Nucleation Behaviors of Adipic Acid in Different Polarity Solvent Based on Metastable Zone Width
Abstract
:1. Introduction
2. Theory
3. Experimental Section
3.1. Materials
3.2. Metastable Zone Width (MSZW) Measurements
4. Results and Discussion
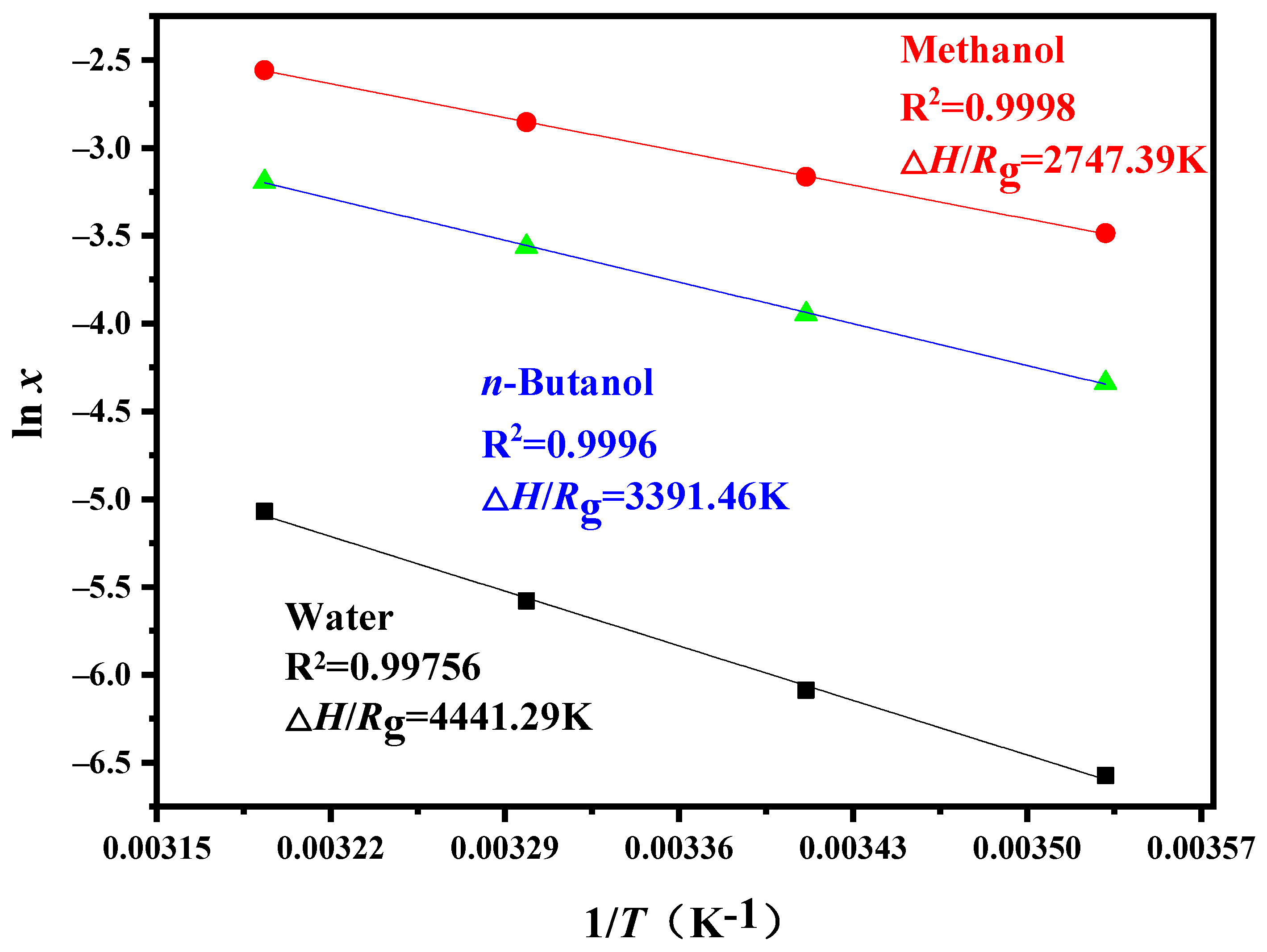
4.1. Solubility
4.2. Effect of Different Cooling Rates, Saturation Temperatures and Polar Solvents on MSZW
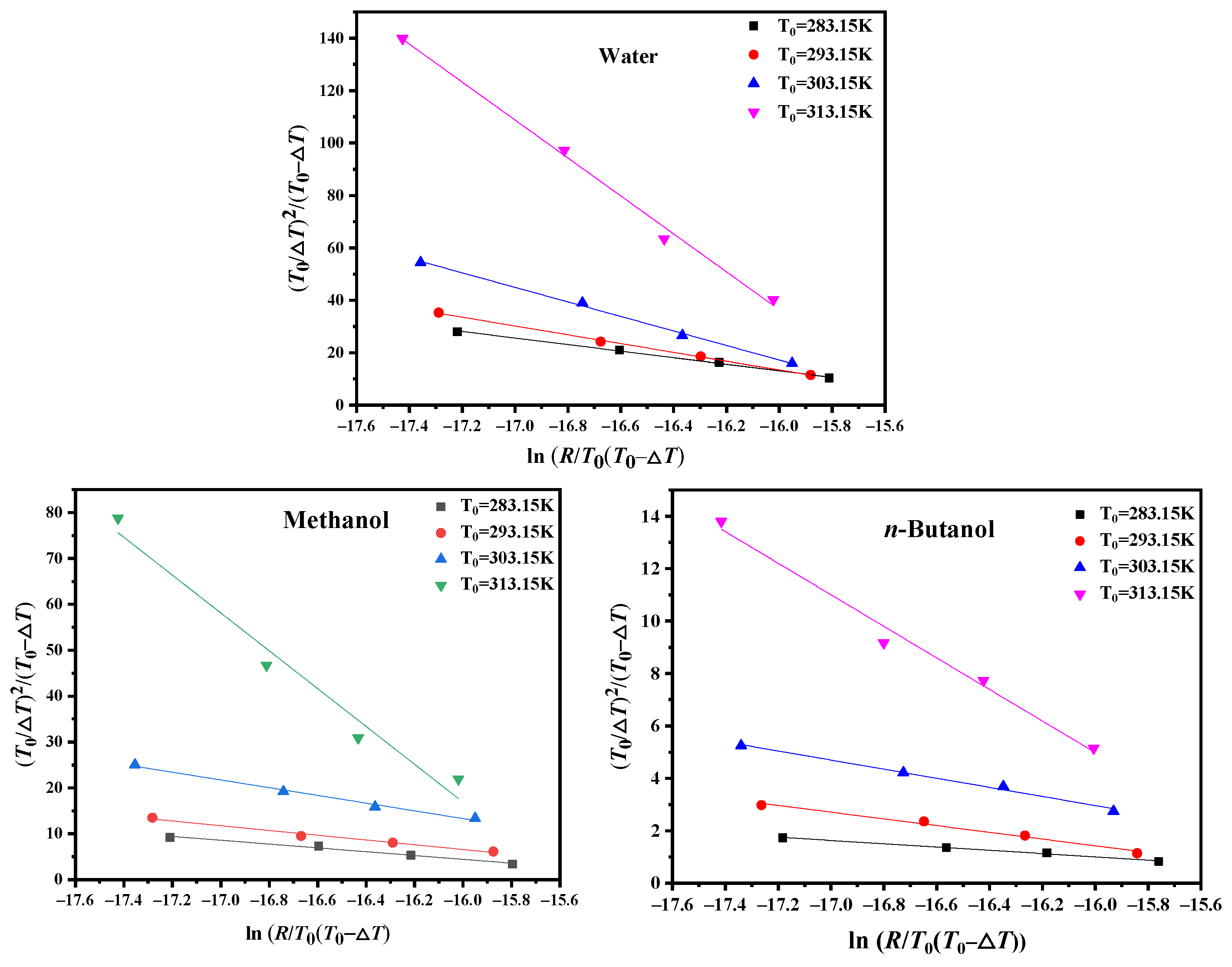
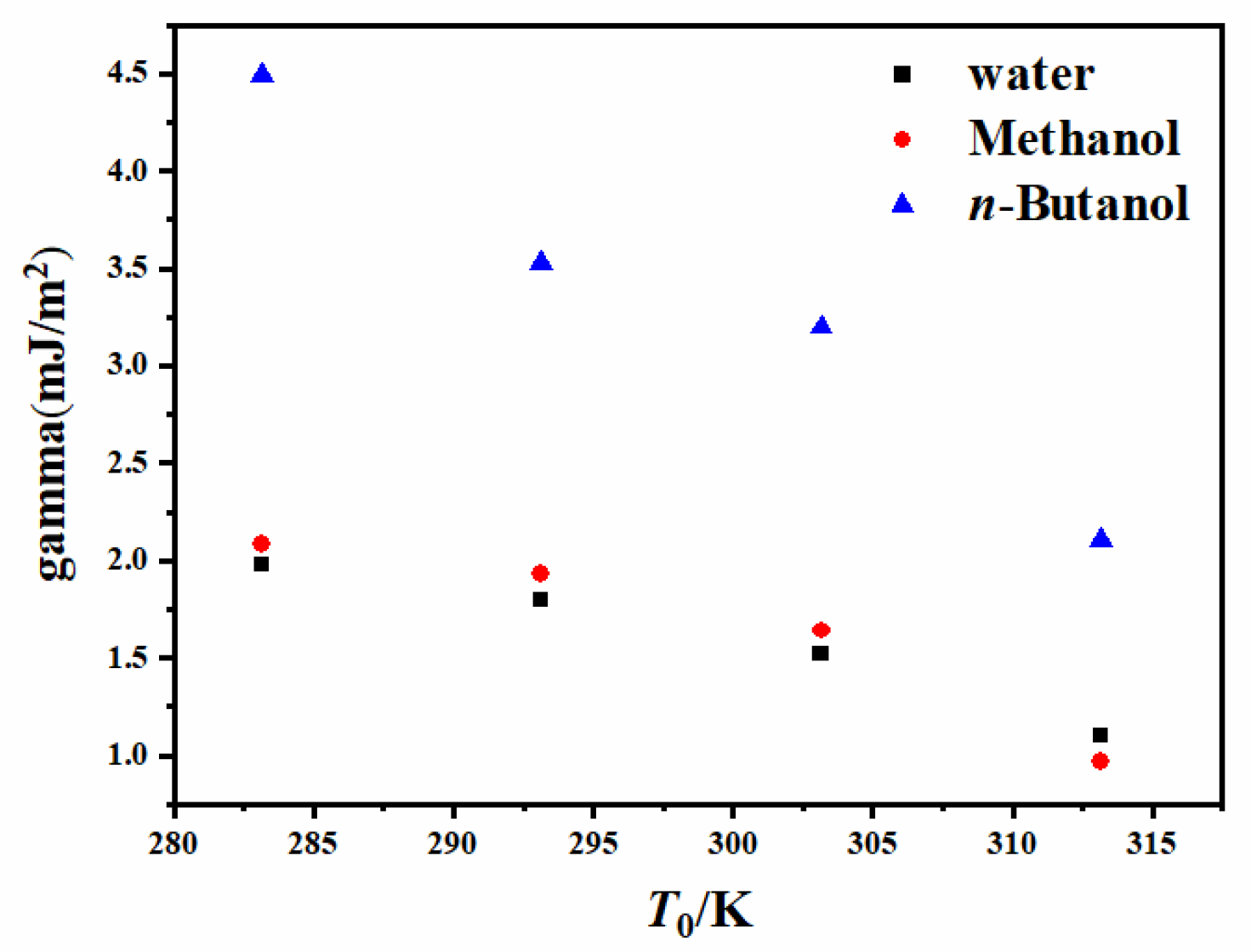
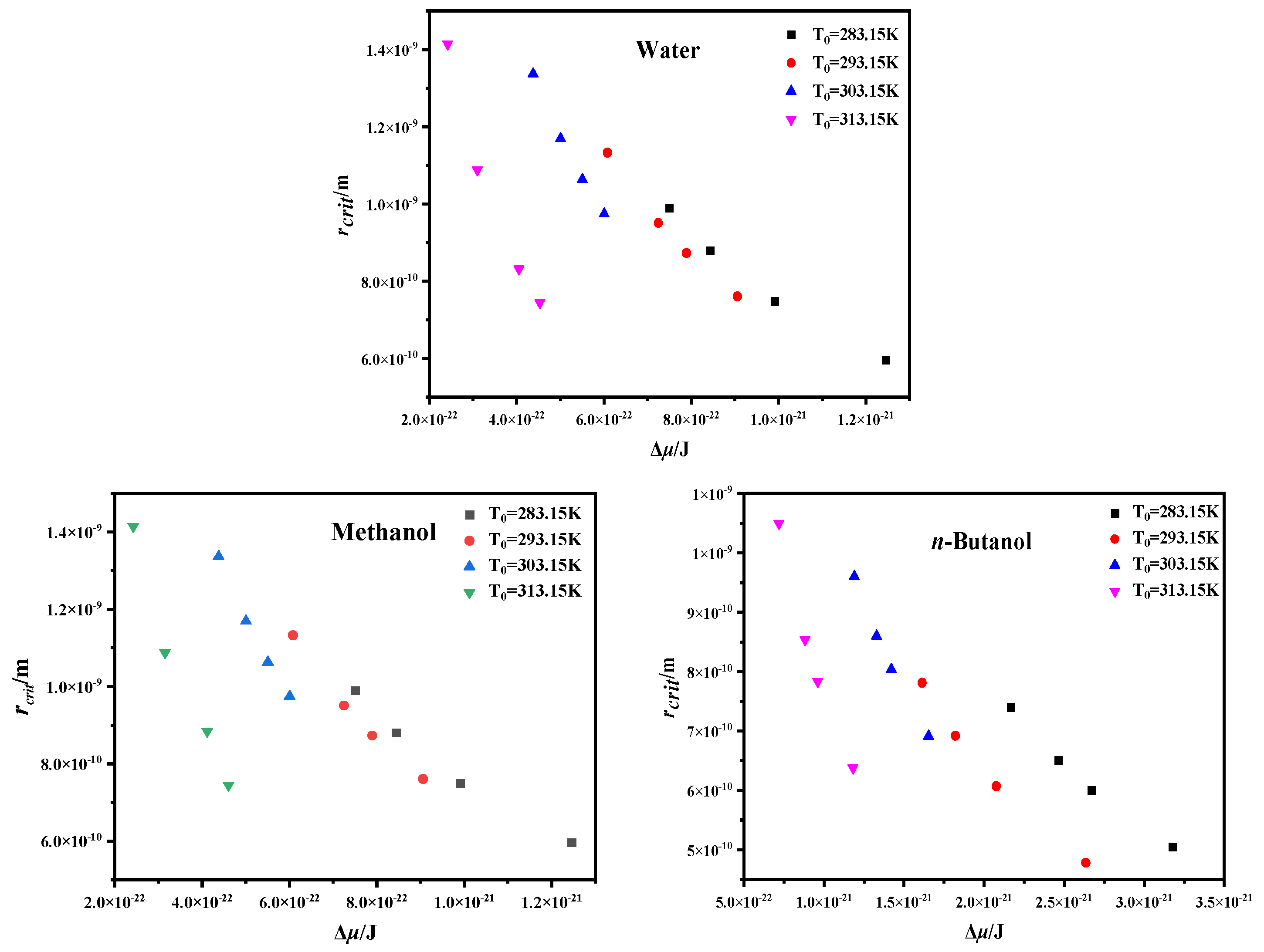
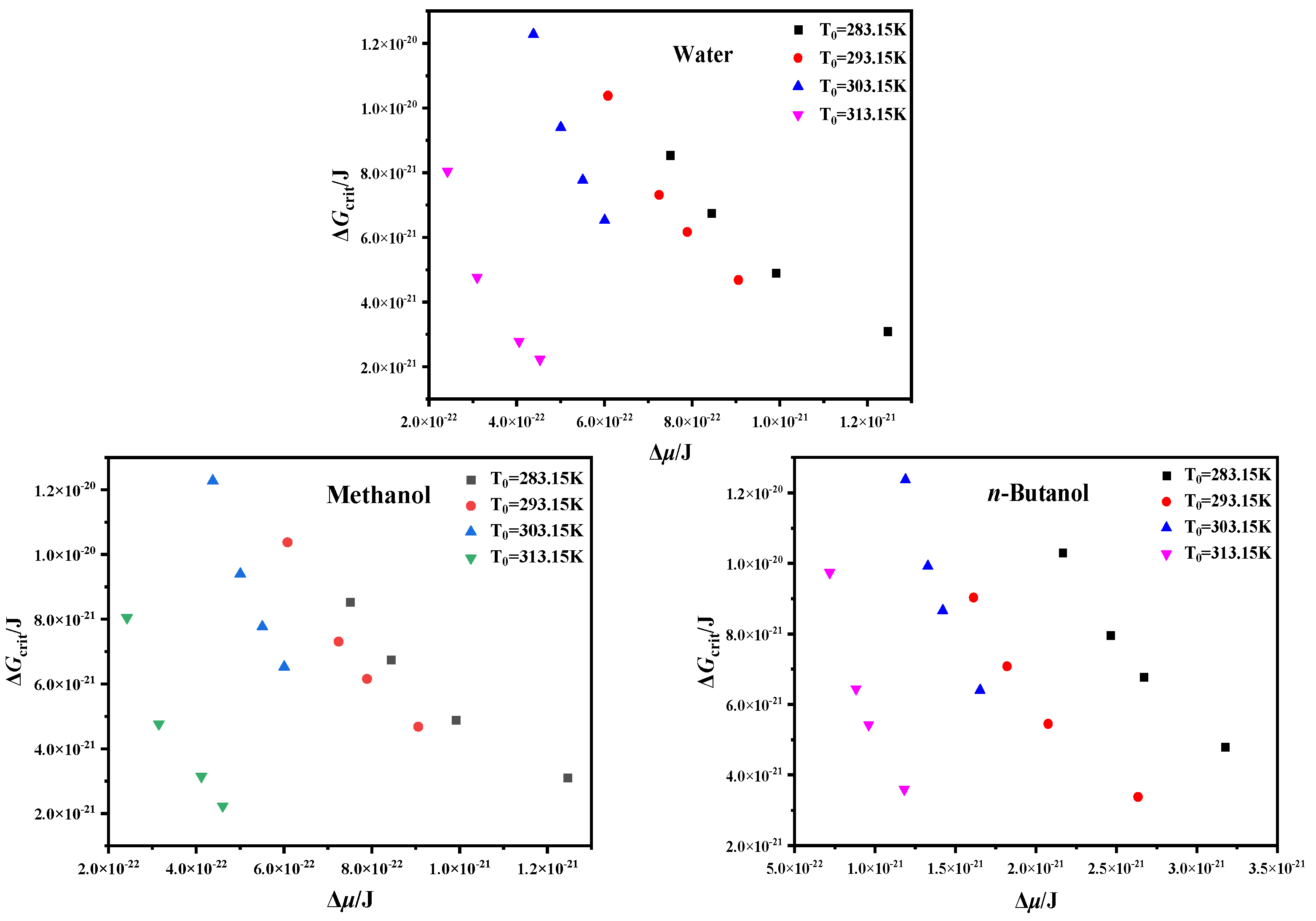
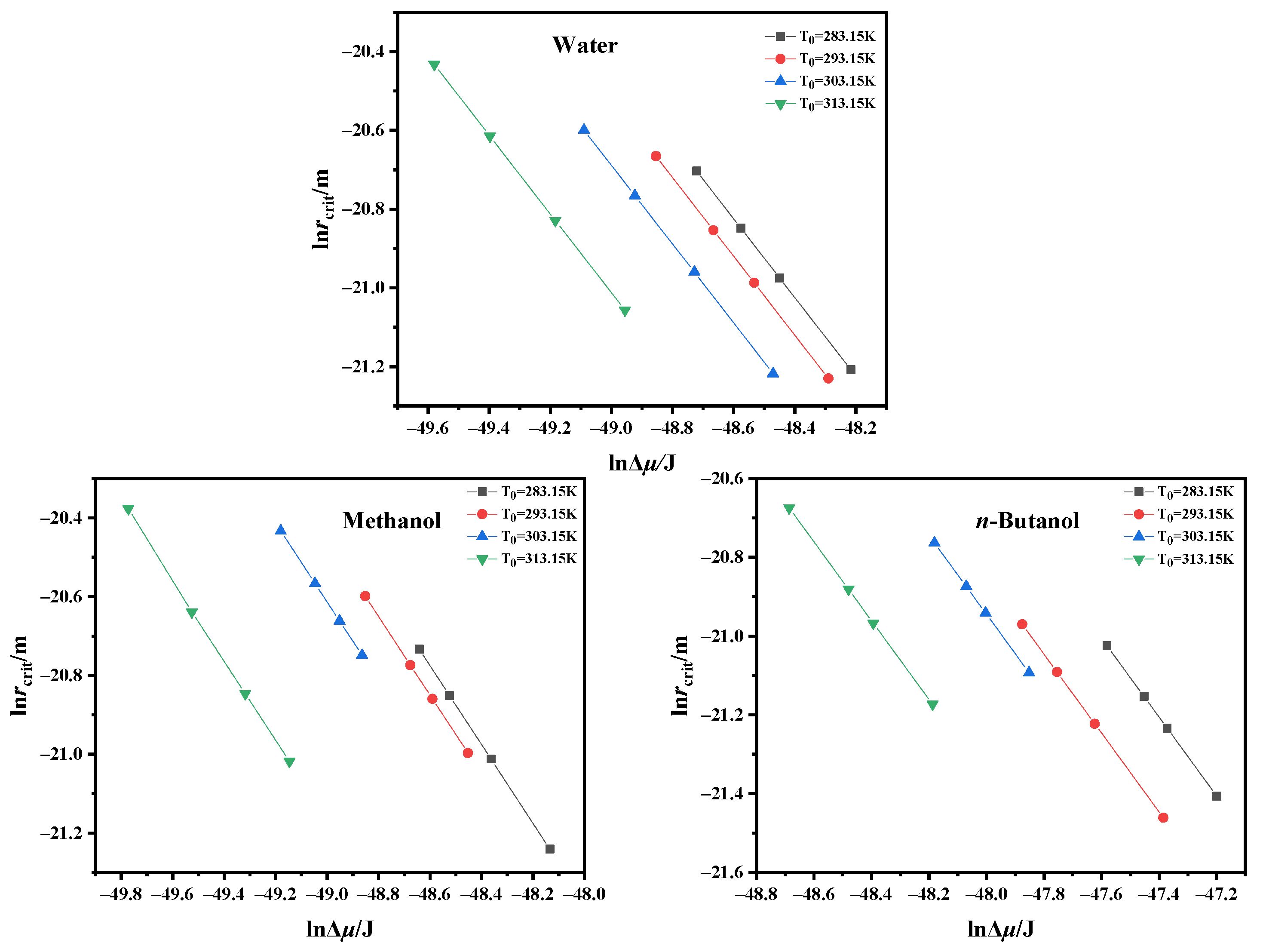
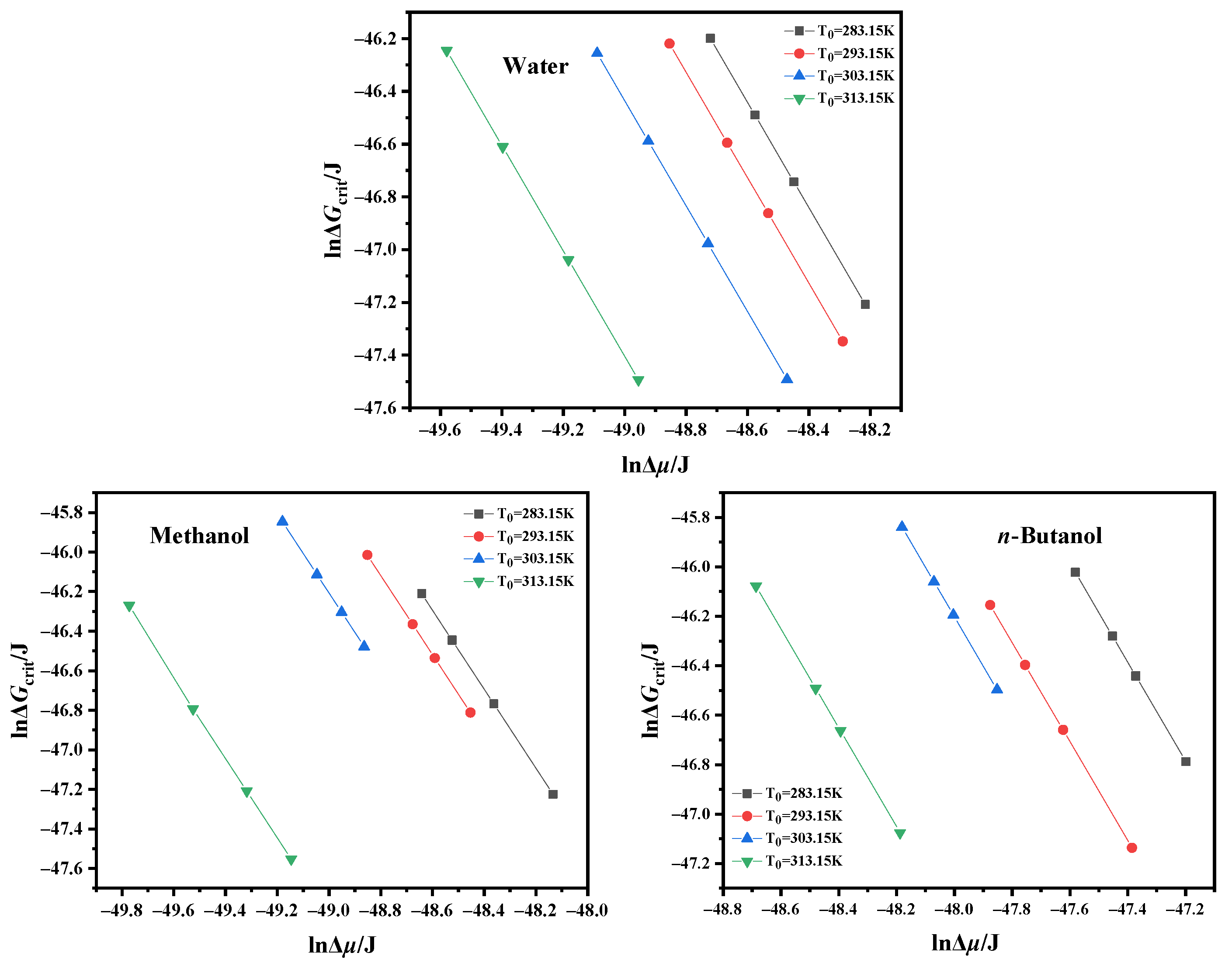
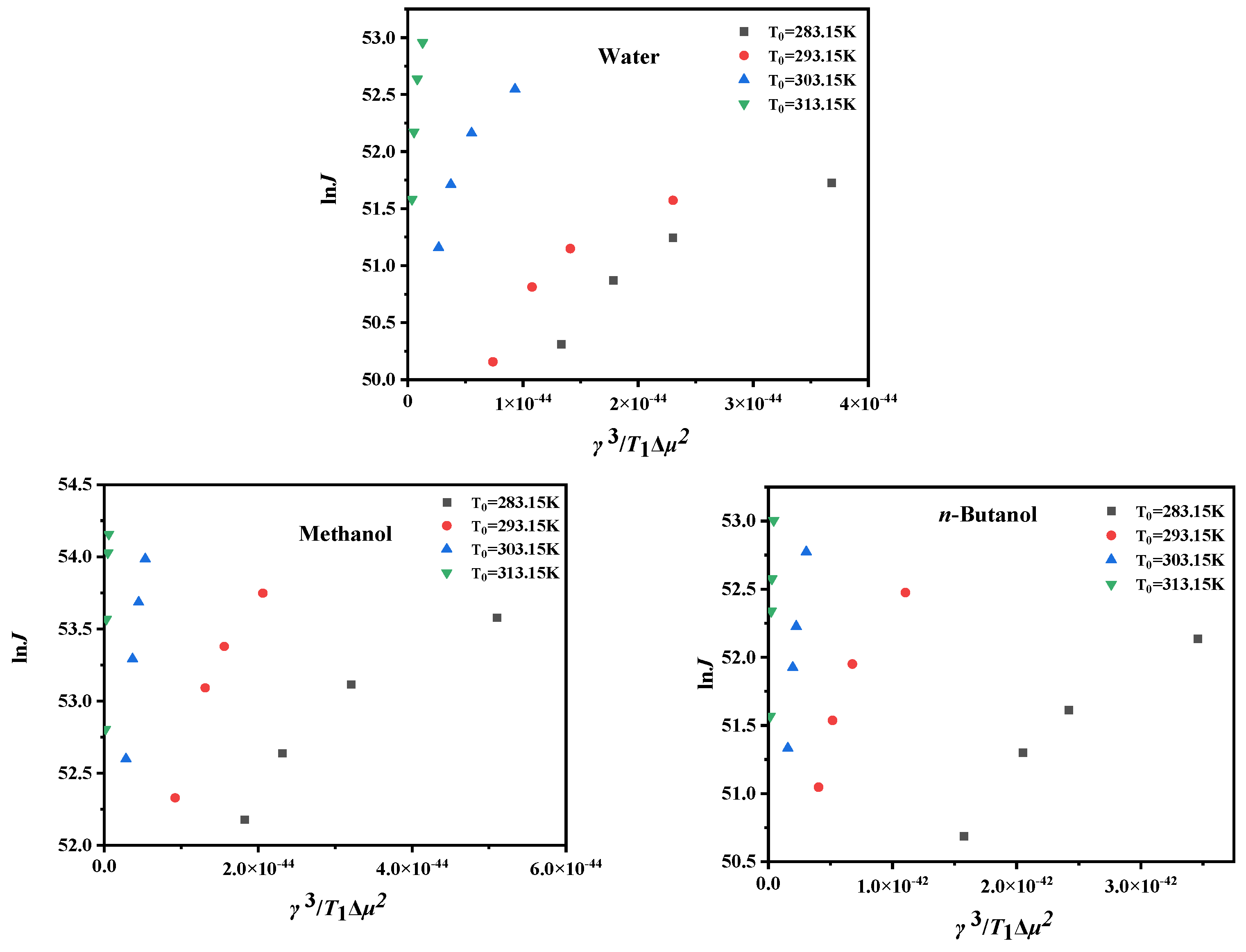
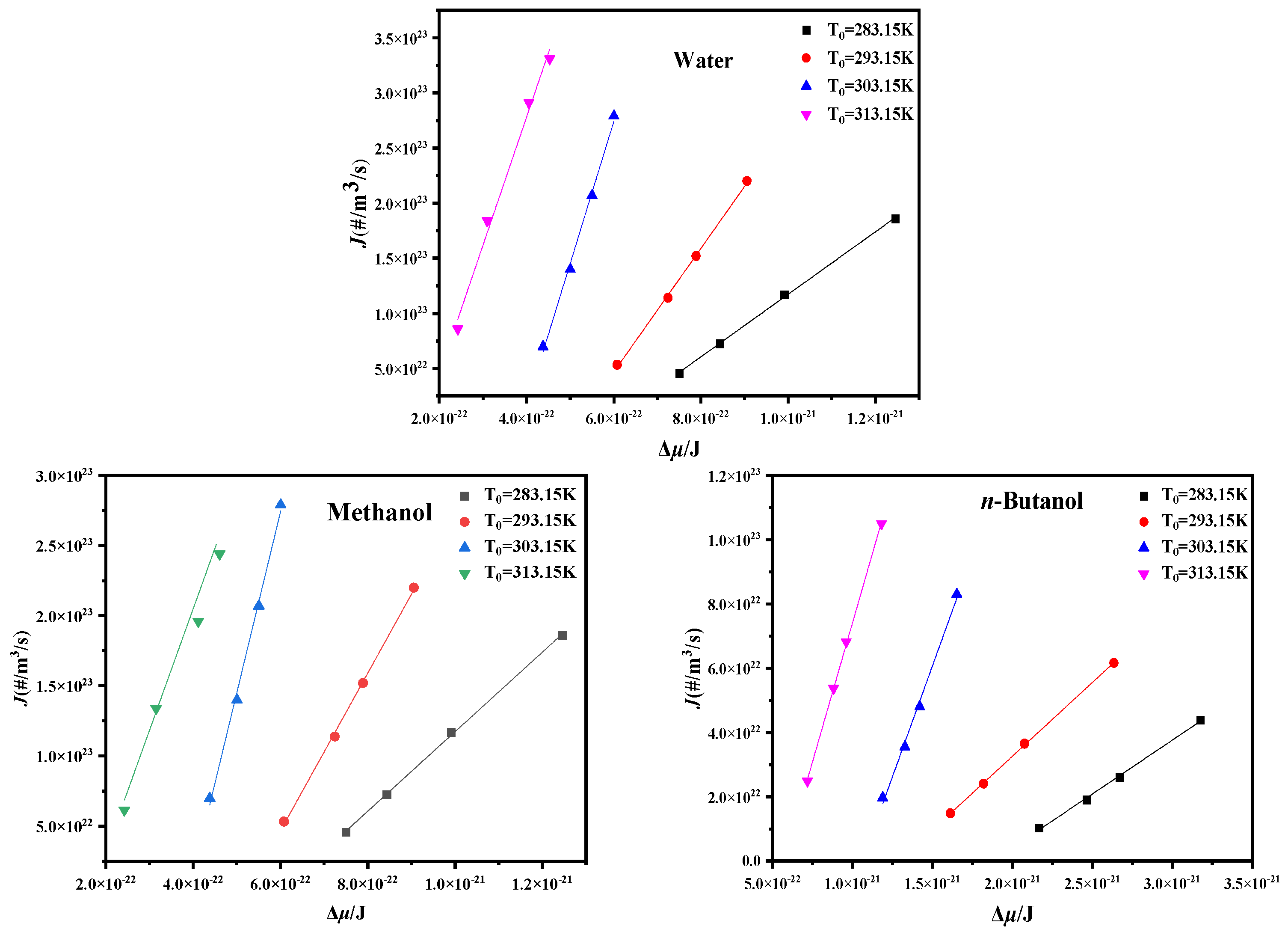
4.3. Critical Nucleation Parameter and Nucleation Kinetic Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Wang, J.; Liu, Q.; Zong, S.; Tian, B.; Huang, X.; Wang, T.; Yin, Q.; Hao, H. Influences and the Mechanism of Additives on Intensifying Nucleation and Growth of p-Methylacetanilide. Cryst. Growth Des. 2020, 20, 973–983. [Google Scholar] [CrossRef]
- Zeglinski, J.; Kuhs, M.; Devi, K.R.; Khamar, D.; Hegarty, A.C.; Thompson, D.; Rasmuson, Å.C. Probing Crystal Nucleation of Fenoxycarb from Solution through the effect of Solvent. Cryst. Growth Des. 2019, 19, 2037–2049. [Google Scholar] [CrossRef]
- Soto, R.; Rasmuson, Å.C. Crystal growth kinetics of Piracetam polymorphs in ethanol and isopropanol. Cryst. Growth Des. 2019, 19, 4273–4286. [Google Scholar] [CrossRef]
- Vekilov, P.G. Nonclassical Nucleation. In Crystallization via Nonclassical Pathways Volume 1: Nucleation, Assembly, Observation & Application; American Chemical Society: Washington, DC, USA, 2020; Volume 1358, pp. 19–46. [Google Scholar]
- Kitamura, M.; Hara, T.; Takimoto-Kamimura, M. Solvent Effect on Polymorphism in Crystallization of BPT Propyl Ester. Cryst. Growth Des. 2006, 6, 381–386. [Google Scholar] [CrossRef]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Qiao, B.; Wang, Y. Review of Liquid–Liquid Phase Separation in Crystallization: From Fundamentals to Application. Cryst. Growth Des. 2021, 21, 7306–7325. [Google Scholar] [CrossRef]
- Xu, S.; Hou, Z.; Chuai, X.; Wang, Y. Overview of Secondary Nucleation: From Fundamentals to Application. Ind. Eng. Chem. Res. 2020, 59, 18335–18356. [Google Scholar] [CrossRef]
- Davey, R.J.; Schroeder, S.L.M.; ter Horst, J.H. Nucleation of Organic CrystalsA Molecular Perspective. Angew. Chem. Int. Ed. 2013, 52, 2166–2179. [Google Scholar] [CrossRef]
- Davey, R.J. Crystallization—How come you look so good? Nature 2004, 428, 374–375. [Google Scholar] [CrossRef]
- Turnbull, D. Kinetics of Solidification of Supercooled Liquid Mercury Droplets. J. Chem. Phys. 1952, 20, 1824. [Google Scholar] [CrossRef]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. Trans. Conn. Acad. Arts Sci. 1878, s3-16, 441–458. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhang, K.; Wu, S.; Liu, S.; Li, K.; Yu, B.; Gong, J. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths. Chem. Eng. Sci. 2016, 155, 248–257. [Google Scholar] [CrossRef]
- Kobari, M.; Kubota, N.; Hirasawa, I. Computer simulation of metastable zone width for unseeded potassium sulfate aqueous solution. J. Cryst. Growth 2011, 317, 64–69. [Google Scholar] [CrossRef]
- Xu, S.; Bu, Y.; Jiang, S.; Yang, P.; Wang, Y. Insights into the Role of Solvents in Nucleation Kinetics of Glutaric Acid from Metastable Zone Widths. Ind. Eng. Chem. Res. 2021, 60, 3073–3082. [Google Scholar] [CrossRef]
- Peters, B. Supersaturation rates and schedules: Nucleation kinetics from isothermal metastable zone widths. J. Cryst. Growth 2011, 317, 79–83. [Google Scholar] [CrossRef]
- Svard, M.; Rasmuson, A.C. m-Hydroxybenzoic Acid: Quantifying Thermodynamic Stability and Influence of Solvent on the Nucleation of a Polymorphic System. Cryst. Growth Des. 2013, 13, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Xu, S.; Wang, J.; Gong, J. Nucleation behavior of ethyl vanillin: Balance between chemical potential difference and saturation temperature. J. Mol. Liq. 2020, 303, 112609. [Google Scholar] [CrossRef]
- Yuan, Y.; Leng, Y.; Huang, C.; Yue, M.; Tan, Q. Effects of cooling rate, saturation temperature, and agitation on the metastable zone width of DL-malic acid-water system. Russ. J. Phys. Chem. A 2015, 89, 1567–1571. [Google Scholar] [CrossRef]
- Kubota, N. Effect of sample volume on metastable zone width and induction time. J. Cryst. Growth 2012, 345, 27–33. [Google Scholar] [CrossRef]
- Rajesh, N.P.; Perumal, C.K.L.; Raghavan, P.S.; Ramasamy, P. Effect of urea on metastable zone width, induction time and nucleation parameters of ammonium dihydrogen orthophosphate. Cryst. Res. Technol. 2001, 36, 55–63. [Google Scholar] [CrossRef]
- Quan, Y.; Yang, Y.; Xu, S.; Zhu, P.; Liu, S.; Jia, L.; Gong, J. Insight into the role of piperazine in the thermodynamics and nucleation kinetics of the triethylenediamine–methyl tertiary butyl ether system. Crystengcomm 2019, 21, 948–956. [Google Scholar] [CrossRef]
- Marciniak, B. Density and ultrasonic velocity of undersaturated and supersaturated solutions of fluoranthene in trichloroethylene, and study of their metastable zone width. J. Cryst. Growth 2002, 236, 347–356. [Google Scholar] [CrossRef]
- Lyczko, N.; Espitalier, F.; Louisnard, O.; Schwartzentruber, J. Effect of ultrasound on the induction time and the metastable zone widths of potassium sulphate. Chem. Eng. J. 2002, 86, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Guerbuez, H.; Oezdemir, B. Experimental determination of the metastable zone width of borax decahydrate by ultrasonic velocity measurement. J. Cryst. Growth 2003, 252, 343–349. [Google Scholar] [CrossRef]
- Khamar, D.; Zeglinski, J.; Mealey, D.; Rasmuson, A.C. Investigating the Role of Solvent-Solute Interaction in Crystal Nucleation of Salicylic Acid from Organic Solvents. J. Am. Chem. Soc. 2014, 136, 11664–11673. [Google Scholar] [CrossRef]
- Sullivan, R.A.; Davey, R.J.; Sadiq, G.; Dent, G.; Back, K.R.; ter Horst, J.H.; Toroz, D.; Hammond, R.B. Revealing the Roles of Desolvation and Molecular Self-Assembly in Crystal Nucleation from Solution: Benzoic and p-Aminobenzoic Acids. Cryst. Growth Des. 2014, 14, 2689–2696. [Google Scholar] [CrossRef] [Green Version]
- Mealey, D.; Zeglinski, J.; Khamar, D.; Rasmuson, A.C. Influence of solvent on crystal nucleation of risperidone. Faraday Discuss 2015, 179, 309–328. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.A.; McGarrity, E.; Meekes, H.; ter Horst, J.H. Isonicotinamide self-association: The link between solvent and polymorph nucleation. Chem. Commun. 2012, 48, 4983–4985. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Yin, Q.; Gong, J.; Bao, Y.; Zhang, X.; Sun, X.; Ding, S.; Xie, C.; Zhang, M.; Hao, H. Effects of Solvent on Polymorph Formation and Nucleation of Prasugrel Hydrochloride. Cryst. Growth Des. 2014, 14, 4519–4525. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, S.; Wu, S.; Du, S.; Cao, Y.; Chen, Y.; Liu, L.; Dong, W.; Gong, J. Temperature and solvent dependent thermodynamic behavior of tetrabromobisphenol A. J. Mol. Liq. 2017, 241, 150–162. [Google Scholar] [CrossRef]
- Azhagan, S.; Marianandhakumar, V. Crystallization of pure adipic acid from methanol solvent and their characterization studies: Intense NLO activity from Centrosymmetric crystal. Opt. Int. J. Light Electron Opt. 2021, 227, 166002. [Google Scholar] [CrossRef]
- Fan, L.; Peisheng, M.A.; Xiang, Z. Measurement and Correlation for Solubility of Adipic Acid in Several Solvents. Chin. J. Chem. Eng. 2007, 15, 110–114. [Google Scholar]
- Rodríguez-Hornedo, N.; Murphy, D. Significance of controlling crystallization mechanisms and kinetics in pharmaceutical systems. J. Pharm. Sci. 1999, 88, 651–660. [Google Scholar] [CrossRef]
- Nývlt, J.; Söhnel, O.; Matuchová, M.; Broul, M. The Kinetics of Industrial Crystallization; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Sangwal, K. A novel self-consistent Nyvlt-like equation for metastable zone width determined by the polythermal method. Cryst. Res. Technol. 2009, 44, 231–247. [Google Scholar] [CrossRef]
- Kubota, N. A new interpretation of metastable zone widths measured for unseeded solutions. J. Cryst. Growth 2008, 310, 629–634. [Google Scholar] [CrossRef]
- Gaivoronskii, A.N.; Granzhan, V.A. Solubility of Adipic Acid in Organic Solvents and Water. Russ. J. Appl. Chem. 2005, 78, 404–408. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Cheng, C.; Cong, Y.; Du, C.; Zhao, H. Solubility and preferential solvation of econazole nitrate in binary solvent mixtures of methanol, ethanol and 1,4-dioxane in water. J. Chem. Thermodyn. 2017, 111, 228–237. [Google Scholar] [CrossRef]
- Welton, T.; Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 175–198. [Google Scholar] [CrossRef]
- Gu, C.H.; Li, H.; Gandhi, R.B.; Raghavan, K. Grouping solvents by statistical analysis of solvent property parameters: Implication to polymorph screening. Int. J. Pharm. 2004, 283, 117–125. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saraswatula, V.G.; Saha, B.K. Thermal Expansion in Alkane Diacids Another Property Showing Alternation in an Odd–Even Series. Cryst. Growth Des. 2013, 13, 3651–3656. [Google Scholar] [CrossRef]
- Gopalan, R.S.; Kumaradhas, P.; Kulkarni, G.U. Structural Phase Transition in Adipic Acid. J. Solid State Chem. 1999, 148, 129–134. [Google Scholar] [CrossRef]




| Materials | Sources | Mass Fraction Purity |
|---|---|---|
| Adipic acid | Shanghai Aladdin Co., Ltd. | ≥0.99 |
| Water | Self-made deionized water | |
| Methanol | Tianjin Jiangtian Chemical Technology Co., Ltd. | ≥0.99 |
| n-Butanol | Tianjin Jiangtian Chemical Technology Co., Ltd. | ≥0.99 |
| Solvent Name | Polarity of Solvent | αa | βb | ΔS/Rg (K) | ΔHs/Rg (K) |
|---|---|---|---|---|---|
| water | 1.00 [40] | 1.17 | 0.47 | 9.08848 | 4441.29 |
| Methanol | 0.60 [41] | 0.43 | 0.47 | 6.21182 | 2747.39 |
| n-Butanol | 0.47 [41] | 0.37 | 0.48 | 7.63163 | 3391.46 |
| Solvent | T0 = 283.15 K | T0 = 293.15 K | T0 = 303.15 K | T0 = 313.15 K | ||||
|---|---|---|---|---|---|---|---|---|
| R (K/h) | ΔT/K | R (K/h) | ΔT/K | R (K/h) | ΔT/K | R (K/h) | ΔT/K | |
| Water | 9.5 | 3.2 | 9.5 | 2.9 | 9.5 | 2.4 | 9.5 | 1.5 |
| 17.5 | 3.7 | 17.5 | 3.5 | 17.5 | 2.8 | 17.5 | 1.8 | |
| 25.5 | 4.2 | 25.5 | 4.0 | 25.5 | 3.4 | 25.5 | 2.2 | |
| 38.5 | 5.3 | 38.5 | 5.1 | 38.5 | 4.4 | 38.5 | 2.8 | |
| Methanol | 9.5 | 5.6 | 9.5 | 4.7 | 9.5 | 3.5 | 9.5 | 2.0 |
| 17.5 | 6.3 | 17.5 | 5.6 | 17.5 | 4.0 | 17.5 | 2.6 | |
| 25.5 | 7.4 | 25.5 | 6.1 | 25.5 | 4.4 | 25.5 | 3.2 | |
| 38.5 | 9.3 | 38.5 | 7.0 | 38.5 | 4.8 | 38.5 | 3.8 | |
| n-Butanol | 9.5 | 13.1 | 9.5 | 10.1 | 9.5 | 7.7 | 9.5 | 4.8 |
| 17.5 | 14.9 | 17.5 | 11.4 | 17.5 | 8.6 | 17.5 | 5.9 | |
| 25.5 | 16.2 | 25.5 | 13.0 | 25.5 | 9.2 | 25.5 | 6.4 | |
| 38.5 | 19.2 | 38.5 | 16.5 | 38.5 | 10.7 | 38.5 | 7.9 | |
| Solvent | ln(R/T0(T0 − Tmax)) | (T0/ΔTmax)2/(T0 − ΔTmax) |
|---|---|---|
| Water | −15.92 | 12.06 |
| Methanol | −13.91 | −4.27 |
| n-Butanol | −15.37 | 0.60 |
| Solvent | T0/K | Slope | Intercept | γ (mJ/m2]) | f/A | f | A (m3]/s) |
|---|---|---|---|---|---|---|---|
| Water | 283.15 | −12.49 | −186.82 | 1.98 | 7.03 × 102 | 4.67 × 1025 | 6.64 × 1022 |
| 293.15 | −16.77 | −263.80 | 1.80 | 1.53 × 103 | 7.60 × 1025 | 4.95 × 1022 | |
| 303.15 | −27.73 | −426.57 | 1.52 | 1.08 × 103 | 1.26 × 1026 | 1.17 × 1023 | |
| 313.15 | −72.45 | −1122.32 | 1.10 | 1.21 × 103 | 2.10 × 1026 | 1.74 × 1023 | |
| Methanol | 283.15 | −4.17 | −62.17 | 2.08 | 1.12 × 103 | 4.70 × 1026 | 4.19 × 1023 |
| 293.15 | −5.19 | −76.45 | 1.93 | 9.15 × 102 | 6.56 × 1026 | 7.17 × 1023 | |
| 303.15 | −8.42 | −121.41 | 1.64 | 6.66 × 102 | 9.10 × 1026 | 1.37 × 1024 | |
| 313.15 | −41.24 | −656.91 | 0.97 | 3.01 × 103 | 1.25 × 1027 | 4.15 × 1023 | |
| n-Butanol | 283.15 | −0.63 | −9.01 | 4.49 | 5.31 × 102 | 8.70 × 1025 | 1.64 × 1023 |
| 293.15 | −1.28 | −19.13 | 3.53 | 8.66 × 102 | 1.30 × 1026 | 1.50 × 1023 | |
| 303.15 | −1.75 | −24.82 | 3.20 | 4.77 × 102 | 1.92 × 1026 | 4.03 × 1023 | |
| 313.15 | −6.02 | −91.39 | 2.11 | 1.15 × 103 | 2.82 × 1026 | 2.46 × 1023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chuai, X.; Li, Y.; Guo, J.; Yang, J.; Liu, Z.; Xu, S. Nucleation Behaviors of Adipic Acid in Different Polarity Solvent Based on Metastable Zone Width. Crystals 2022, 12, 202. https://doi.org/10.3390/cryst12020202
Wang Y, Chuai X, Li Y, Guo J, Yang J, Liu Z, Xu S. Nucleation Behaviors of Adipic Acid in Different Polarity Solvent Based on Metastable Zone Width. Crystals. 2022; 12(2):202. https://doi.org/10.3390/cryst12020202
Chicago/Turabian StyleWang, Yanfei, Xiaoyu Chuai, Yifei Li, Jia Guo, Jing Yang, Zhixue Liu, and Shijie Xu. 2022. "Nucleation Behaviors of Adipic Acid in Different Polarity Solvent Based on Metastable Zone Width" Crystals 12, no. 2: 202. https://doi.org/10.3390/cryst12020202
APA StyleWang, Y., Chuai, X., Li, Y., Guo, J., Yang, J., Liu, Z., & Xu, S. (2022). Nucleation Behaviors of Adipic Acid in Different Polarity Solvent Based on Metastable Zone Width. Crystals, 12(2), 202. https://doi.org/10.3390/cryst12020202










