Crystal Structures of Gallium(III) Halides with Bulky Ligands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
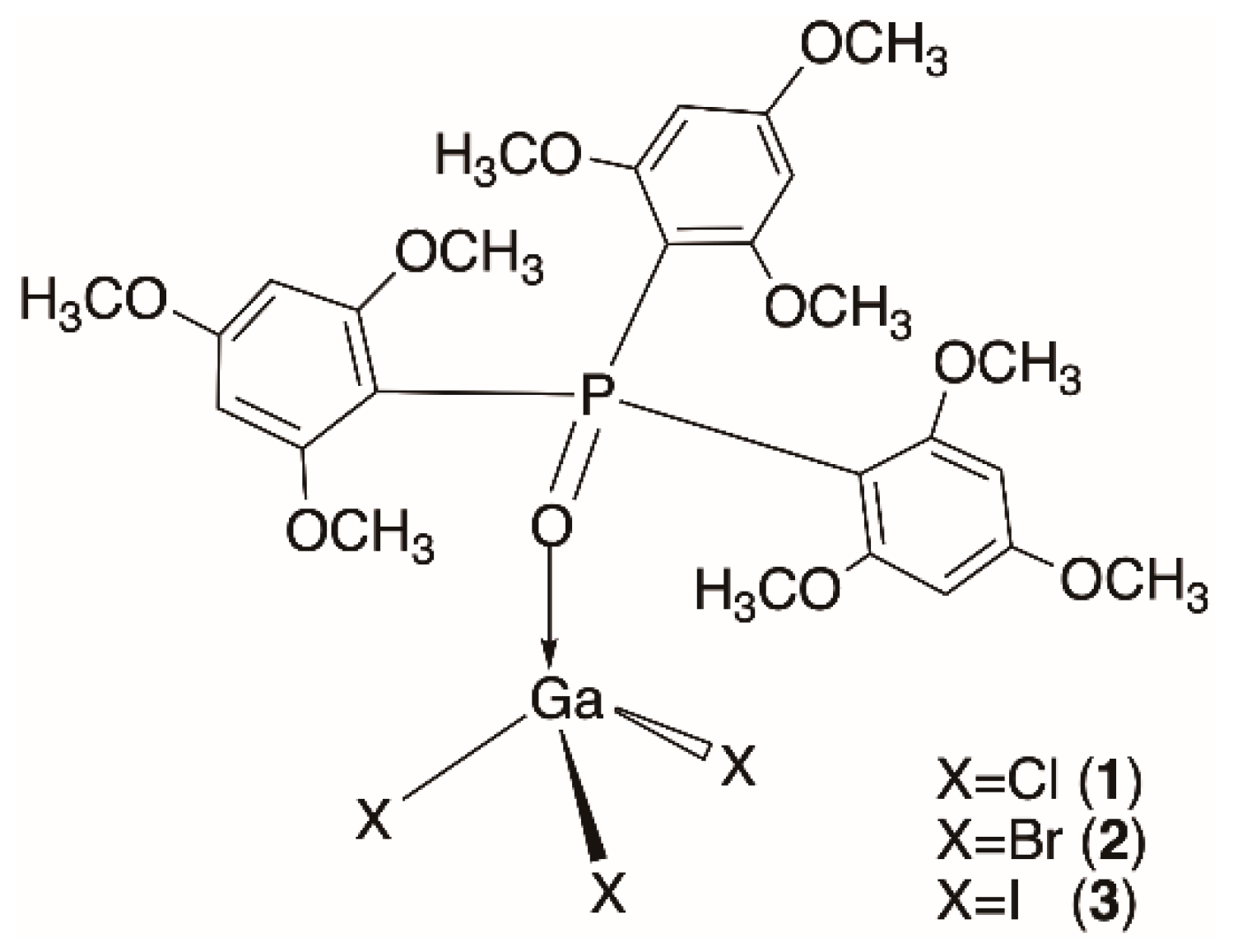
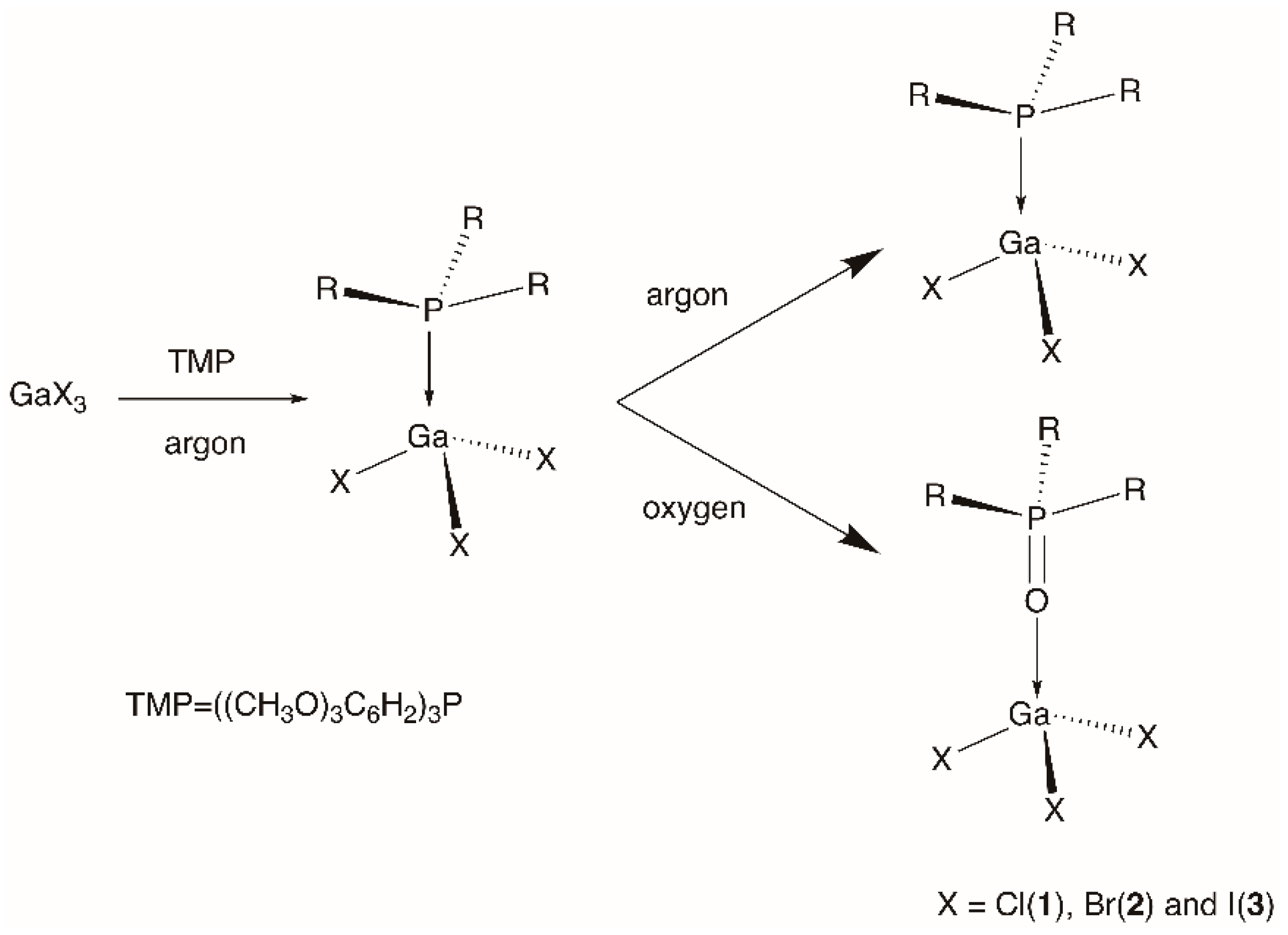
2.2. Synthesis of the Complexes
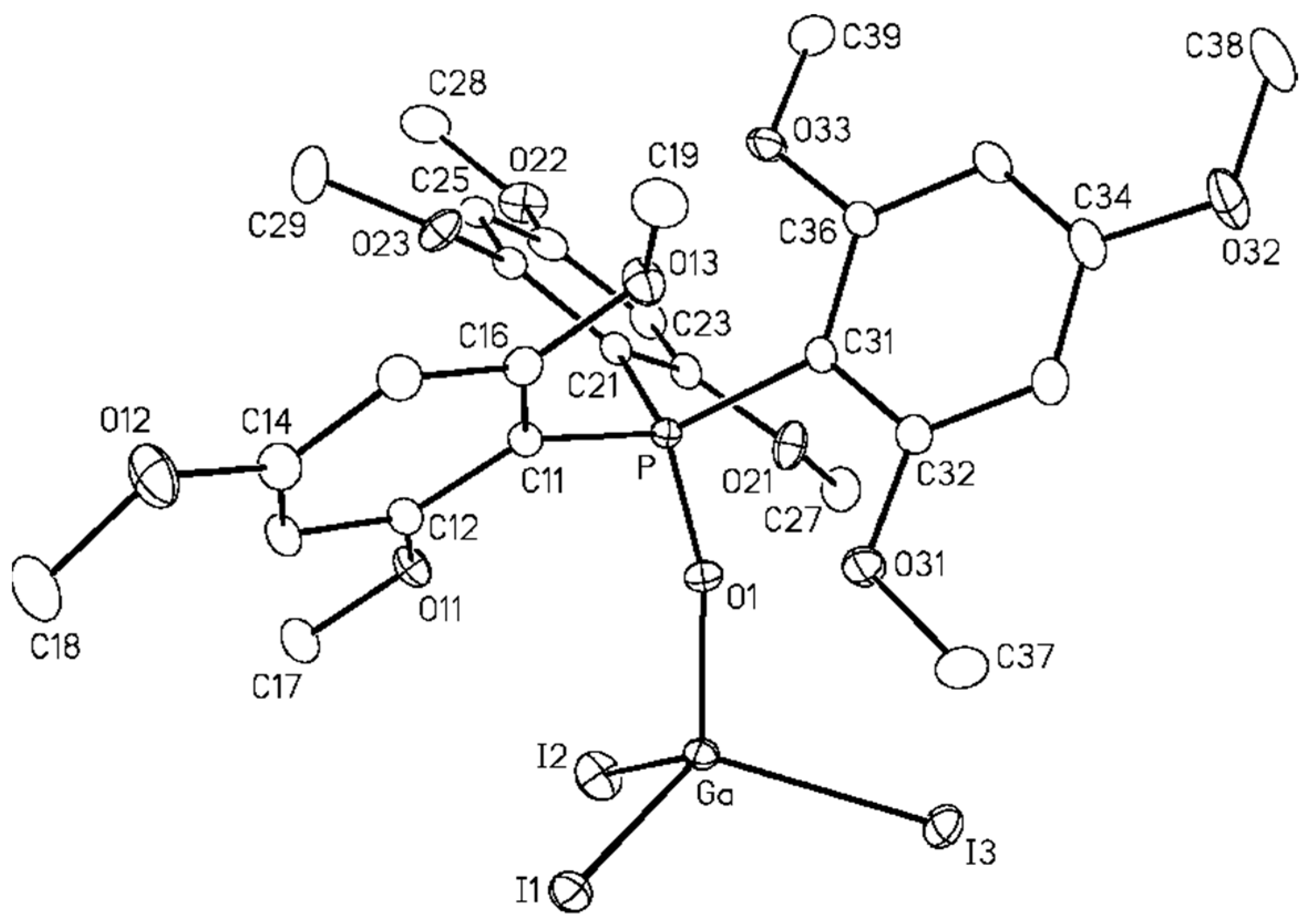
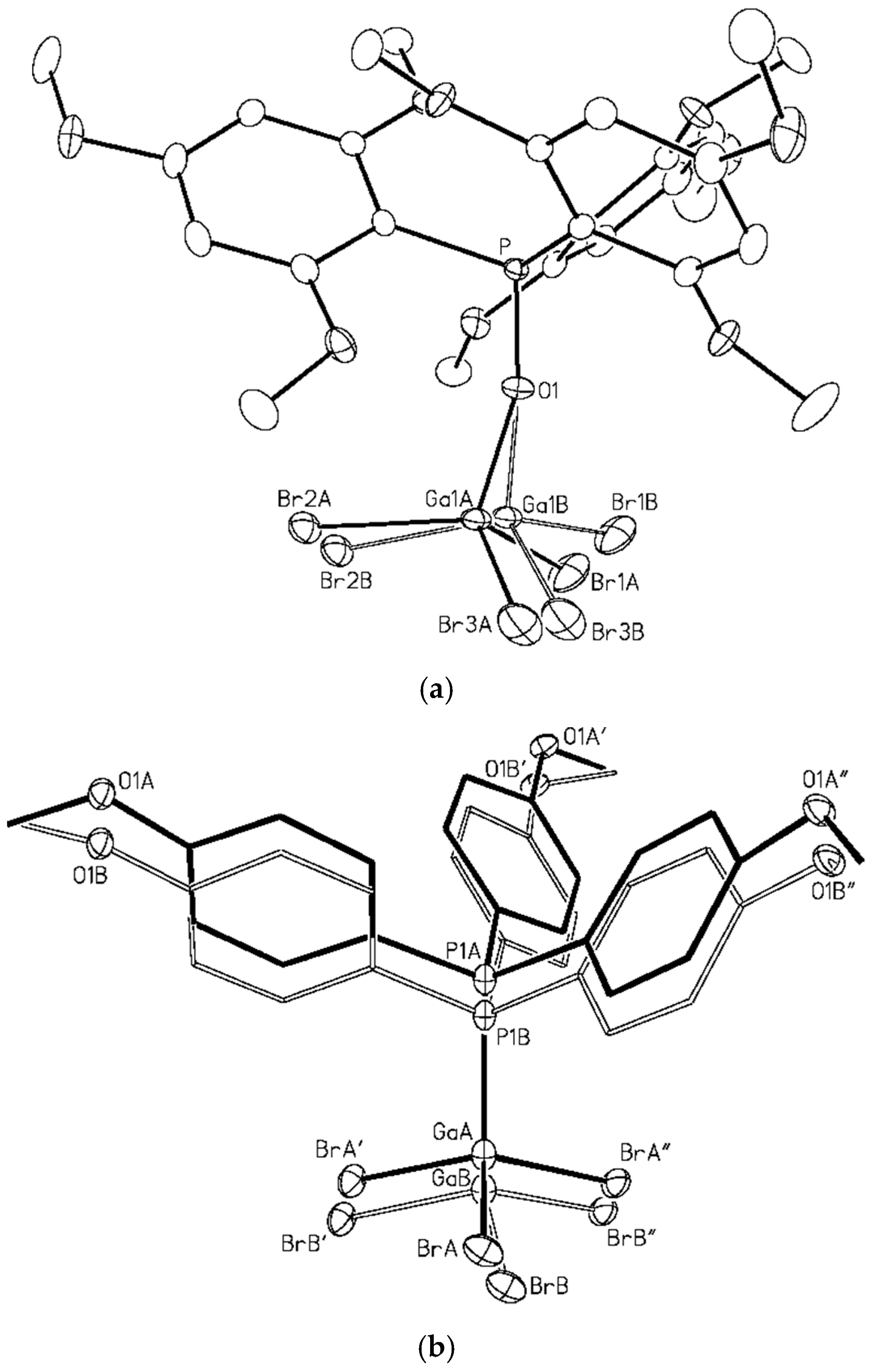
2.3. Crystal Structure Determination
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonas, V.; Frenking, G. Studies on the boron–nitrogen bond length of the classical donor–acceptor complex H3N–BF3. J. Chem. Soc. Chem. Commun. 1994, 1489–1490. [Google Scholar] [CrossRef]
- Timoshkin, A.Y.; Suvorov, A.V.; Bettinger, H.F.; Schaefer, H.F. Role of the Terminal Atoms in the Donor-Acceptor Complexes MX3-D (M = Al, Ga, In; X = F, Cl, Br, I.; D = YH3, YX3, X−; Y = N, P, As). J. Am. Chem. Soc. 1999, 121, 5687–5699. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, S.; Zhao, J.; Ulhaq, I.; Ferguson, M.J.; Mcdonald, R.; Ma, G.; Ronald, C.G. Synthesis and Structures of Bis(iminophosphorano)methanide Chelate Complexes with Zinc and Group 13. Polyhedron 2019, 159, 167–175. [Google Scholar] [CrossRef]
- Ge, S.; Zhao, J.; Ferguson, M.J.; Ma, G.; Cavell, R.G. Rare Carbon-Bridged Bimetallic Lanthanide (Nd or Sm) and Tl(I) Geminal Carbon Derivatives of a Bis(iminophosphorano)methanediide. Organometallics 2020, 39, 478–486. [Google Scholar] [CrossRef]
- Ma, G.; Kritikos, M.; Maliarik, M.; Glaser, J. Modification of Binuclear PtTl Bonded Complexes by Attaching Bipyridine Ligands to the Thallium Site. Inorg. Chem. 2004, 43, 4328–4340. [Google Scholar] [CrossRef]
- Ma, G.; Fischer, A.; Glaser, J. Synthesis and Structure of Monomeric and Platinum-Bonded (1,10-Phenanthroline)thallium Complexes. Eur. J. Inorg. Chem. 2002, 2002, 1307–1314. [Google Scholar] [CrossRef]
- Carty, A.J. Coordination complexes of gallium(III) and indium(III) halides. II. Far-infrared spectra of gallium(III) halide complexes with triarylphosphines. Can. J. Chem. 1967, 45, 3187–3192. [Google Scholar] [CrossRef]
- Carty, A.J.; Hinsperger, T.; Boorman, P.M. Coordination complexes of gallium(III) and indium(III) halides. V. Trialkyl and mixed alkyl\u2013aryl phosphine complexes of indium(III) chloride, bromide, and iodide. Can. J. Chem. 1970, 48, 1959–1970. [Google Scholar] [CrossRef]
- Chen, F.; Ma, G.; Bernard, G.M.; Cavell, R.G.; McDonald, R.; Ferguson, M.J.; Wasylishen, R.E. Solid-state 115In and 31P NMR studies of triarylphosphine indium trihalide adducts. J. Am. Chem. Soc. 2010, 132, 5479–5493. [Google Scholar] [CrossRef]
- Chen, F.; Ma, G.; Bernard, G.M.; Wasylishen, R.E.; Ferguson, M.J. An investigation of 1:1 adducts of gallium trihalides with triarylphosphines by solid-state (69/71)Ga and 31P NMR spectroscopy. Chem.—Eur. J. 2013, 19, 2826–2838. [Google Scholar] [CrossRef]
- Burford, N.; Royan, B.W.; Spence, R.E.v.H.; Cameron, T.S.; Linden, A.; Gogers, R.D. Linear co-ordinative bonding at oxygen: A spectroscopic and structural study of phosphine oxide-group 12 Lewis acid adducts. J. Chem. Soc. Dalton Trans. 1990, 21, 1521–1528. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A.; Ziller, J.W. Synthesis and characterization of gallium-containing silsequioxanes. Inorg. Chem. 1997, 36, 4082–4086. [Google Scholar] [CrossRef]
- McMahon, C.N.; Bott, S.G.; Barron, A.R. Observation of an unusual amine oxidation reaction during the oxidation and hydrolysis of [(tBu)2Ga(o-C6H4NMe2)]: Molecular structures of [(tBu)2Ga(o-C6H4N(O)Me2)] and [(tBu)2Ga(o-C6H4NMe2)(O=PPh3)]. Polyhedron 1997, 16, 3407–3413. [Google Scholar] [CrossRef]
- Peppe, C.; Nobrega, J.A.; Hernandes, M.Z.; Longo, R.L.; Tuck, D.G. Preparation, crystal structure determination, and properties of adducts of halogenomethyl compounds of indium with group 16 donors. J. Organomet. Chem. 2001, 626, 68–75. [Google Scholar] [CrossRef]
- Peppe, J.; Mello, M.d.A.; Wioppiold, T.A.; Lang, E.S. Synthesis of dihalogenidoindium(III) ions by halide transfer involving tellurium compounds in the presence of hard and soft ligands. J. Organomet. Chem. 2012, 718, 52–56. [Google Scholar] [CrossRef]
- Weiss, J.; Prermeier, T.; Fischer, R.A. Reactions of elemental indium and indium(I) bromide with nickel-bromine bonds: Structure of (η5-C5H4)(Ph3P)Ni-InBr2(O=PPh3). Inorg. Chem. 1996, 35, 71–75. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Minch Michael, J. An Introduction to Hydrogen Bonding (Jeffrey, George A.). J. Chem. Educ. 1999, 76, 759. [Google Scholar] [CrossRef] [Green Version]
- Stewart, B.; Harriman, A.; Higham, L.J. Predicting the Air Stability of Phosphines. Organometallics 2011, 30, 5338–5343. [Google Scholar] [CrossRef]
- Sokolov, V.G.; Koptseva, T.S.; Moskalev, M.V.; Bazyakina, N.L.; Fedushkin, I.L. Gallium Hydrides with a Radical-Anionic Ligand. Inorg. Chem. 2017, 56, 13401–13410. [Google Scholar] [CrossRef]
- Kimura, S.; Bill, E.; Bothe, E.; Weyhermuller, T.; Weighardt, K. Phenylthiyl Radical Complexes of Gallium(III), Iron(III), and Cobalt(III) and Comparison with. J. Am. Chem. Soc. 2001, 123, 6025–6039. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Codgbrook, H.L.; Hector, A.L.; Levason, W.; Reid, G.; Webster, M.; Zhang, W. Gallium(III) halide complexes with phosphines, arsines and phosphine oxides—A comparative study. Polyhedron 2007, 26, 4147–4155. [Google Scholar] [CrossRef]
- Cobb, J.E.; Cribbs, C.M.; Henke, B.R.; Uehling, D.E.; Hernan, A.G.; Matin, C.; Rayner, C.M. Triphenylphosphine. In Encyclopedia of Regents for Organic Synthesis; Raquette, L., Ed.; J. Wiley & Sons: New York, NY, USA, 2004; ISBN 0471936235. [Google Scholar] [CrossRef]
- Bresien, J.; Faust, K.; Schulz, A.; Villinger, A. Low-Temperature Isolation of the Bicyclic Phosphinophosphonium Salt [Mes*2P4Cl][GaCl4]. Angew. Chem. Int. Ed. 2015, 54, 6926–6930. [Google Scholar] [CrossRef] [PubMed]
- Samanamu, C.R.; Olmstead, M.M.; Montchamp, J.L.; Richards, A.F. Convenient Synthesis of Aluminum and Gallium Phosphonate Cages. Inorg. Chem. 2008, 47, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Stephan, D.W. An imine–gallium Lewis pair stabilized oxophosphinidene via an unexpected phosphirene rearrangement. Chem. Commun. 2018, 54, 1041–1044. [Google Scholar] [CrossRef] [Green Version]




| Empirical Formula | C27H33Cl3GaO10P (1) | C27H33Br3GaO10P (2) | C27H33GaI3O10P (3) |
|---|---|---|---|
| Formula weight | 724.57 | 857.95 | 998.92 |
| Crystal system Crystal Dimensions | Triclinic 0.38 × 0.32 × 0.24 mm | Triclinic 0.36 × 0.25 × 0.15 mm | Triclinic 0.36 × 0.28 × 0.10 mm |
| Space group | Pī(No.2) | Pī(No.2) | Pī(No.2) |
| Unit cell parameters a (Å) | 11.0002(10) | 11.0820(11) | 11.2385(6) |
| b (Å) | 12.1039(11) | 12.7203(14) | 12.6376(7) |
| c (Å) | 14.0488(13) | 13.8490(16) | 13.3734(6) |
| α (°) | 72.6161(11) | 71.6261(14) | 104.4313(6) |
| β (°) | 65.1529(11) | 68.7071(13) | 101.9588(6) |
| γ (°) | 70.7251(11) | 69.7549(14) | 102.5358(6) |
| Volume (Å3) | 1614.9(3) | 1666.0(3) | 1725.65(16) |
| Z | 2 | 2 | 2 |
| Calculated density (g cm−3) | 1.490 | 1.710 | 1.922 |
| Temperature, K | 173.2(1) | 173.2(1) | 173.2(1) |
| μ (MoKα), (mm−1) | 1.201 | 4.522 | 3.576 |
| θ range for data collection (°) | 0.3 to 27.49 | 0.3 to 26.61 | 0.3 to 27.48 |
| Total data collected | 14,087 | 13,675 | 15,248 |
| Index ranges | −14 ≤ h ≤ 14 | −13 ≤ h ≤ 13 | −14 ≤ h ≤ 14 |
| −15 ≤ k ≤ 15 | −16 ≤ k ≤ 16 | −16 ≤ k ≤ 16 | |
| −18 ≤ l ≤ 18 | −17 ≤ l ≤ 17 | −17 ≤ l ≤ 17 | |
| Independent reflections | 7290 (Rint = 0.0207) | 6946 (Rint = 0.0205) | 7842 (Rint = 0.0126) |
| Observed reflections | 5695 | 5475 | 7029 |
| Data/restraints/parameters | 7290/0/379 | 6946/10/400 | 7842/0/379 |
| Goodness-of-fit on F2 | 1.060 | 1.042 | 1.025 |
| Final R indices | |||
| [F02 ≥ 2σ(F0)] | R1 = 0.0502 | R1 = 0.0346 | R1 = 0.0274 |
| wR2 [F02 ≥ −3σ(F02)] | wR2 = 0.1336 | wR2 = 0.0913 | wR2 = 0.0754 |
| Large difference peak and hole | −1.388 and 1.546 e/Å3 | −0.947 and 0.674 e/Å3 | −0.829 and 1.495 e/Å3 |
| D–H—A | H—A |
|---|---|
| O(13)–C(19)–H—Cl(1) | 3.074 (3) |
| O(31)–C(37)–H—Cl(1) | 3.084 (4) |
| O(31)–C(37)–H—Cl(2) | 2.964 (3) |
| O(33)–C(39)–H—Br(1A) | 3.155 (4) |
| O(33)–C(39)–H—Br(1B) | 3.056 (3) |
| O(13)–C(19)–H—Br(2A) | 3.097 (4) |
| O(13)–C(19)–H—Br(2B) | 3.485 (4) |
| O(13)-C(19)–H—Br(3A) | 3.517 (4) |
| O(11)–C(17)–H—I(1) | 3.497 (4) |
| O(21)–C(27)–H—I(2) | 3.473 (4) |
| O(31)–C(37)–H—I(3) | 3.366 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Ge, S.; Zhao, J.; McDonald, R.; Ferguson, M.; Ma, G. Crystal Structures of Gallium(III) Halides with Bulky Ligands. Crystals 2022, 12, 330. https://doi.org/10.3390/cryst12030330
Sun M, Ge S, Zhao J, McDonald R, Ferguson M, Ma G. Crystal Structures of Gallium(III) Halides with Bulky Ligands. Crystals. 2022; 12(3):330. https://doi.org/10.3390/cryst12030330
Chicago/Turabian StyleSun, Manluan, Sai Ge, Jianguo Zhao, Robert McDonald, Michael Ferguson, and Guibin Ma. 2022. "Crystal Structures of Gallium(III) Halides with Bulky Ligands" Crystals 12, no. 3: 330. https://doi.org/10.3390/cryst12030330
APA StyleSun, M., Ge, S., Zhao, J., McDonald, R., Ferguson, M., & Ma, G. (2022). Crystal Structures of Gallium(III) Halides with Bulky Ligands. Crystals, 12(3), 330. https://doi.org/10.3390/cryst12030330





