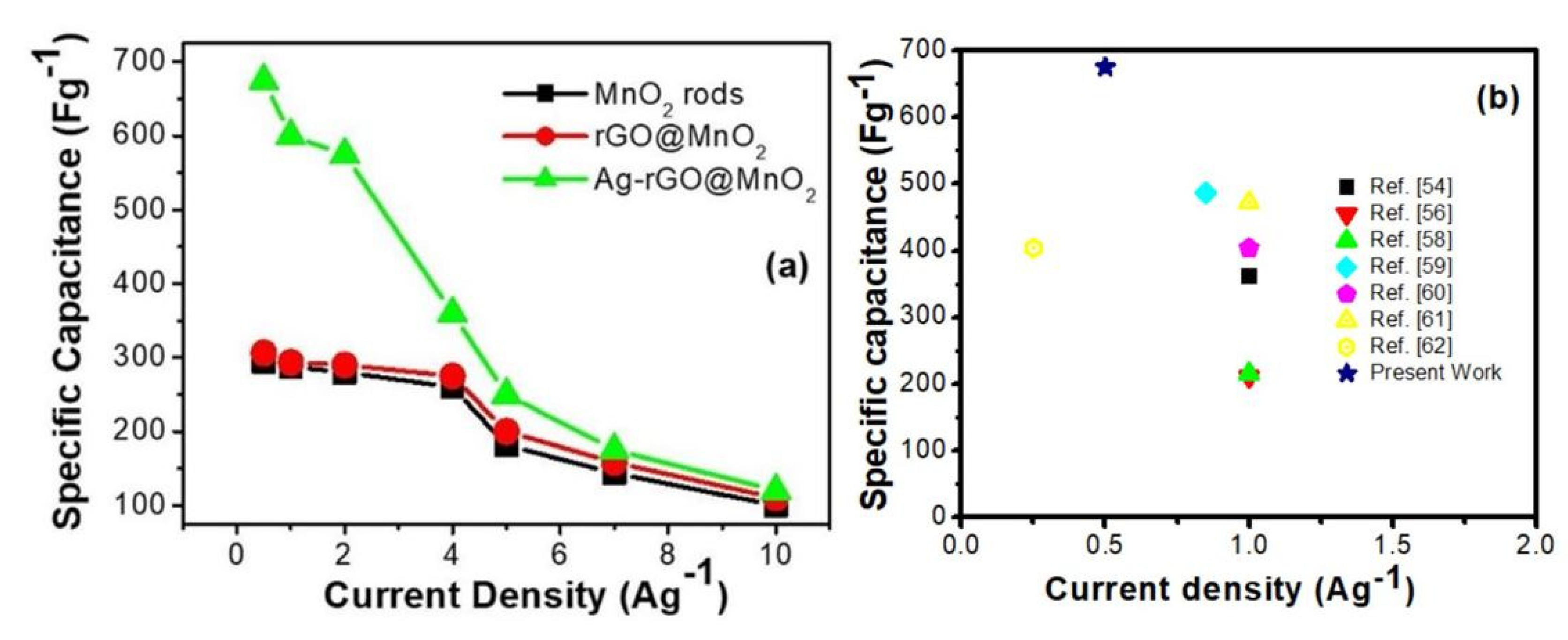
3.5. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires. Energy Environ. Sci. 2011, 4, 1813–1819. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Sci. Mag. 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.A.; Parveen, N.; Han, T.H.; Ansari, M.O.; Cho, M.H. Fibrous polyaniline@ manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053–9060. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Chang, D.W.; Dai, L.; Baek, J.-B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Manthiram, A. Nanocrystalline manganese oxides for electrochemical capacitors with neutral electrolytes. J. Electrochem. Soc. 2002, 149, A1419. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, L.; Zhang, M.; Guo, S.; Liu, Z.-H.; Yang, Z.; Wang, Z. Preparation of Ag-nanoparticle-loaded MnO2 nanosheets and their capacitance behavior. Energy Fuels 2012, 26, 618–623. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile synthesis of three-dimensional ternary ZnCo2O4/reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Iwama, E.; Kisu, K.; Naoi, W.; Simon, P.; Naoi, K. Enhanced hybrid supercapacitors utilizing nanostructured metal oxides. In Metal Oxides in Supercapacitors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 247–264. [Google Scholar]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Shahid, M.M.; Ismail, A.H.; AL-Mokaram, A.M.A.A.; Vikneswaran, R.; Ahmad, S.; Hamza, A.; Numan, A. A single-step synthesis of nitrogen-doped graphene sheets decorated with cobalt hydroxide nanoflakes for the determination of dopamine. Prog. Nat. Sci. Mater. Int. 2017, 27, 582–587. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Wang, L.; Qin, X.; Tian, J.; Lu, W.; Chang, G.; Sun, X. One-pot green synthesis of Ag nanoparticles-graphene nanocomposites and their applications in SERS, H2 O2, and glucose sensing. RSC Adv. 2012, 2, 538–545. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J. Basic principles for observing the photosensitizer role of graphene in the graphene–semiconductor composite photocatalyst from a case study on graphene–ZnO. J. Phys. Chem. C 2013, 117, 21724–21734. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Yang, X.; Dou, X.; Rouhanipour, A.; Zhi, L.; Räder, H.J.; Müllen, K. Two-dimensional graphene nanoribbons. J. Am. Chem. Soc. 2008, 130, 4216–4217. [Google Scholar] [CrossRef]
- Buchsteiner, A.; Lerf, A.; Pieper, J. Water dynamics in graphite oxide investigated with neutron scattering. J. Phys. Chem. B 2006, 110, 22328–22338. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Ali, G.A.; Makhlouf, S.A.; Yusoff, M.M.; Chong, K.F. Structural and electrochemical characteristics of graphene nanosheets as supercapacitor electrodes. Rev. Adv. Mater. Sci. 2015, 41, 35–43. [Google Scholar]
- Ali, G.A.; Yusoff, M.M.; Chong, K.F. Graphene: Electrochemical production and its energy storage properties. ARPN J. Eng. Appl. Sci. 2016, 11, 9712–9717. [Google Scholar]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G. Flexible graphene devices related to energy conversion and storage. Energy Environ. Sci. 2015, 8, 790–823. [Google Scholar] [CrossRef]
- Yang, J.; Gunasekaran, S. Electrochemically reduced graphene oxide sheets for use in high performance supercapacitors. Carbon 2013, 51, 36–44. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Wang, Y.; Yuan, H. Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J. Power Sources 2014, 245, 101–106. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene oxide− MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Huang, N.; Lim, H. Solvothermal synthesis of SnO2/graphene nanocomposites for supercapacitor application. Ceram. Int. 2013, 39, 6647–6655. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Yun, G.; Shi, K.; Lv, X.; Yang, B. High-performance solid-state supercapacitors based on graphene-ZnO hybrid nanocomposites. Nanoscale Res. Lett. 2013, 8, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, D.; Zhu, J.; Liang, X. Preparation of Co3O4 nanoplate/graphene sheet composites and their synergistic electrochemical performance. Ionics 2013, 19, 215–220. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Marina, P.E.; Ali, G.A.; See, L.M.; Teo, E.Y.L.; Ng, E.-P.; Chong, K.F. In situ growth of redox-active iron-centered nanoparticles on graphene sheets for specific capacitance enhancement. Arab. J. Chem. 2019, 12, 3883–3889. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.M.; He, Z.Q.; Chen, S.; Ma, M.Y.; Xiao, Z.B.; Liu, J.B. Silver-doped lithium manganese oxide thin films prepared by solution deposition. Mater. Lett. 2006, 60, 2497–2500. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Sung, Y.-E.; Kim, W.B.; Seong, T.-Y. Crystalline Ag nanocluster-incorporated RuO2 as an electrode material for thin-film micropseudocapacitors. Electrochem. Solid-State Lett. 2008, 11, A112. [Google Scholar] [CrossRef]
- Pasricha, R.; Gupta, S.; Srivastava, A.K. A facile and novel synthesis of Ag–graphene-based nanocomposites. Small 2009, 5, 2253–2259. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Yang, J.; Zhu, G.; Chen, K. A novel reduced graphene oxide/Ag/CeO2 ternary nanocomposite: Green synthesis and catalytic properties. Appl. Catal. B 2014, 144, 454–461. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Wang, Y.; Ma, Y.; Liu, Z.; Chen, Y. Synthesis and characterization of a graphene–C60 hybrid material. Carbon 2009, 47, 334–337. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Cho, M.H. Facile electrochemical assisted synthesis of ZnO/graphene nanosheets with enhanced photocatalytic activity. RSC Adv. 2015, 5, 97788–97797. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. A comparison study on Raman scattering properties of α-and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Prasad, K.G.; Sen, A.; Maiyalagan, T. Enhanced pseudocapacitance from finely ordered pristine α-MnO2 nanorods at favourably high current density using redox additive. Appl. Surf. Sci. 2018, 449, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, S.; Hosseini, S.R.; Boore-Talari, O. Sonochemical assisted synthesis MnO2/RGO nanohybrid as effective electrode material for supercapacitor. Ultrason. Sonochem. 2018, 40, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Numan, A.; Jafer, R.; Bashir, S.; Jilani, A.; Mohammad, S.; Khalid, M.; Ramesh, K.; Ramesh, S. Ternary nanocomposite of cobalt oxide nanograins and silver nanoparticles grown on reduced graphene oxide conducting platform for high-performance supercapattery electrode material. J. Alloys Compd. 2020, 821, 153452. [Google Scholar] [CrossRef]
- Iqbal, J.; Li, L.; Numan, A.; Rafique, S.; Jafer, R.; Mohamad, S.; Khalid, M.; Ramesh, K.; Ramesh, S. Density functional theory simulation of cobalt oxide aggregation and facile synthesis of a cobalt oxide, gold and multiwalled carbon nanotube based ternary composite for a high performance supercapattery. New J. Chem. 2019, 43, 13183–13195. [Google Scholar] [CrossRef]
- Cui, F.; Xu, L.; Cui, T.; Yao, T.; Yu, J.; Zhang, X.; Sun, K. Facile synthesis of ultrasmall TiO2 nanocrystals/porous carbon composites in large quantity and their photocatalytic performance under visible light. RSC Adv. 2014, 4, 33408–33415. [Google Scholar] [CrossRef]
- Xia, H.; Hong, C.; Shi, X.; Li, B.; Yuan, G.; Yao, Q.; Xie, J. Hierarchical heterostructures of Ag nanoparticles decorated MnO2 nanowires as promising electrodes for supercapacitors. J. Mater. Chem. A 2015, 3, 1216–1221. [Google Scholar] [CrossRef]
- Çıplak, Z.; Yıldız, N. The effect of Ag loading on supercapacitor performance of graphene based nanocomposites. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 65–76. [Google Scholar] [CrossRef]
- Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver Nanoparticles Embedded on Reduced Graphene Oxide@Copper Oxide Nanocomposite for High Performance Supercapacitor Applications. Materials 2021, 14, 5032. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.U.; Wang, F.; Javed, M.S.; Saleem, R.; Nazir, M.S.; Zhan, J.; Khan, Z.U.H.; Farooq, M.U.; Ali, S. Synthesis, characterization and electrochemical properties of α-MnO2 nanowires as electrode material for supercapacitors. Int. J. Electrochem. Sci. 2018, 13, 6426–6435. [Google Scholar] [CrossRef]
- Raj, B.G.S.; Asiri, A.M.; Qusti, A.H.; Wu, J.J.; Anandan, S. Sonochemically synthesized MnO2 nanoparticles as electrode material for supercapacitors. Ultrason. Sonochem. 2014, 21, 1933–1938. [Google Scholar]
- Chang, X.; Zhai, X.; Sun, S.; Gu, D.; Dong, L.; Yin, Y.; Zhu, Y. MnO2/g-C3N4 nanocomposite with highly enhanced supercapacitor performance. Nanotechnology 2017, 28, 135705. [Google Scholar] [CrossRef]
- Naderi, H.R.; Mortaheb, H.R.; Zolfaghari, A. Supercapacitive properties of nanostructured MnO2/exfoliated graphite synthesized by ultrasonic vibration. J. Electroanal. Chem. 2014, 719, 98–105. [Google Scholar] [CrossRef]
- Racik, M.; Manikandan, A.; Mahendiran, M.; Madhavan, J.; Raj, M.V.A.; Mohamed, M.G.; Maiyalagan, T. Hydrothermal synthesis and characterization studies of α-Fe2O3/MnO2 nanocomposites for energy storage supercapacitor application. Ceram. Int. 2020, 46, 6222–6233. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.O.; Cho, M.H. Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J. Colloid Interface Sci. 2017, 506, 613–619. [Google Scholar] [CrossRef]
- Lu, L.; Xu, S.; An, J.; Yan, S. Electrochemical performance of CNTs/RGO/MnO2 composite material for supercapacitor. Nanomater. Nanotechnol. 2016, 6, 1847980416663687. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Wang, C.; Mo, Y.; Wang, X.; Fan, J.; Xu, Q.; Min, Y. A ternary composite with manganese dioxide nanorods and graphene nanoribbons embedded in a polyaniline matrix for high-performance supercapacitors. RSC Adv. 2017, 7, 33591–33599. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Liu, Y.; Kan, E.; Tang, J.; Zhang, L.; Wang, H.; Tang, W. Sandwich-structured MnO2 /polypyrrole/reduced graphene oxide hybrid composites for high-performance supercapacitors. RSC Adv. 2014, 4, 9898–9904. [Google Scholar] [CrossRef]


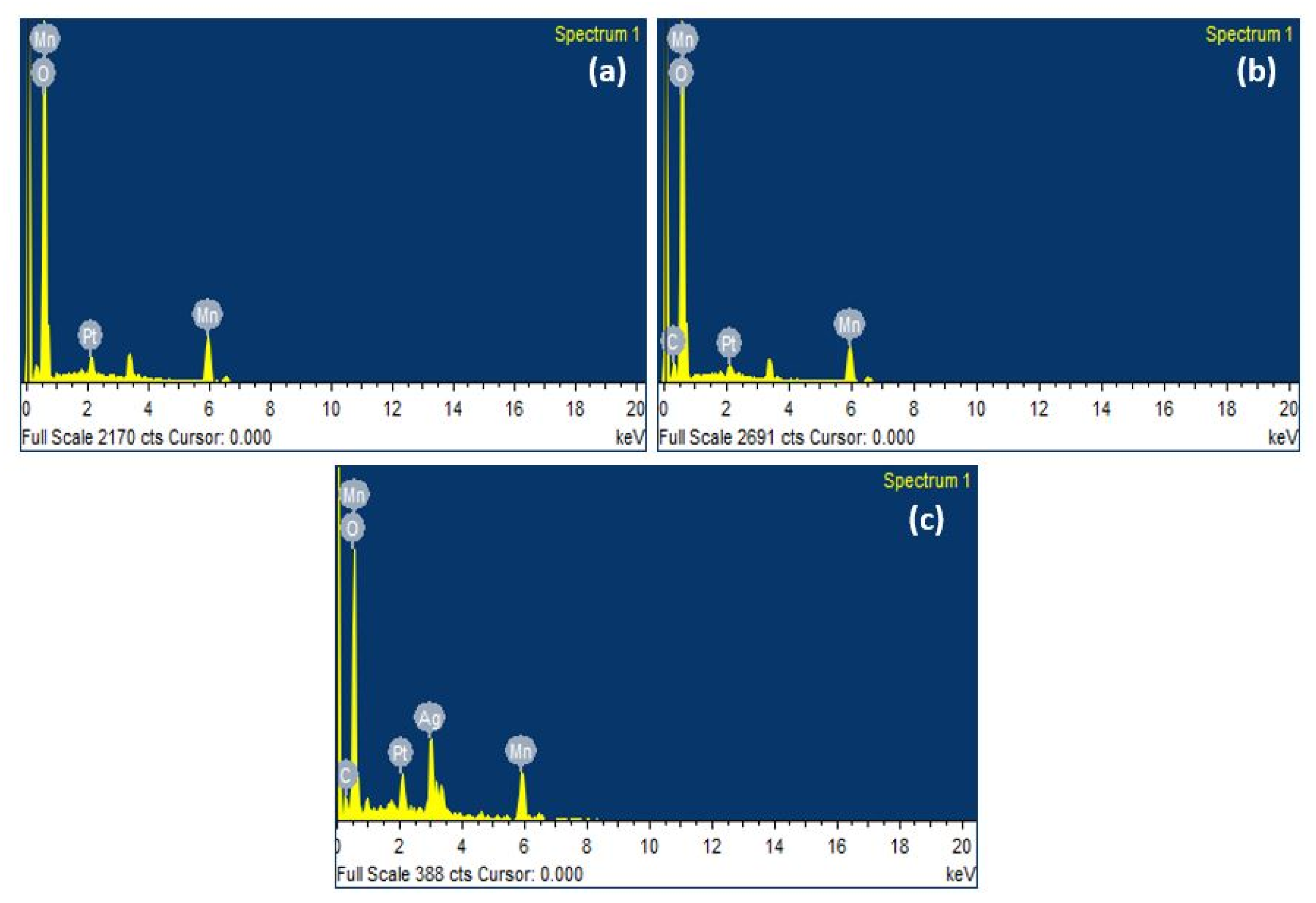
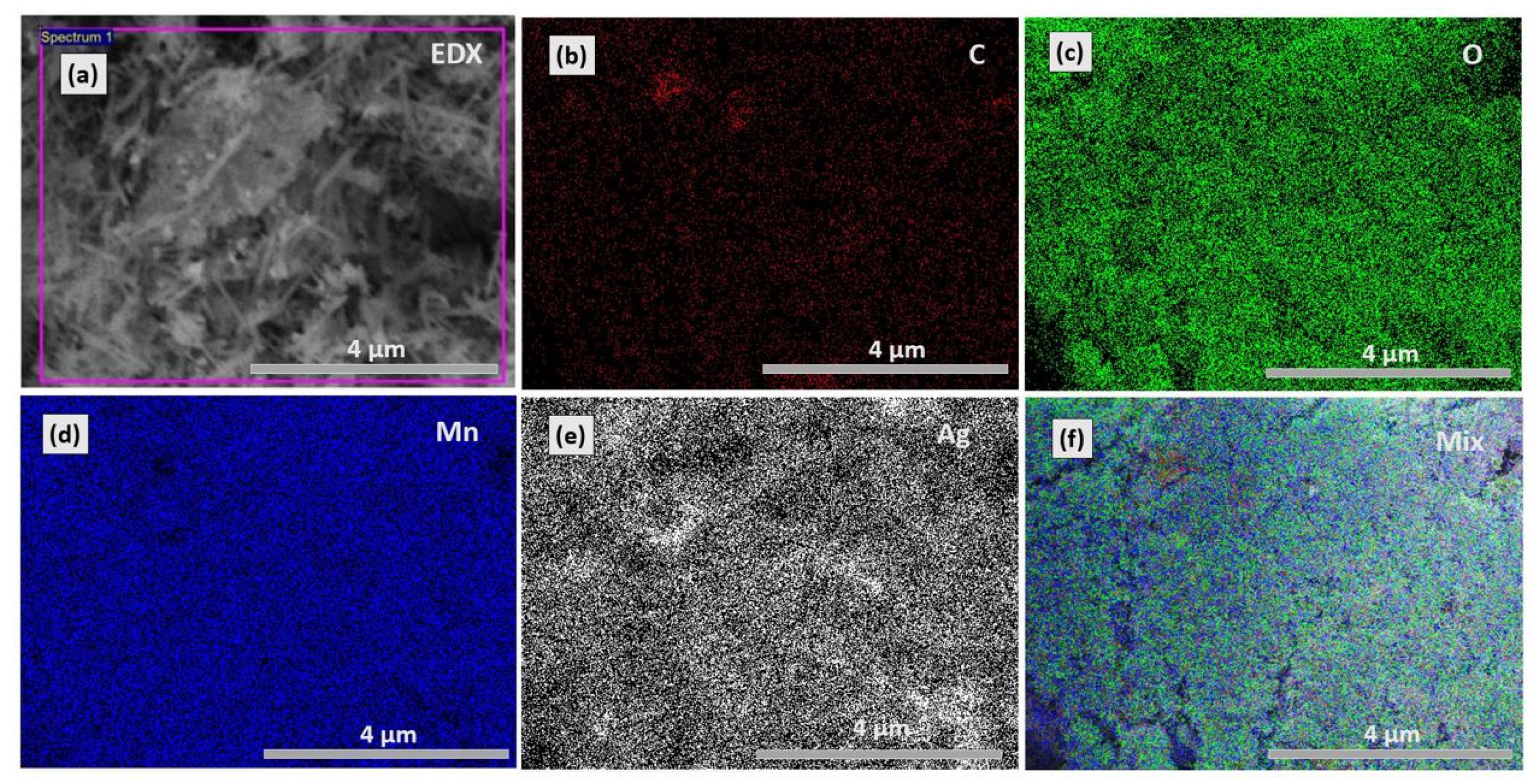

| Element | Weight% | Atomic% |
|---|---|---|
| C K | 1.93 | 5.44 |
| O K | 30.20 | 63.85 |
| Mn L | 34.93 | 21.51 |
| Ag L | 24.89 | 1.81 |
| Pt M | 8.05 | 1.40 |
| Current Density (Ag−1) | Capacitance (Fg−1) | ||
|---|---|---|---|
| MnO2 rods | rGO@MnO2 | Ag-rGO@MnO2 | |
| 0.5 | 293.5 | 305.25 | 675 |
| 1 | 287.5 | 293.5 | 600 |
| 2 | 280 | 290 | 575 |
| 4 | 260 | 275 | 360 |
| 5 | 181.25 | 200 | 250 |
| 7 | 143.5 | 158 | 175 |
| 10 | 100 | 110 | 120 |
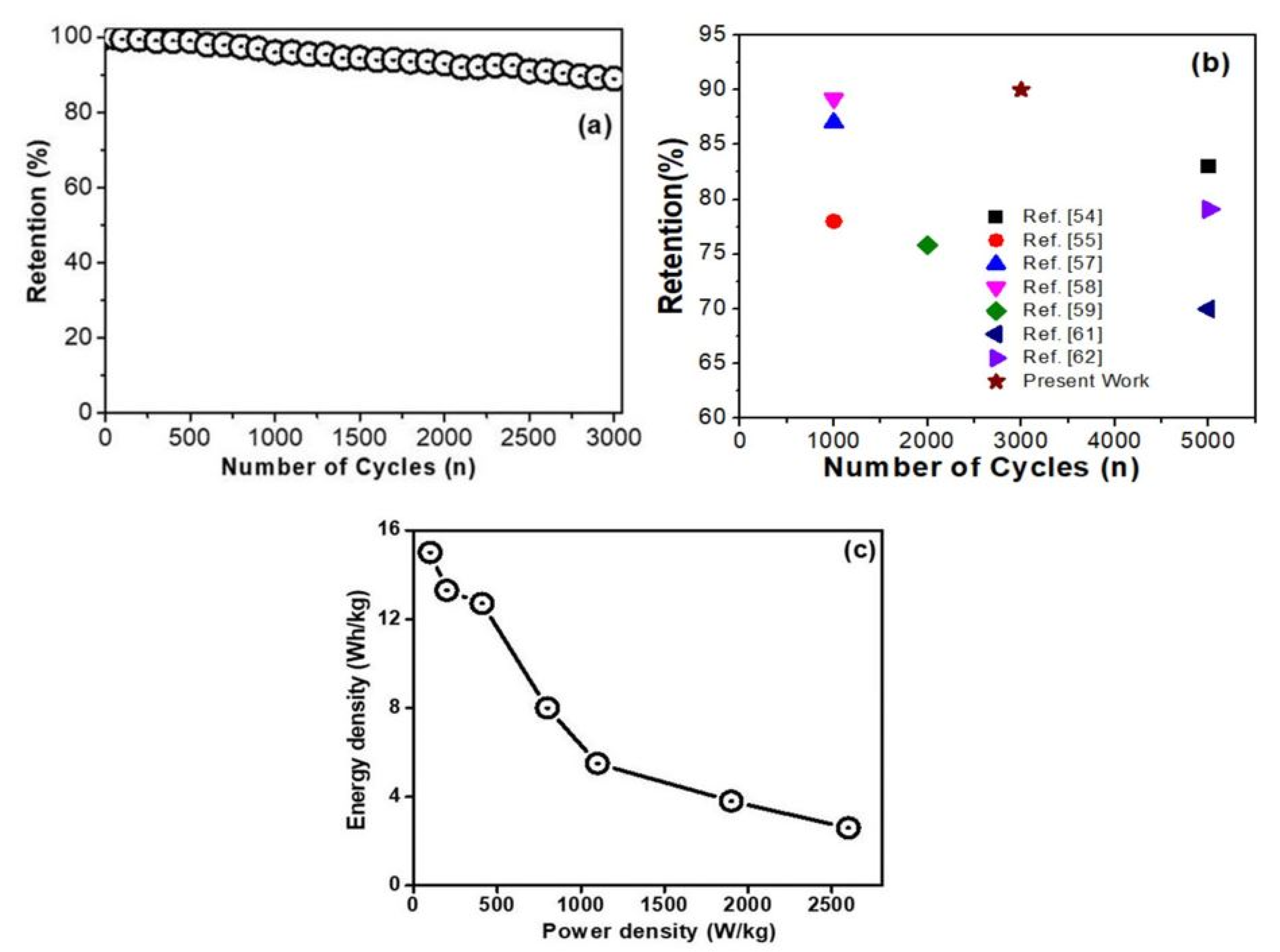
| S. No. | Electrode Material | Electrolyte | Current Density (Ag−1) | Specific Capacitance (Csp) Fg−1 | No. of Cycles | Retention% | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | MnO2 NRr | 1M Na2 SO4 | 1 | 362 | 5000 | 83 | [54] |
| 2. | MnO2 nanoparticles | 1M Ca(NO3)2 | 0.5 mA cm−2 | 283 | 1000 | 78 | [55] |
| 3. | MnO2/g-C3N4 | 1M Na2 SO4 | 1 | 211 | 1000 | 90 | [56] |
| 4. | MnO2/EG | 1M Na2 SO4 | 2 mV s−1 | 358 | 1000 | 87 | [57] |
| 5. | α-Fe2O3/MnO2 | 1M KOH | 1 | 216 | 1000 | 89.2 | [58] |
| 6. | rGO/MnO2 | 6KOH | 0.85 | 486.48 | 2000 | 75.8 | [59] |
| 7. | CNT/rGO/MnO2 | 2M Na2 SO4 | 1 | 404 | 5000 | 70 | [60] |
| 8. | MnO2/PANI/GN | 1M Na2 SO4 | 1 | 472 | 5000 | 79.7 | [61] |
| 9. | MnO2/PPy/rGO | 1M Na2 SO4 | 0.25 | 404 | 5000 | 91 | [62] |
| 10. | Ag-rGO@MnO2 | 2M KOH | 0.5 | 675 | 3000 | 90 | Present case |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices. Crystals 2022, 12, 389. https://doi.org/10.3390/cryst12030389
Ansari AR, Ansari SA, Parveen N, Ansari MO, Osman Z. Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices. Crystals. 2022; 12(3):389. https://doi.org/10.3390/cryst12030389
Chicago/Turabian StyleAnsari, Akhalakur Rahman, Sajid Ali Ansari, Nazish Parveen, Mohammad Omaish Ansari, and Zurina Osman. 2022. "Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices" Crystals 12, no. 3: 389. https://doi.org/10.3390/cryst12030389
APA StyleAnsari, A. R., Ansari, S. A., Parveen, N., Ansari, M. O., & Osman, Z. (2022). Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices. Crystals, 12(3), 389. https://doi.org/10.3390/cryst12030389







