Surface Modification with Phosphate and Hydroxyapatite of Porous Magnesium Scaffolds Fabricated by Binder Jet Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Dense and Porous Mg Samples
2.2. Coating of Dense and Porous Mg Samples
2.3. Materials Characterisation
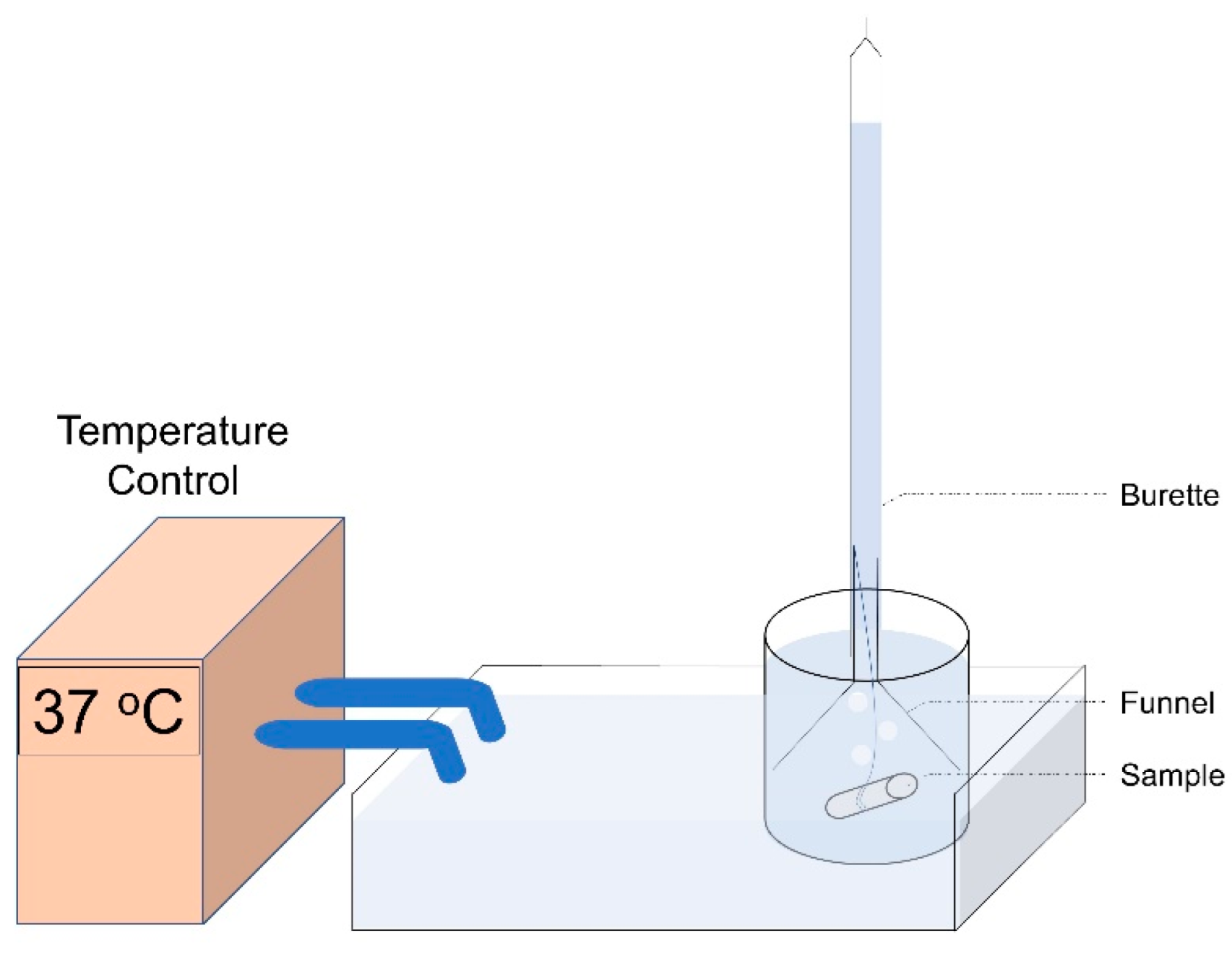
2.4. Corrosion Study
3. Results and Discussions
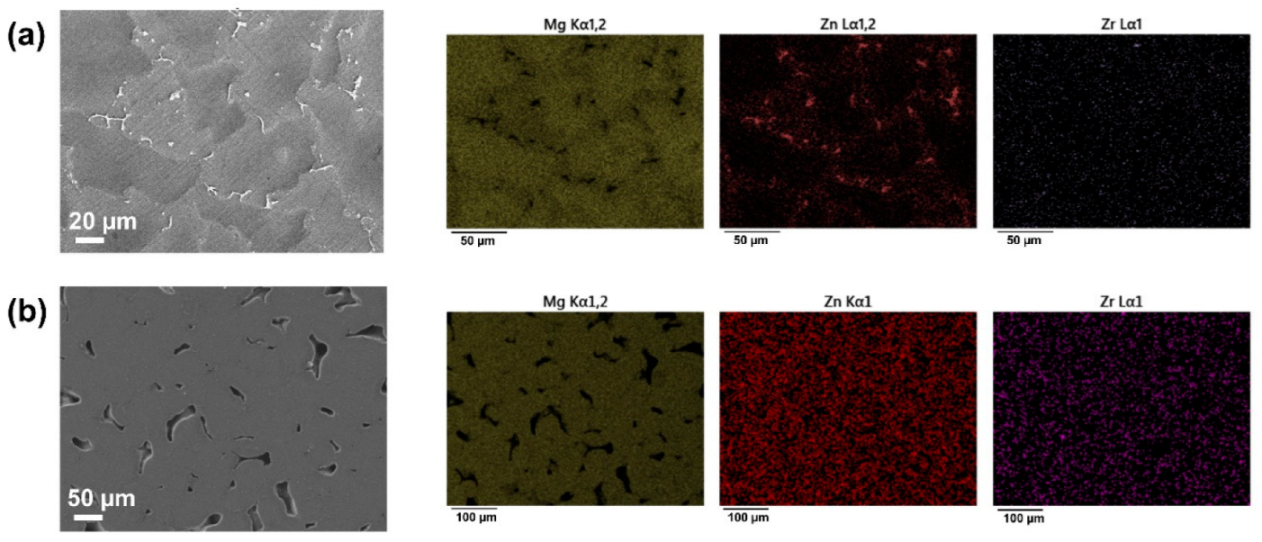
3.1. Characterisation of Dense and Porous Mg-Zn-Zr Samples
3.2. Coating of Dense Samples
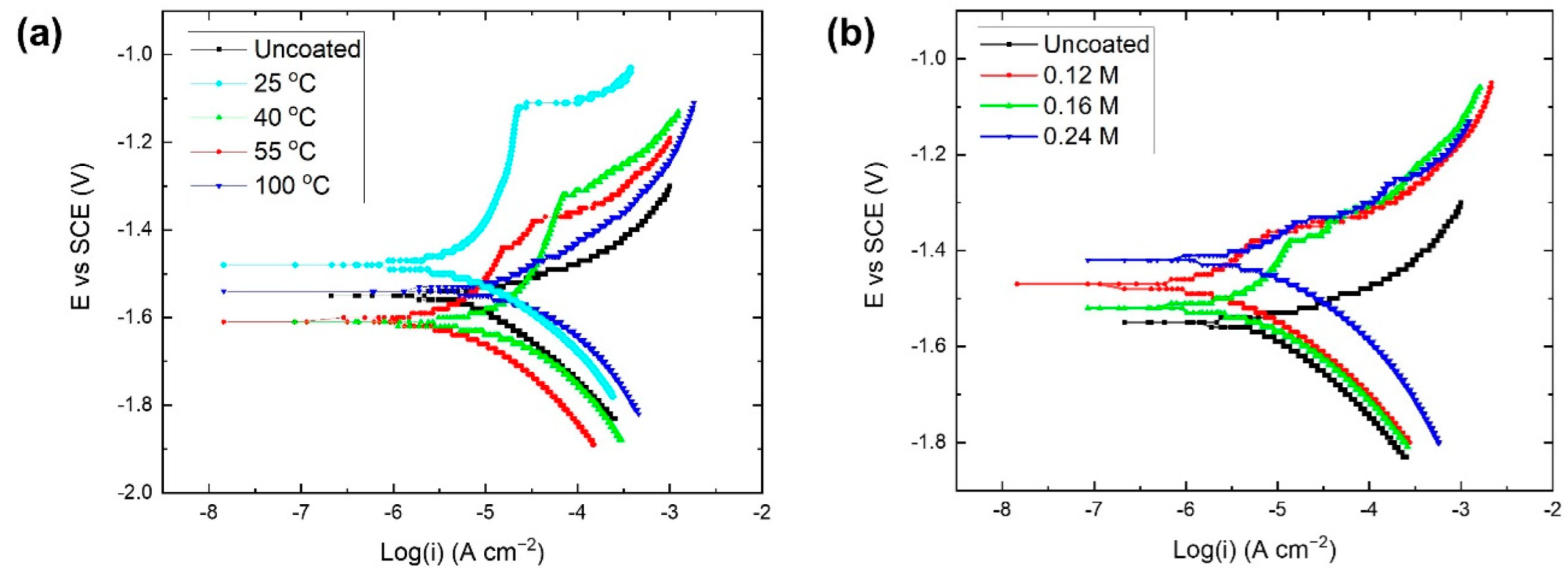
3.3. Corrosion of Coated Dense Samples
3.3.1. Hydroxyapatite Coating
3.3.2. Phosphate Conversion Coating
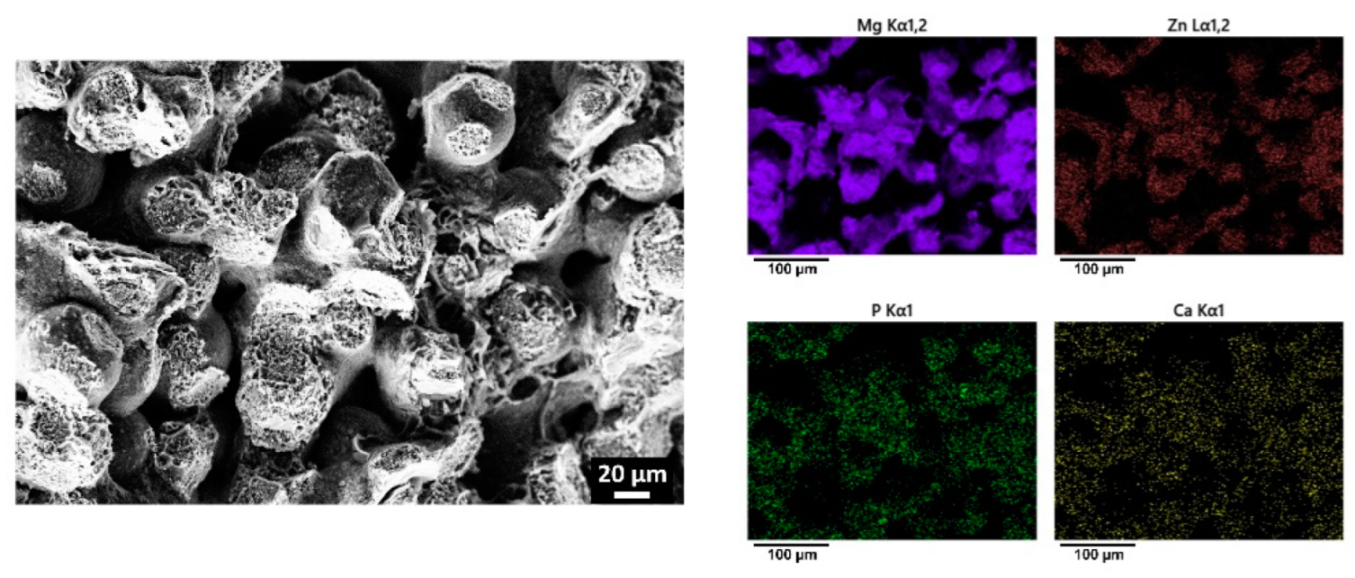
3.4. Coating of Porous Mg-Zn-Zr Scaffolds
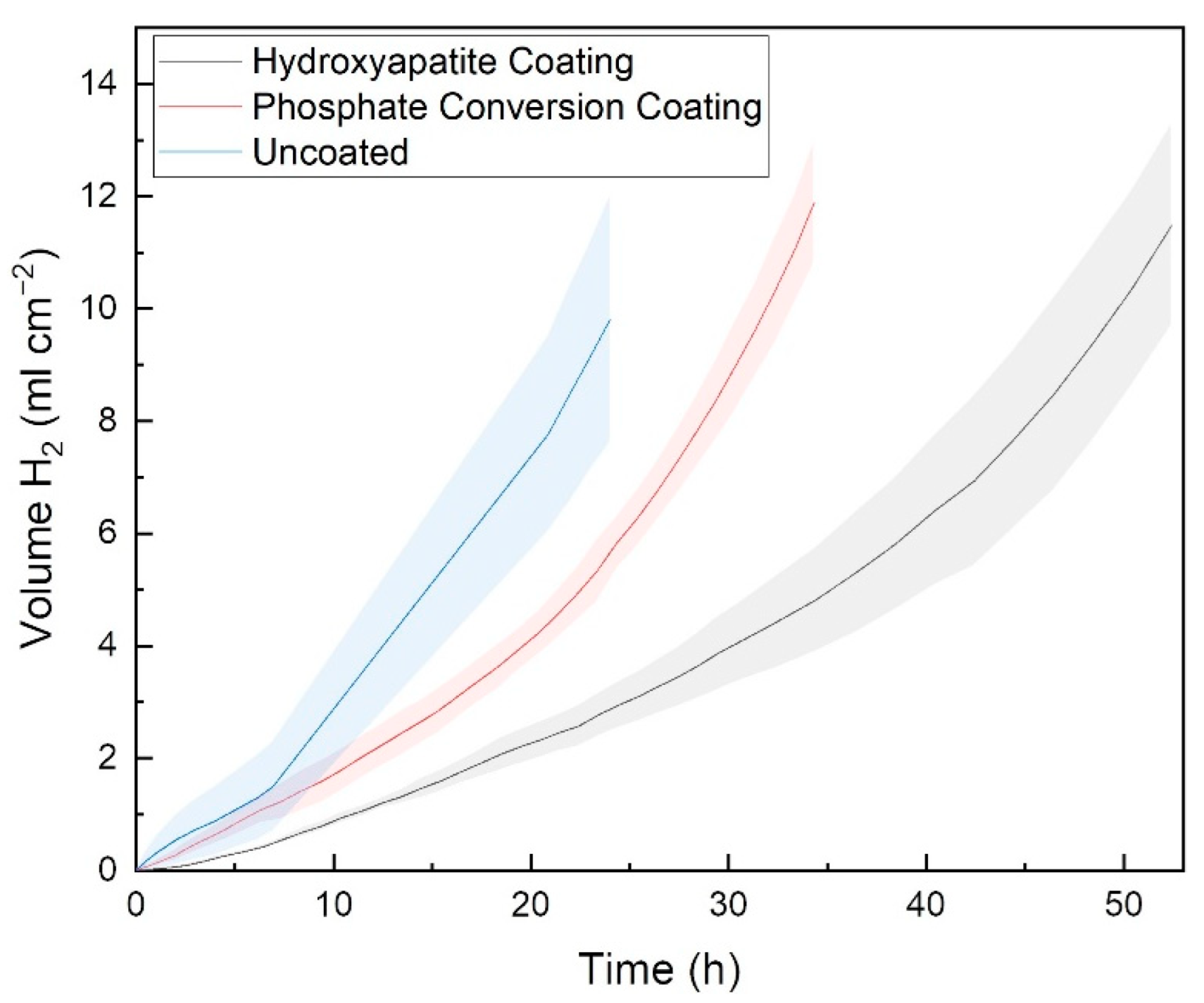
3.5. Corrosion of Coated Porous Scaffolds
4. Challenges of Coating and Corrosion Testing of Porous Mg-Zn-Zr Scaffolds
4.1. Influence of H2 Evolution during Coating Process
4.2. Limited Diffusion within the Pore Channel during the Coating and Corrosion Processes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malda, J.; Woodfield, T.B.F.; Van Der Vloodt, F.; Wilson, C.; Martens, D.E.; Tramper, J.; Van Blitterswijk, C.A.; Riesle, J. The effect of PEGT/PBT scaffold architecture on the composition of tissue engineered cartilage. Biomaterials 2005, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magn. Alloys 2022, 10, 627–669. [Google Scholar] [CrossRef]
- Ding, P.; Liu, Y.; He, X.; Liu, D.; Chen, M. In vitro and in vivo biocompatibility of Mg–Zn–Ca alloy operative clip. Bioact. Mater. 2019, 4, 236–244. [Google Scholar] [CrossRef]
- Hiromoto, S.; Inoue, M.; Taguchi, T.; Yamane, M.; Ohtsu, N. In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 2015, 11, 520–530. [Google Scholar] [CrossRef]
- Benn, F.; Kröger, N.; Zinser, M.; van Gaalen, K.; Vaughan, T.J.; Yan, M.; Smeets, R.; Bibiza, E.; Malinov, S.; Buchanan, F.; et al. Influence of surface condition on the degradation behaviour and biocompatibility of additively manufactured WE43. Mater. Sci. Eng. C 2021, 124, 112016. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Koç, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magn. Alloys 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. Corrosion rate modelling of biodegradable porous iron scaffold considering the effect of porosity and pore morphology. Mater. Sci. Eng. C 2019, 103, 109776. [Google Scholar] [CrossRef]
- Md Saad, A.P.; Jasmawati, N.; Harun, M.N.; Abdul Kadir, M.R.; Nur, H.; Hermawan, H.; Syahrom, A. Dynamic degradation of porous magnesium under a simulated environment of human cancellous bone. Corros. Sci. 2016, 112, 495–506. [Google Scholar] [CrossRef]
- Chen, X.-B.; Li, C.; Xu, D. Biodegradation of Mg-14Li alloy in simulated body fluid: A proof-of-concept study. Bioact. Mater. 2018, 3, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Li, Y.; Wen, C. HA coating on Mg alloys for biomedical applications: A review. J. Magn. Alloys 2020, 8, 929–943. [Google Scholar] [CrossRef]
- Johari, N.A.; Alias, J.; Zanurin, A.; Mohamed, N.S.; Alang, N.A.; Zain, M.Z.M. Anti-corrosive coatings of magnesium: A review. Mater. Today Proc. 2022, 48, 1842–1848. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Tokunaga, T.; Ohno, M.; Matsuura, K. Coatings on Mg alloys and their mechanical properties: A review. J. Mater. Sci. Technol. 2018, 34, 1119–1126. [Google Scholar] [CrossRef]
- Zeng, R.; Lan, Z.; Kong, L.; Huang, Y.; Cui, H. Characterization of calcium-modified zinc phosphate conversion coatings and their influences on corrosion resistance of AZ31 alloy. Surf. Coat Technol. 2011, 205, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Van Phuong, N.; Moon, S. Comparative corrosion study of zinc phosphate and magnesium phosphate conversion coatings on AZ31 Mg alloy. Mater. Lett. 2014, 122, 341–344. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. A simple route towards a hydroxyapatite-Mg(OH)2 conversion coating for magnesium. Corros. Sci. 2011, 53, 2263–2268. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Diodati, S.; Pigato, M.; Bonesso, M.; Dabalà, M. Microstructural and corrosion properties of hydroxyapatite containing peo coating produced on az31 mg alloy. Materials 2021, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasniyati, M.; Zuhailawati, H.; Sivakumar, R.; Dhindaw, B.K.; Noor, S.N.F.M. Cold spray deposition of hydroxyapatite powder onto magnesium substrates for biomaterial applications. Surf. Eng. 2015, 31, 867–874. [Google Scholar] [CrossRef]
- Noorakma, A.C.W.; Zuhailawati, H.; Aishvarya, V.; Dhindaw, B.K. Hydroxyapatite-Coated Magnesium-Based Biodegradable Alloy: Cold Spray Deposition and Simulated Body Fluid Studies. J. Mater. Eng. Perform. 2013, 22, 2997–3004. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S.; Lee, M.H. A simple strategy to modify the porous structure of plasma electrolytic oxidation coatings on magnesium. RSC Adv. 2016, 6, 16100–16114. [Google Scholar] [CrossRef]
- Dong, J.; Tümer, N.; Putra, N.E.; Zhu, J.; Li, Y.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zadpoor, A.A.; et al. Extrusion-based 3D printed magnesium scaffolds with multifunctional MgF2 and MgF2–CaP coatings. Biomater. Sci. 2021, 9, 7159–7182. [Google Scholar] [CrossRef]
- Jayaraj, J.; Amruth Raj, S.; Srinivasan, A.; Ananthakumar, S.; Pillai, U.T.S.; Dhaipule, N.G.K.; Mudali, U.K. Composite magnesium phosphate coatings for improved corrosion resistance of magnesium AZ31 alloy. Corros. Sci. 2016, 113, 104–115. [Google Scholar] [CrossRef]
- Zai, W.; Su, Y.; Man, H.C.; Lian, J.; Li, G. Effect of pH value and preparation temperature on the formation of magnesium phosphate conversion coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2019, 492, 314–327. [Google Scholar] [CrossRef]
- Shirokova, A.G.; Bogdanova, E.A.; Skachkov, V.M.; Pasechnik, L.A.; Borisov, S.V.; Sabirzyanov, N.A. Bioactive coatings of porous materials: Fabrication and properties. J. Surf. Investig. 2017, 11, 107–113. [Google Scholar] [CrossRef]
- Ghasemi, A.; Kamrani, S.; Hübler, D.; Fleck, C. Corrosion behavior of porous magnesium coated by plasma electrolytic oxidation in simulated body fluid. Mater. Corros. 2019, 70, 1561–1569. [Google Scholar] [CrossRef]
- Daavari, M.; Atapour, M.; Mohedano, M.; Sánchez, H.M.; Rodríguez-Hernández, J.; Matykina, E.; Arrabal, R.; Taherizadeh, A. Quasi-in vivo corrosion behavior of AZ31B Mg alloy with hybrid MWCNTs-PEO/PCL based coatings. J. Magn. Alloys 2021, 10, 3217–3233. [Google Scholar] [CrossRef]
- Castro, Y.; Durán, A. Control of degradation rate of Mg alloys using silica sol–gel coatings for biodegradable implant materials. J. Solgel Sci. Technol. 2019, 90, 198–208. [Google Scholar] [CrossRef]
- Kirkland, N.; Birbilis, N.; Staiger, M. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 61, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kuah, K.X.; Blackwood, D.J.; Ong, W.K.; Salehi, M.; Seet, H.L.; Nai, M.L.S.; Wijesinghe, S. Analysis of the corrosion performance of binder jet additive manufactured magnesium alloys for biomedical applications. J. Magn. Alloys 2022, 10, 1296–1310. [Google Scholar] [CrossRef]
- Kleger, N.; Cihova, M.; Masania, K.; Studart, A.R.; Löffler, J.F. 3D Printing of Salt as a Template for Magnesium with Structured Porosity. Adv. Mater. 2019, 31, 1903783. [Google Scholar] [CrossRef]
- Kapłon, H.; Blawert, C.; Chęcmanowski, J.; Naplocha, K. Development of open-porosity magnesium foam produced by investment casting. J. Magn. Alloys 2022, 10, 1941–1956. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, H.; Jang, T.S.; Seong, Y.J.; Kim, H.E.; Koh, Y.H.; Song, J.; do Jung, H. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019, 84, 453–467. [Google Scholar] [CrossRef]
- Kucharczyk, A.; Naplocha, K.; Kaczmar, J.W.; Dieringa, H.; Kainer, K.U. Current Status and Recent Developments in Porous Magnesium Fabrication. Adv. Eng. Mater. 2018, 20, 1700562. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Nai, S.M.L.; Meenashisundaram, G.K.; Goh, M.H.; Gupta, M. A paradigm shift towards compositionally zero-sum binderless 3D printing of magnesium alloys via capillary-mediated bridging. Acta Mater. 2019, 165, 294–306. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; bin Sapari, M.A.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Salehi, M.; Seet, H.L.; Gupta, M.; Farnoush, H.; Maleksaeedi, S.; Nai, M.L.S. Rapid densification of additive manufactured magnesium alloys via microwave sintering. Addit. Manuf. 2021, 37, 101655. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Nai, M.L.S.; Gupta, M. Towards additive manufacturing of magnesium alloys through integration of binderless 3D printing and rapid microwave sintering. Addit. Manuf. 2019, 29, 100790. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; StJohn, D. An Hydrogen Evolution Method for the Estimation of the Corrosion Rate of Magnesium Alloys. In Magnesium Technology 2001; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 254–262. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Farnoush, H.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. An investigation into interaction between magnesium powder and Ar gas: Implications for selective laser melting of magnesium. Powder Technol. 2018, 333, 252–261. [Google Scholar] [CrossRef]
- Kuah, K.X.; Salehi, M.; Ong, W.K.; Seet, H.L.; Nai, M.L.S.; Wijesinghe, S.; Blackwood, D.J. Insights into the influence of oxide inclusions on corrosion performance of additive manufactured magnesium alloys. NPJ Mater. Degrad. 2022, 6, 36. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Abbott, T.; Birbilis, N. The influence of zirconium additions on the corrosion of magnesium. Corros. Sci. 2014, 81, 27–35. [Google Scholar] [CrossRef]
- Clark, J.B. Transmission electron microscopy study of age hardening in a Mg-5 wt.% Zn alloy. Acta Metall. 1965, 13, 1281–1289. [Google Scholar] [CrossRef]
- Chun, J.S.; Byrne, J.G. Precipitate strengthening mechanisms in magnesium zinc alloy single crystals. J. Mater. Sci. 1969, 4, 861–872. [Google Scholar] [CrossRef]
- Zhang, X.; Böhm, S.; Bosch, A.J.; van Westing, E.P.M.; de Wit, J.H.W. Influence of drying temperature on the corrosion performance of chromate coatings on galvanized steel. Mater. Corros. 2004, 55, 501–510. [Google Scholar] [CrossRef]
- Fouladi, M.; Amadeh, A. Comparative study between novel magnesium phosphate and traditional zinc phosphate coatings. Mater. Lett. 2013, 98, 1–4. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, B.; Hou, Y.; Duan, G.; Yang, L.; Zhang, B.; Zhang, T.; Wang, F. Revisiting the cracking of chemical conversion coating on magnesium alloys. Corros. Sci. 2021, 178, 109069. [Google Scholar] [CrossRef]
- Hua, T.S.; Song, R.G.; Zong, Y.; Cai, S.W.; Wang, C. Effect of solution pH on stress corrosion and electrochemical behaviour of aluminum alloy with micro-arc oxidation coating. Mater. Res. Express. 2019, 6, 096441. [Google Scholar] [CrossRef]
- Sadeghi, S.; Ebrahimifar, H. Effect of Electrolyte pH on Microstructure, Corrosion Behavior, and Mechanical Behavior of Ni-P-W-TiO2 Electroplated Coatings. J. Mater. Eng. Perform. 2021, 30, 2409–2421. [Google Scholar] [CrossRef]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Wagener, V.; Schilling, A.; Mainka, A.; Hennig, D.; Gerum, R.; Kelch, M.L.; Keim, S.; Fabry, B.; Virtanen, S. Cell Adhesion on Surface-Functionalized Magnesium. ACS Appl. Mater. Interfaces 2016, 8, 11998–12006. [Google Scholar] [CrossRef]



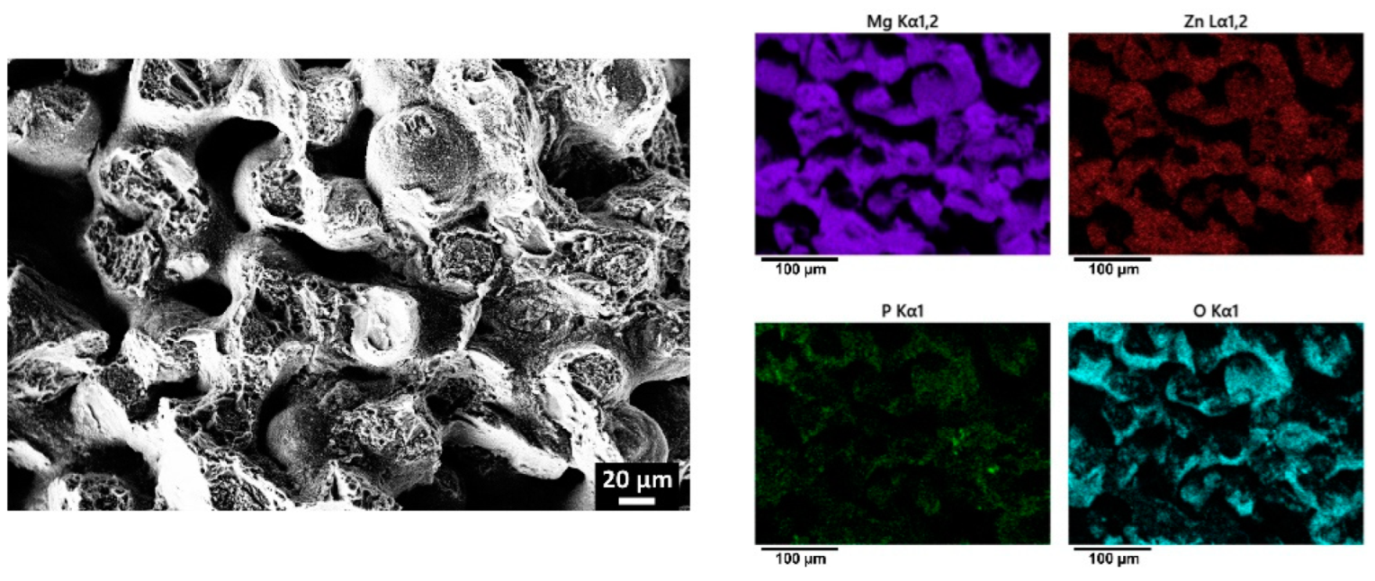

| Sample | Zn (wt.%) | Zr (wt.%) | O (wt.%) | Mg (wt.%) |
|---|---|---|---|---|
| As cased dense | 5.49 | 0.73 | - | Bal. |
| BJP porous | 5.16 | 0.18 | 0.12 | Bal. |
| Sample | Corrosion Current Density (×10−6 A cm−2) |
|---|---|
| Uncoated dense Mg-Zn-Zr | 15.0 |
| Hydroxyapatite dried at 25 °C | 5.0 |
| Hydroxyapatite dried at 40 °C | 52.6 |
| Hydroxyapatite dried at 55 °C | 6.2 |
| Hydroxyapatite dried at 100 °C | 22.0 |
| 0.12 M H3PO4 | 3.1 |
| 0.16 M H3PO4 | 4.7 |
| 0.24 M H3PO4 | 5.9 |
| Sample | Average Hydrogen Evolution Rate (mL cm−2 hr−1) |
|---|---|
| Uncoated porous | 0.41 ± 0.09 |
| Phosphate Conversion Coating | 0.35 ± 0.03 |
| Hydroxyapatite Coating | 0.22 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuah, K.X.; Salehi, M.; Huang, Z.; Zhang, S.X.; Seet, H.L.; Nai, M.L.S.; Blackwood, D.J. Surface Modification with Phosphate and Hydroxyapatite of Porous Magnesium Scaffolds Fabricated by Binder Jet Additive Manufacturing. Crystals 2022, 12, 1850. https://doi.org/10.3390/cryst12121850
Kuah KX, Salehi M, Huang Z, Zhang SX, Seet HL, Nai MLS, Blackwood DJ. Surface Modification with Phosphate and Hydroxyapatite of Porous Magnesium Scaffolds Fabricated by Binder Jet Additive Manufacturing. Crystals. 2022; 12(12):1850. https://doi.org/10.3390/cryst12121850
Chicago/Turabian StyleKuah, Kai Xiang, Mojtaba Salehi, Zihan Huang, Su Xia Zhang, Hang Li Seet, Mui Ling Sharon Nai, and Daniel John Blackwood. 2022. "Surface Modification with Phosphate and Hydroxyapatite of Porous Magnesium Scaffolds Fabricated by Binder Jet Additive Manufacturing" Crystals 12, no. 12: 1850. https://doi.org/10.3390/cryst12121850
APA StyleKuah, K. X., Salehi, M., Huang, Z., Zhang, S. X., Seet, H. L., Nai, M. L. S., & Blackwood, D. J. (2022). Surface Modification with Phosphate and Hydroxyapatite of Porous Magnesium Scaffolds Fabricated by Binder Jet Additive Manufacturing. Crystals, 12(12), 1850. https://doi.org/10.3390/cryst12121850











