Comparison of Dynamical and Empirical Simulation Methods of Secondary Dendrite Arm Coarsening
Abstract
1. Introduction
2. Theory of Coarsening
2.1. Effect of the Radius of the Solid Phase on the Equilibrium Concentrations
2.2. Process of the Coarsening
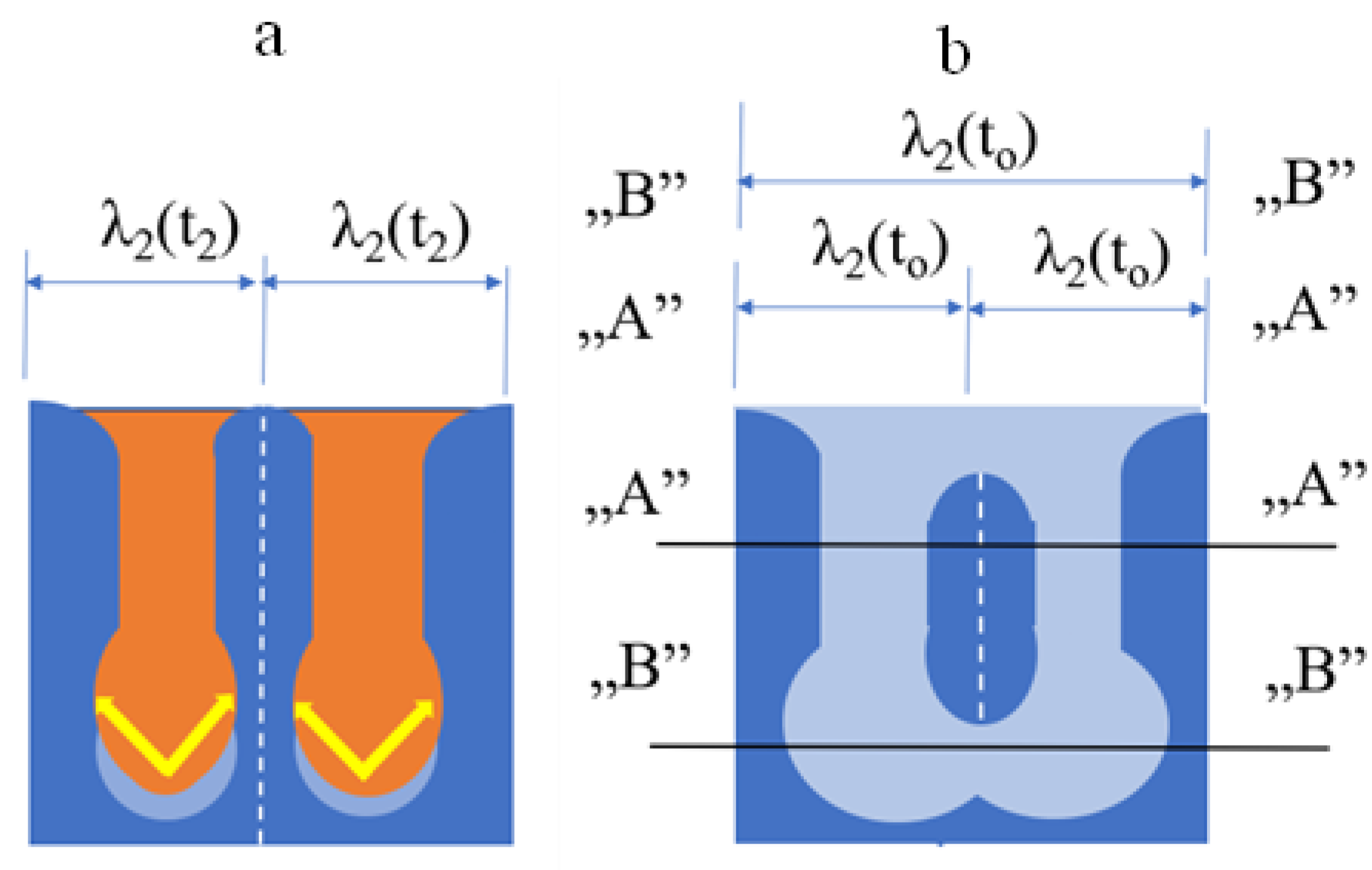
2.3. Models of the Coarsening of Secondary Dendrite Arms
3. Experiments
3.1. Alloy
3.2. Solidification Experiments
3.3. Measuring Method of Secondary Dendrite Arm Spacing (SDAS)
3.4. Cooling Curves
4. Simulation of the Secondary Dendrite Arm Spacing (SDAS)
4.1. Empirical Simulation Method
- (i)
- The change of the SDAS during solidification cannot be calculated, so this method is not good enough for the simulation of microsegregation;
- (ii)
- This simulation method is usable for only directional solidification when the temperature gradient and the solid/liquid front velocity can be determined.
4.2. Dynamical Simulation Method
5. Results and Discussion
5.1. Material Constants
5.2. Simulation Method
5.3. Capability of the Dynamical Method
5.4. Comparison of the Results of Two Simulation Methods and the Measured SDAS
5.5. Effect of Form of Cooling Curves
6. Summary and Conclusions
- (i)
- The two simulation methods describe well enough the measured final SDAS (at t0). With the empirical method, it is possible to calculate the final SDAS in an arbitrary position of the sample, while because the dynamical simulation must know the cooling curve, with the dynamical method, it is possible to calculate the final SDAS, and the cooling curves were determined.
- (ii)
- As with the empirical method, it is possible to calculate only the final SDAS; with the dynamical method, the SDAS can be calculated during solidification too. So, using the dynamical method, the results of the solidification simulation (the microsegregation) will be more correct.
- (iii)
- The dynamical simulation proved that the final SDAS depends on the form of the cooling curve, which could change during solidification. The dynamical method can consider the form of the cooling curve, and then it can be usable for any type of solidification.
- (iv)
- In contrast with the current fact that one SDAS belongs to one local solidification time, it was shown that it is not valid; the difference between the SDAS can be either ~50%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roósz, A.; Gácsi, Z.; Fuchs, E. Solute redistribution during solidification and homogenization of binary solid solution. Acta Metall. 1984, 32, 1745–1754. [Google Scholar] [CrossRef]
- Osório, W.R.; Goulart, P.R.; Garcia, A.; Santos, G.A.; Moura-Neto, C. Effect of dendritic arm spacing on mechanical properties and corrosion resistance of Al 9 Wt Pct Si and Zn 27 Wt Pct Al alloys. Metall. Mater. Trans. A 2006, 37, 2525–2538. [Google Scholar] [CrossRef]
- Rao, D.H.; Tagore, G.R.N.; Janardhana, G.R. Evolution of Artificial Neural Network (ANN) model for predicting secondary dendrite arm spacing in aluminium alloy casting. J. Braz. Soc. Mech. Sci. Eng. 2010, 32, 276–281. [Google Scholar] [CrossRef]
- Zamani, M. Al-Si Cast Alloys -Microstructure and Mechanical Properties at Ambient and Elevated Temperature. Ph.D. Thesis, Research Series from the School of Engineering, Jönköping University, Department of Materials and Manufacturing, Jönköping, Sweden, 2015. Dissertation Series No. 7. [Google Scholar]
- Kattamis, T.Z.; Coughlin, J.M.; Flemings, M.C. Influence of Coarsening on Dendrite Arm Spacing of Aluminum-Copper Alloys. Trans. TMS-AIME 1967, 239, 1504. [Google Scholar]
- Chernov, A.A. Estimation of transformation times for inclusions and dendritic crystals. Kirstallografiya 1956, 1, 583. [Google Scholar]
- Kahlweit, M. On the ageing of dendrites. Scr. Metall. 1968, 2, 251–254. [Google Scholar] [CrossRef]
- Huang, S.C.; Glicksman, M.E. Fundamental of Dendritic solidification II, Development of Sidebranch Structure. Acta Metall. 1981, 29, 717–734. [Google Scholar] [CrossRef]
- Ramirez-Vidaurri, L.E.; Castro-Rom, M.; Herrera-Trejo, M.; Fraga-Chavez, K.L. Secondary dendritic arm spacing and cooling rate relationship for an ASTM F75 alloy. Mater. Res. Technol. 2022, 19, 5049–5065. [Google Scholar] [CrossRef]
- Üstün, E.; Airline. Effect of Growth Rate on Coarsening of Secondary Dendrite Arm Spacings, in Directionally Solidified of Al-8.8La-1.2Ni Ternary Alloy. Arch. Metall. Mater. 2022, 6, 931–938. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Castroa, J.A.; Ferreira, J.A. Predicting Secondary-Dendrite Arm Spacing of the Al-4.5wt%Cu Alloy During Unidirectional Solidification. Mat. Res. 2017, 20, 68–75. [Google Scholar] [CrossRef]
- Natsume, Y.; Ishida, H. Characterization of Dendrite Morphology for Al-Based Alloy by Phase-Field Model; The Japan Institute of Light Metals: Tokyo, Japan, 2010; pp. 1746–1751. [Google Scholar]
- Milenkovic, S.; Rahimian, M.; Sabirov, I.; Maestro, L. Effect of solidification parameters on the secondary dendrite arm spacing in MAR M-247 superalloy determined by a novel approach. MATEC Web Conf. 2014, 14, 13004. [Google Scholar] [CrossRef]
- Rontó, V.; Roósz, A. The effect of cooling rate and composition on the secondary dendrite arm spacing during solidification. Part I: Al-Cu-Si alloys. Int. J. Cast Met. Res. 2001, 13, 337–342. [Google Scholar] [CrossRef]
- Rontó, V.; Roósz, A. The effect of the cooling rate or the local solidification time and composition on the secondary dendrite arm spacing during solidification PART II: Al–Mg–Si alloys. Int. J. Cast Met. Res. 2001, 14, 131–135. [Google Scholar] [CrossRef]
- Kirkwood, D.H. A simple model for dendrite arm coarsening during solidification. Mater. Sci. Eng. 1985, 73, L1–L4. [Google Scholar] [CrossRef]
- Glicksman, M.; (University of Florida, Gainesville, FL, USA). Personal notification, 2022.
- Roósz, A.; Rónaföldi, A.; Li, Y.; Mangelinck-Noël, N.; Zimmermann, G.; Nguyen-Thi, H.; Svéda, M.; Veres, Z. Influence of Solidification Parameters on the Amount of Eutectic and Secondary Arm Spacing of Al–7wt% Si Alloy Solidified under Microgravity. Crystals 2022, 12, 414. [Google Scholar] [CrossRef]
- Roósz, A.; Rónaföldi, A.; Li, Y.; Mangelinck-Noël, N.; Zimmermann, G.; Nguyen-Thi, H.; Svéda, M.; Veres, Z. Microstructure Analysis of Al-7 wt% Si Alloy Solidified on Earth Compared to Similar Experiments in Microgravity. Crystals 2022, 12, 1226. [Google Scholar] [CrossRef]
- Roósz, A. The Effect of Temperature Gradient and Primary Arm Tip Velocity on Secondary Dendrite Arm Spacing at Steady-state Conditions Solidification. Cast Metals 1988, 1, 223–226. [Google Scholar] [CrossRef]
- Halder, E.; Roósz, A.; Exner, H.E.; Fischmeister, H.F. Numerical calculation of the concentration distribution during the solidification of binary alloys allowing for dendrite arm coarsening. Mater. Sci. Forum 1987, 13–14, 547–558. [Google Scholar] [CrossRef]
- Kumoto, E.A.; Alhadeff, R.O.; Martorano, M.A. Microsegregation and dendrite arm coarsening in tin bronze. Mater. Sci. Technol. 2002, 18, 1001. [Google Scholar] [CrossRef]
- Paliwal, M.; Kang, D.H.; Essadiqi, E.; Jung, I. Variations of Microsegregation and Second Phase Fraction of Binary Mg-Al Alloys with Solidification Parameters. Metall. Mater. Trans. A 2014, 45, 3308–3320. [Google Scholar] [CrossRef]
- Paliwal, M.; Kang, D.H.; Essadiqi, E.; Jung, I. The Evolution of As-cast Microstructure of Ternary Mg-Al-Zn Alloys: An Experimental and Modeling Study. Metall. Mater. Trans. A 2014, 45, 3596–3608. [Google Scholar] [CrossRef]
- Battle, T.P.; Pehlke, R.D. Mathematical Modeling of Microsegregation in Binary Metallic Alloys. Metall. Trans. 1990, 21, 357–375. [Google Scholar] [CrossRef]





















| Sample | Alloy | Initial G K/mm | Stage I | Stage II | |||
|---|---|---|---|---|---|---|---|
| v1 mm/s | z1 mm | v2 mm/s | z2 mm | RMF | |||
| GB1 | Al + 7wt%Si +GR | 4 | 0.02 | 50 | 0.2 | 60 | No |
| GB2 | Al + 7wt%Si | 4 | 0.02 | 50 | 0.2 | 60 | 0.5 mT |
| GB5 | Al + 7wt%Si +GR | 4 | 0.02 | 50 | 0.02 | 60 | No |
| GB6 | Al + 7wt%Si | 4 | 0.02 | 50 | 0.02 | 60 | 0.5 mT |
| Partition Coefficient | k | 0.13 | wt%/wt% |
|---|---|---|---|
| Liquidus slope | mL | −6.587 | K/wt% |
| Geometric factor | G | 33 | - |
| Interfacial energy | σ | 9.3 × 10−2 | J/m2 |
| Latent heat | L | 3.97 × 105 | J/kg |
| Si liquid diffusion | D0 | 1.3 × 10−7 | m2/s |
| Q | 30,000 | J/mol | |
| Density | ρ | 2.475 × 103 | kg/m3 |
| Alloy concentration | C0 | 7.0 | wt% |
| Sample | T1 start °C | T Stop, °C | Cool. rate K/s | Hold s | T2 Start °C | T2 Stop °C | Cool. Rate K/s | SDAS µm | Fs % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 614 | 574 | 0.384 | - | - | - | 16.2 | ||
| 2 | 614 | 604 | 10 | 100 | 604 | RT | 10 | 23.4 | 16 |
| 3 | 614 | 594 | 10 | 100 | 594 | RT | 10 | 21.8 | 31 |
| 4 | 614 | 584 | 10 | 100 | 584 | RT | 10 | 20.4 | 42 |
| 5 | 614 | 575 | 10 | 100 | 575 | RT | 10 | 19.3 | 51 |
| SDASmax/SDASmin = 23.4/16.2 = 1.44 | |||||||||
| Sample | T1 Start °C | T Stop, °C | Cool. Rate K/s | Hold s | T2 Start °C | T2 Stop °C | Cool. Rate K/s | SDAS µm | Fs % |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 614 | RT | 0.1 | - | - | - | 34.6 | - | |
| 7 | 614 | 594 | 1 | 180 | 594 | RT | 1 | 33 | 31 |
| 8 | 614 | 575 | 1 | 360 | - | - | - | 29.3 | 51 |
| 9 | 614 | 594 | 0.052 | - | 594 | RT | 10 | 36.7 | 31 |
| SDASmax/SDASmin = 36.7/29.3 = 1.25 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roósz, A.; Rónaföldi, A.; Svéda, M.; Veres, Z. Comparison of Dynamical and Empirical Simulation Methods of Secondary Dendrite Arm Coarsening. Crystals 2022, 12, 1834. https://doi.org/10.3390/cryst12121834
Roósz A, Rónaföldi A, Svéda M, Veres Z. Comparison of Dynamical and Empirical Simulation Methods of Secondary Dendrite Arm Coarsening. Crystals. 2022; 12(12):1834. https://doi.org/10.3390/cryst12121834
Chicago/Turabian StyleRoósz, András, Arnold Rónaföldi, Mária Svéda, and Zsolt Veres. 2022. "Comparison of Dynamical and Empirical Simulation Methods of Secondary Dendrite Arm Coarsening" Crystals 12, no. 12: 1834. https://doi.org/10.3390/cryst12121834
APA StyleRoósz, A., Rónaföldi, A., Svéda, M., & Veres, Z. (2022). Comparison of Dynamical and Empirical Simulation Methods of Secondary Dendrite Arm Coarsening. Crystals, 12(12), 1834. https://doi.org/10.3390/cryst12121834






