Study of the Applicability of Magnetic Iron-Containing Nanoparticles in Hyperthermia and Determination of Their Resistance to Degradation Processes
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Objects of Study
2.3. Characterization
2.4. Hyperthermia Research
2.5. Corrosion Resistance Tests
3. Results and Discussion
3.1. Results of Characterization of the Studied Nanostructures
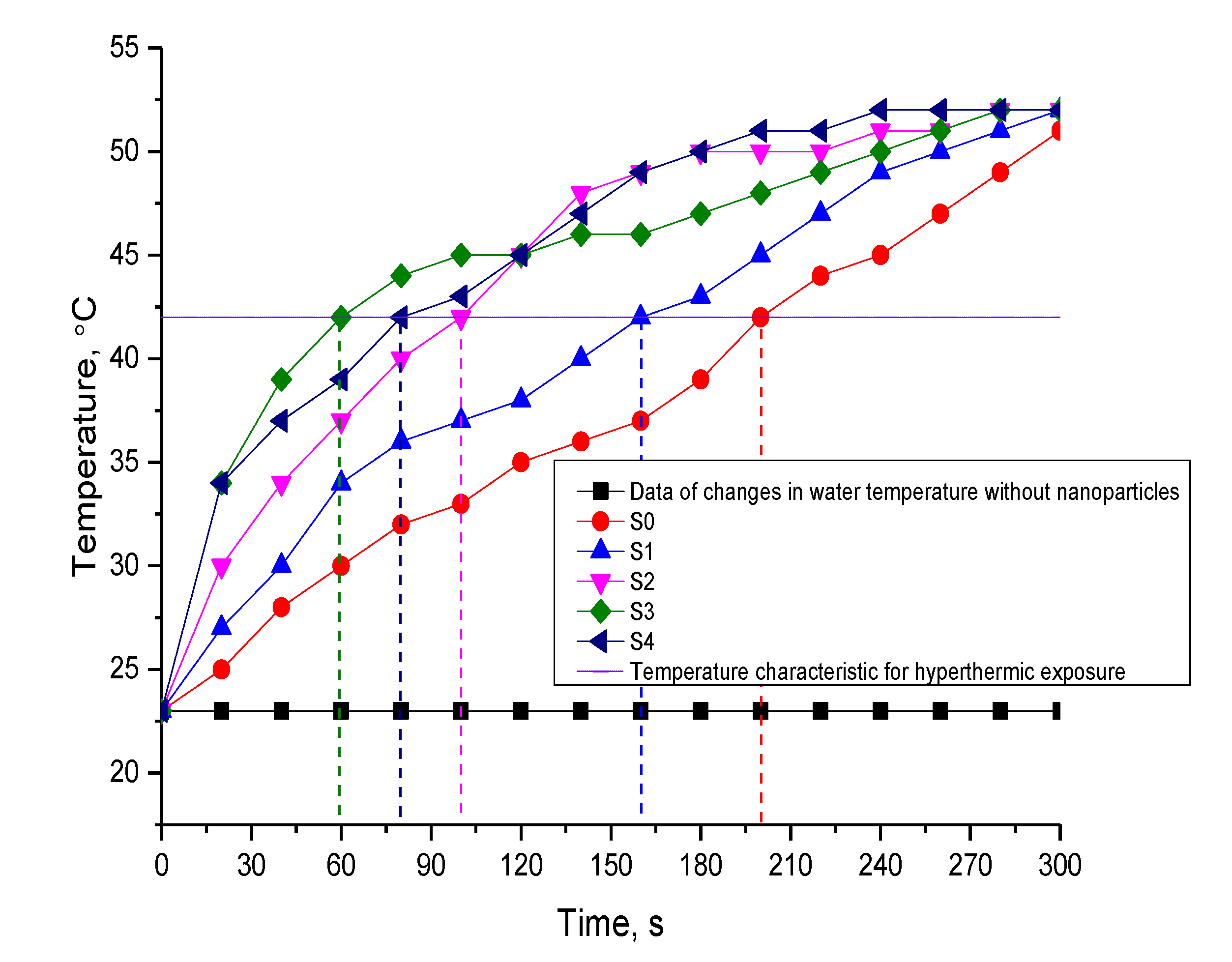
3.2. Results of Hyperthermic Studies
3.3. Results of a Comparative Analysis of Magnetic Hyperthermia with Literature Data
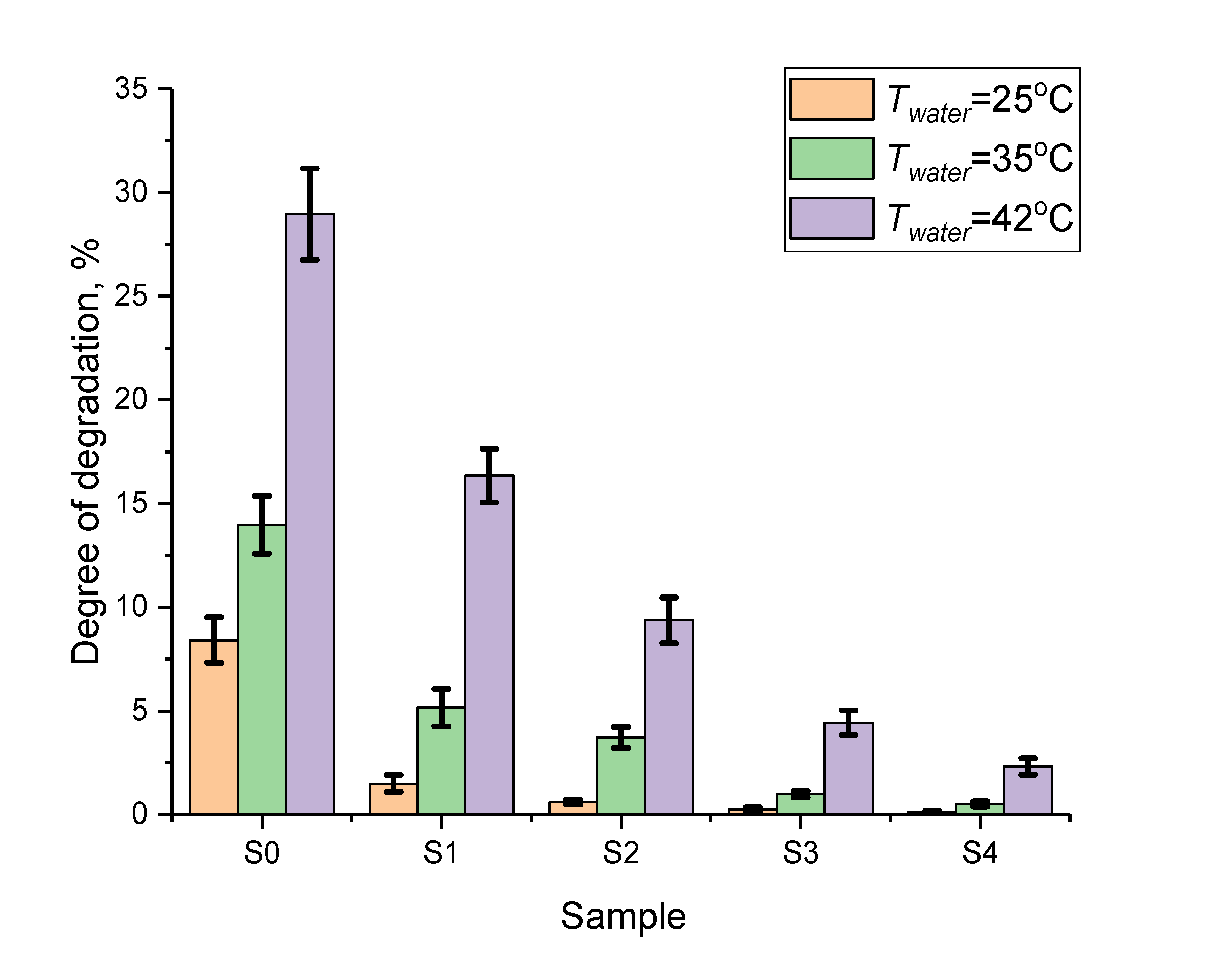
3.4. Results of Nanoparticle Corrosion Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Grüttner, C.; Müller, K.; Teller, J.; Westphal, F. Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int. J. Hyperth. 2013, 29, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; James, F.H. Intravenous magnetic nanoparticle cance hyperthermia. Int. J. Nanomed. 2013, 8, 2521. [Google Scholar]
- Ramana, P.V.; Rao, K.S.; Kumar, K.R.; Kapusetti, G.; Choppadandi, M.; Kiran, J.N.; Rao, K.H. A study of uncoated and coated nickel-zinc ferrite nanoparticles for magnetic hyperthermia. Mater. Chem. Phys. 2021, 266, 124546. [Google Scholar] [CrossRef]
- Ramirez, D.; Oliva, J.; Cordova-Fraga, T.; Basurto-Islas, G.; Benal-Alvarado, J.J.; Mtz-Enriquez, A.I.; Quintana, M.; Gomez-Solis, C. High Heating Efficiency of Magnetite Nanoparticles Synthesized with Citric Acid: Application for Hyperthermia Treatment. J. Electron. Mater. 2022, 51, 4425–4436. [Google Scholar] [CrossRef]
- Islam, M.S.; Kusumoto, Y.; Kurawaki, J.; Abdulla-Al-Mamun, M.D.; Manaka, H. A comparative study on heat dissipation, morphological and magnetic properties of hyperthermia suitable nanoparticles prepared by co-precipitation and hydrothermal methods. Bull. Mater. Sci. 2012, 35, 1047–1053. [Google Scholar] [CrossRef]
- Dey, C.; Baishya, K.; Ghosh, A.; Goswami, M.M.; Ghosh, A.; Mandal, K. Improvement of drug delivery by hyperthermia treatment using magnetic cubic cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 2017, 427, 168–174. [Google Scholar] [CrossRef]
- Macías-Martínez, B.I.; Cortés-Hernández, D.A.; Zugasti-Cruz, A.; Cruz-Ortíz, B.R.; Múzquiz-Ramos, E.M. Heating ability and hemolysis test of magnetite nanoparticles obtained by a simple co-precipitation method. J. Appl. Res. Technol. 2016, 14, 239–244. [Google Scholar] [CrossRef]
- Jang, D.-H.; Lee, Y.-I.; Kim, K.-S.; Park, E.-S.; Kang, S.-C.; Yoon, T.-J.; Choa, Y.-H. Induced heat property of polyethyleneglycol-coated iron oxide nanoparticles with dispersion stability for hyperthermia. J. Nanosci. Nanotechnol. 2013, 13, 6098–6102. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Ermekova, A.E.; Korolkov, I.V.; Chudoba, D.; Jazdzewska, M.; Ludzik, K.; Zdorovets, M.V. Study of phase transformations, structural, corrosion properties and cytotoxicity of magnetite-based nanoparticles. Vacuum 2019, 163, 236–247. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Xu, S.H.; Fei, G.T.; Ouyang, H.M.; Zhang, Y.; Huo, P.C.; De Zhang, L. Controllable fabrication of nickel nanoparticle chains based on electrochemical corrosion. J. Mater. Chem. C 2014, 3, 2072–2079. [Google Scholar] [CrossRef]
- Durgadsimi, S.U.; Kattimani, V.R.; Maruti, N.S.; Kulkarni, A.B.; Mathad, S.N. Synthesis and structural analysis of nickel ferrite synthesized by co-precipitation method. Eurasian Phys. Tech. J. 2021, 18, 14–19. [Google Scholar] [CrossRef]
- Fayazzadeh, S.; Khodaei, M.; Arani, M.; Mahdavi, S.R.; Nizamov, T.; Majouga, A. Magnetic Properties and Magnetic Hyperthermia of Cobalt Ferrite Nanoparticles Synthesized by Hydrothermal Method. J. Supercond. Nov. Magn. 2020, 33, 2227–2233. [Google Scholar] [CrossRef]
- Ebrahimisadr, S.; Aslibeiki, B.; Asadi, R. Magnetic hyperthermia properties of iron oxide nanoparticles: The effect of concentration. Phys. C Supercond. Its Appl. 2018, 549, 119–121. [Google Scholar] [CrossRef]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic Review of the Preparation Techniques of Iron Oxide Magnetic Nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ren, H.; Zhang, Y.; Gu, N.; Lin, X. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J. Soils Sediments 2011, 11, 1408–1417. [Google Scholar] [CrossRef]
- Stary, O.; Malyshev, A.V.; Lysenko, E.N.; Petrova, A. Formation of magnetic properties of ferrites during radiation-thermal sintering. Eurasian Phys. Tech. J. 2021, 17, 6–10. [Google Scholar] [CrossRef]
- Smilgies, D.-M. Scherrer grain-size analysis adapted to grazing-incidence scattering with area detectors. J. Appl. Crystallogr. 2009, 42, 1030–1034. [Google Scholar] [CrossRef]
- León Félix, L.; Coaquira, J.A.H.; Martínez, M.A.R.; Goya, G.F.; Mantilla, J.; Sousa, M.H.; Morais, P.C. Structural and magnetic properties of core-shell Au/Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 41732. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Hamishehkar, H.; Arsalani, N.; Entezami, A.A. Preparation of thermo and pH-responsive polymer@ Au/Fe3O4 core/shell nanoparticles as a carrier for delivery of anticancer agent. J. Nanoparticle Res. 2015, 17, 305. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, A.; Yang, X.; He, H.; Fan, Y.; Yao, C. Cubic GdFeO3 particle by a simple hydrothermal synthesis route and its photoluminescence and magnetic properties. Crystengcomm 2012, 14, 8432–8439. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Stibor, I. Pentenoic Acid-Stabilized Magnetic Nanoparticles for Nanomedicine Applications. J. Dispers. Sci. Technol. 2016, 37, 1793–1798. [Google Scholar] [CrossRef]
- Fadeev, M.; Kozlovskiy, A.; Korolkov, I.; Egizbek, K.; Nazarova, A.; Chudoba, D.; Rusakov, V.; Zdorovets, M. Iron oxide @ gold nanoparticles: Synthesis, properties and potential use as anode materials for lithium-ion batteries. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 603, 125178. [Google Scholar] [CrossRef]
- Korolkov, I.; Ludzik, K.; Kozlovskiy, A.; Fadeev, M.; Shumskaya, A.; Gorin, Y.; Marciniak, B.; Jazdzewska, M.; Chudoba, D.; Kontek, R.; et al. Carboranes immobilization on Fe3O4 nanocomposites for targeted delivery. Mater. Today Commun. 2020, 24, 101247. [Google Scholar] [CrossRef]
- Korolkov, I.; Ludzik, K.; Kozlovskiy, A.; Fadeev, M.; Shumskaya, A.; Gorin, Y.; Jazdzewska, M.; Anisovich, M.; Rusakov, V.; Zdorovets, M. Immobilization of carboranes on Fe3O4-polymer nanocomposites for potential application in boron neutron cancer therapy. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 601, 125035. [Google Scholar] [CrossRef]
- Mashlan, M.; Zboril, R.; Machala, L.; Vujtek, M.; Walla, J.; Nomura, K. Mössbauer spectroscopy in study of thermally induced crystallization of amorphous Fe2O3 nanoparticles. J. Metastable Nanocryst. Mater. 2004, 20–21, 641–647. [Google Scholar]
- Ristić, M.; De Grave, E.; Musić, S.; Popović, S.; Orehovec, Z. Transformation of low crystalline ferrihydrite to α-Fe2O3 in the solid state. J. Mol. Struct. 2006, 834–836, 454–460. [Google Scholar] [CrossRef]
- Paesano, A.; Zanatta, S.C.; De Medeiros, S.N.; Cótica, L.F.; Da Cunha, J.B.M. Mechanosynthesis of YIG and GdIG: A Structural and Mössbauer Study. Hyperfine Interact. 2005, 161, 211–220. [Google Scholar] [CrossRef]
- Silva, C.L.S.; Marchetti, S.G.; Faro, A.C.; Silva, T.F.; Assaf, J.M.; Rangel, M.C. Effect of gadolinium on the catalytic properties of iron oxides for WGSR. Catal. Today 2013, 213, 127–134. [Google Scholar] [CrossRef][Green Version]
- Kasenov, B.K.; Kasenova, S.B.; Sagintaeva, Z.I.; Nukhuly, A.; Turtubaeva, M.O.; Bekturganov, Z.S.; Issabaeva, M.A. Synthesis and X-ray investigation of novel nanostructured copper-zinc manganites of lanthanum and alkali metals. Eurasian Phys. Tech. J. 2021, 18, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Musić, S.; Popović, S.; Ristic, M.; Sepiol, B. Chemical and structural properties of the system Fe2O3-Nd2O3. J. Mater. Sci. 1994, 29, 1714–1718. [Google Scholar] [CrossRef]
- Lazurova, J.; Mihalik, M.; Mihalik, M.; Vavra, M.; Zentkova, M.; Briancin, J.; Perovic., M.; Kusigerski, V.; Schneeweiss, O.; Roupcova, P.; et al. Magnetic Properties and Mössbauer spectroscopy of NdFe1−xMnxO3. J. Phys. Conf. Ser. 2015, 592, 012117. [Google Scholar] [CrossRef]
- Scrimshire, A.; Lobera, A.; Bell, A.M.T.; Jones, A.H.; Sterianou, I.; Forder, S.D.; Bingham, P.A. Determination of Debye temperatures and Lamb–Mössbauer factors for LnFeO3 orthoferrite perovskites (Ln = La, Nd, Sm, Eu, Gd). J. Phys. Condens. Matter 2018, 30, 105704. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Young Lee, J.; Yoon, J. Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance. Nanomaterials 2020, 10, 991. [Google Scholar] [CrossRef]
- Darwish, M.S.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef]
- Nikam, D.; Jadhav, S.V.; Khot, V.; Phadatare, M.; Pawar, S. Study of AC magnetic heating characteristics of Co0.5Zn0.5Fe2O4 nanoparticles for magnetic hyperthermia therapy. J. Magn. Magn. Mater. 2013, 349, 208–213. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Fernández, B.L.C.; Delgado, Á.V.; Jimenez-Lopez, C. Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles. Pharmaceutics 2019, 11, 273. [Google Scholar] [CrossRef]
- Hirosawa, F.; Iwasaki, T.; Watano, S. Synthesis and magnetic induction heating properties of Gd-substituted Mg–Zn ferrite nanoparticles. Appl. Nanosci. 2017, 7, 209–214. [Google Scholar] [CrossRef]
- Fotukian, S.M.; Barati, A.; Soleymani, M.; Alizadeh, A.M. Solvothermal synthesis of CuFe2O4 and Fe3O4 nanoparticles with high heating efficiency for magnetic hyperthermia application. J. Alloys Compd. 2020, 816, 152548. [Google Scholar] [CrossRef]

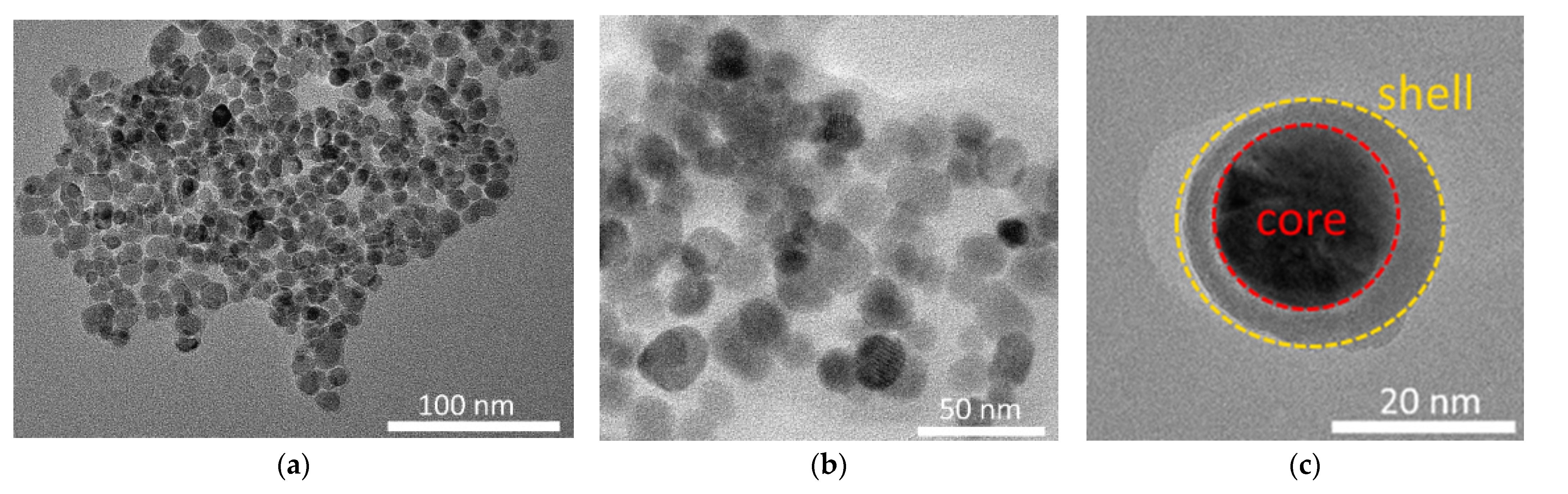


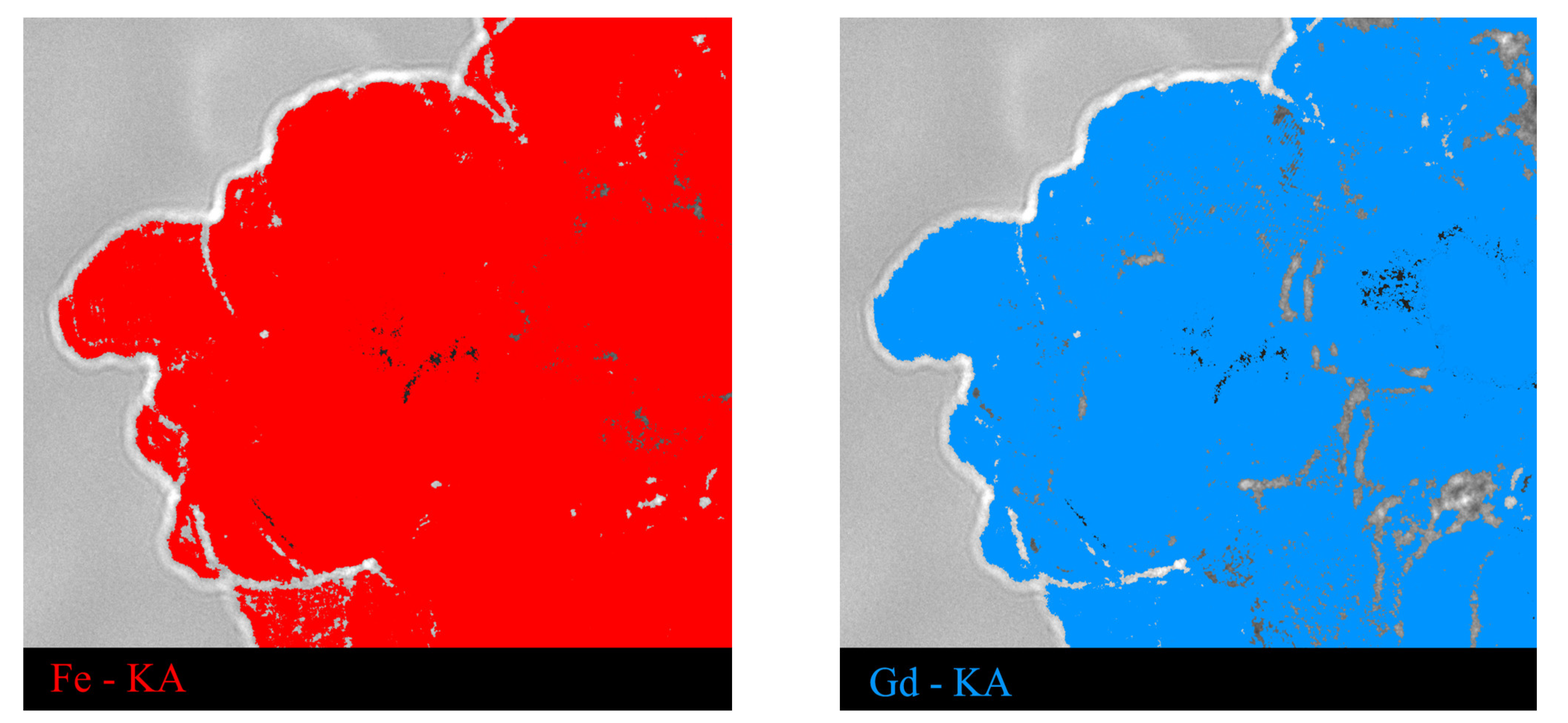


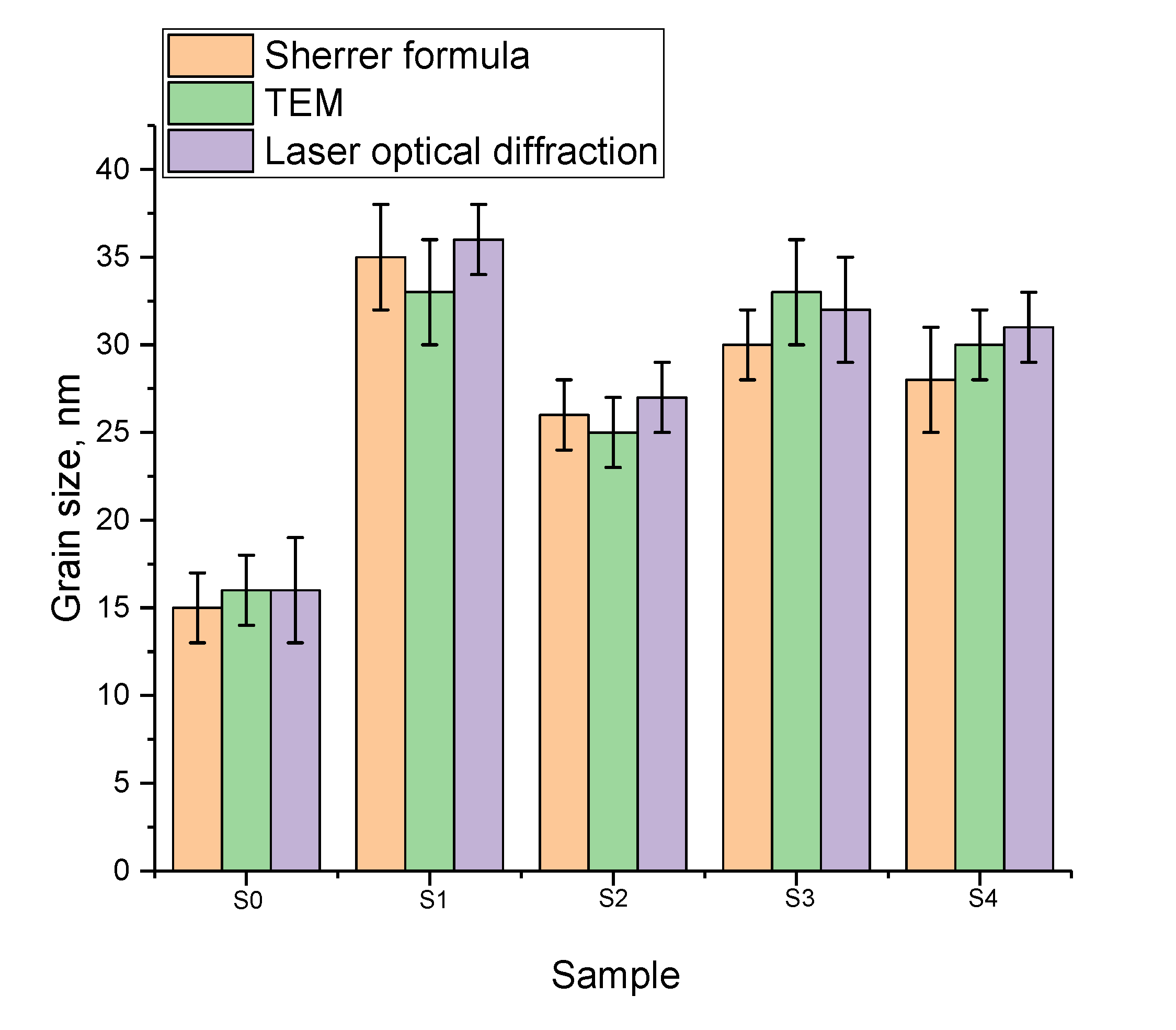




| Sample | Atom State | , % | , mm/s | , mm/s | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | Fe3O4 (initial) | 36.7 ± 0.2 | 0.26 ± 0.02 | 0.02 ± 0.02 | 476.8 ± 1.0 | 6.39 ± 0.12 | 0.165 ± 0.005 | 0.49 ± 0.02 | 11.4 ± 0.8 | 16.4 ± 0.4 | |
| 28.4 ± 0.1 | 0.39 ± 0.02 | −0.02 ± 0.03 | 480.6 ± 1.4 | ||||||||
| 34.9 ± 0.2 | 0.66 ± 0.01 | −0.01 ± 0.01 | 447.9 ± 0.7 | ||||||||
| S1 | Fe3O4 (600 °C) | 3.0 ± 0.2 | 0.19-fix | 0.01-fix | 499-fix | 7.75-fix | 0.314 ± 0.003 | 0.94 ± 0.01 | 5.0 ± 0.9 | 23.0 ± 1.5 | |
| 4.7 ± 0.2 | 0.43-fix | −0.04-fix | 495-fix | ||||||||
| 0.00 ± 0.01 | 0.66-fix | −0.01-fix | 448-fix | ||||||||
| 92.3 ± 0.2 | 0.372 ± 0.002 | −0.108 ± 0.002 | 513.9 ± 0.2 | - | - | - | - | - | |||
| S2 | Fe2O3@Au (600 °C) | 3.1 ± 0.6 | 0.24-fix | 0.03-fix | 488-fix | 6.90-fix | 0.332 ± 0.009 | 1.00 ± 0.26 | 5.0 ± 0.8 | 22.1 ± 1.2 | |
| 4.9 ± 0.2 | 0.37-fix | 0.00-fix | 489-fix | ||||||||
| 0.00 ± 0.01 | 0.66-fix | −0.01-fix | 448-fix | ||||||||
| 92.0 ± 0.6 | 0.372 ± 0.002 | −0.106 ± 0.002 | 513.3 ± 0.2 | - | - | - | - | - | |||
| S3 | Fe3O4/ Gd(NO3)3·6H2O (600 °C) | 32.7 ± 1.3 | 0.28 ± 0.04 | 0.16 ± 0.04 | 486.9 ± 2.9 | 2.08 ± 0.12 | 0.333 ± 0.012 | 1.00 ± 0.05 | 5.0 ± 0.6 | 14.8 ± 0.6 | |
| 51.3 ± 0.7 | 0.36 ± 0.07 | −0.08 ± 0.07 | 482.7 ± 4.0 | ||||||||
| 0.00 ± 0.02 | 0.66-fix | −0.01-fix | 448-fix | ||||||||
| 1.4 ± 1.8 | 0.36-fix | −0.04-fix | 494-fix | - | - | - | - | - | |||
| doublet | 14.6 ± 0.8 | 0.29 ± 0.02 | 0.55 ± 0.02 | - | - | - | - | - | - | ||
| S4 | Fe2O3/Nd2O3 (600 °C) | 6.8 ± 0.4 | 0.28-fix | 0.06 ± 0.03 | 487-fix | 3.61 ± 0.22 | 0.333 ± 0.025 | 1.00 ± 0.08 | 5.0 ± 0.9 | 17.8 ± 1.0 | |
| 10.6 ± 0.1 | 0.36-fix | −0.10 ± 0.02 | 483-fix | ||||||||
| 0.00 ± 0.01 | 0.66-fix | −0.01-fix | 448-fix | ||||||||
| 58.7 ± 1.9 | 0.373 ± 0.002 | −0.097 ± 0.002 | 515.1 ± 0.2 | - | - | - | - | - | |||
| 24.0 ± 2.2 | 0.361 ± 0.005 | −0.052 ± 0.005 | 499.5 ± 0.8 | - | - | - | - | - | |||
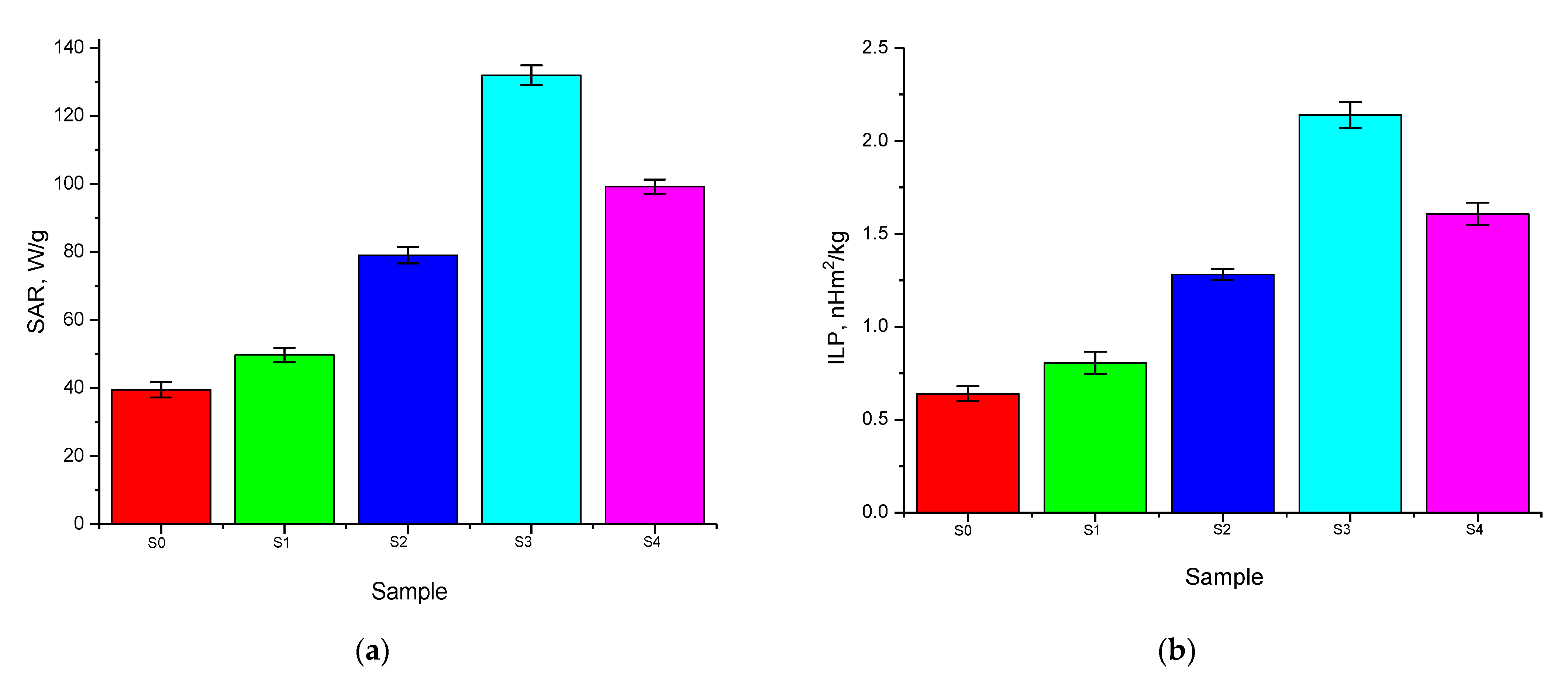
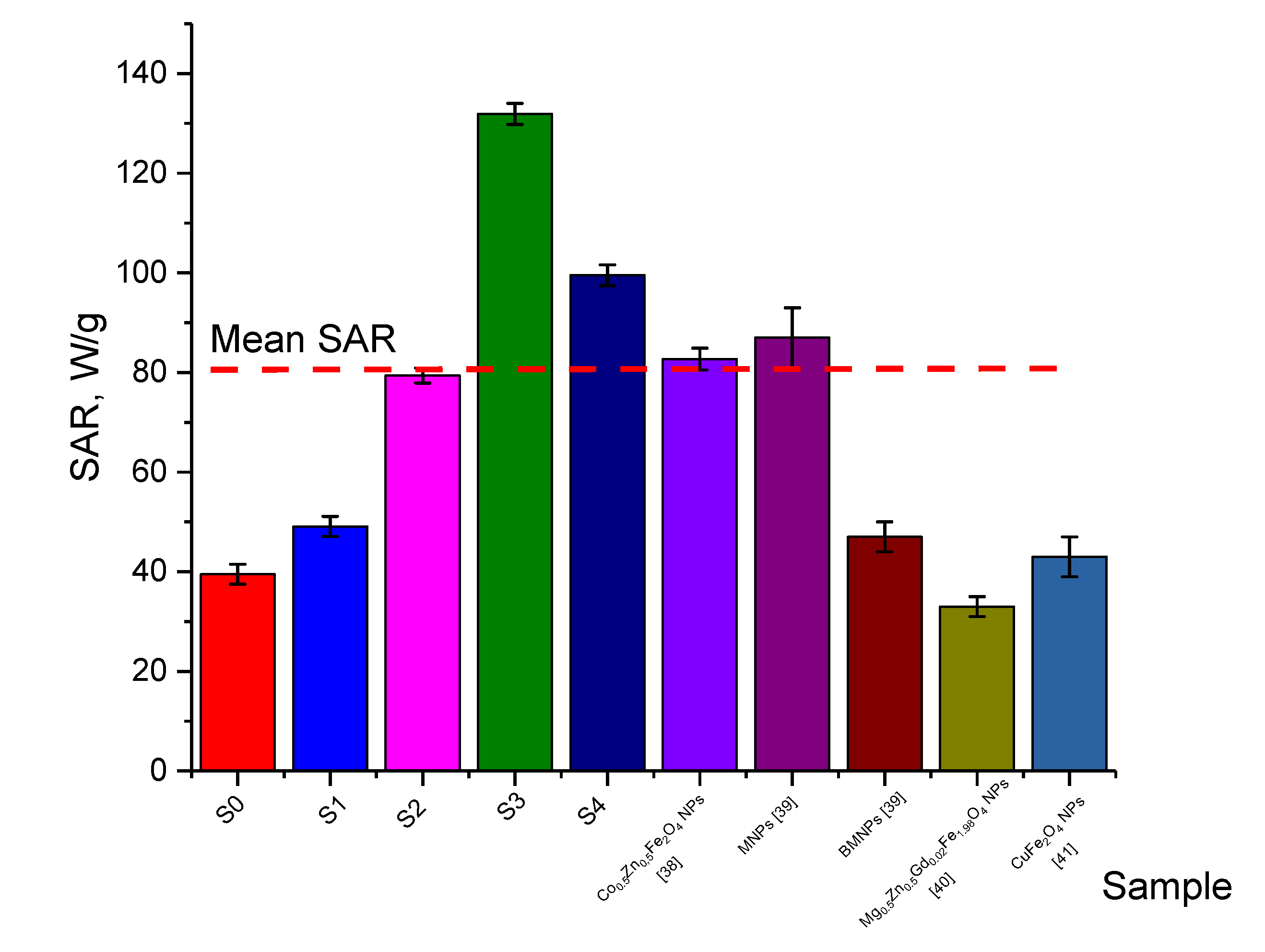
| Sample | Amplitude of the Magnetic Field | The Frequency, kHz | SAR, W/g | ILP, nHm2/kg | Ref. |
|---|---|---|---|---|---|
| S0 | 210 Oe | 320 | 39 ± 2 | 0.6 ± 0.1 | Present work |
| S1 | 49 ± 2 | 0.8 ± 0.2 | |||
| S2 | 79 ± 2 | 1.3 ± 0.1 | |||
| S3 | 132 ± 5 | 2.1 ± 0.3 | |||
| S4 | 99 ± 3 | 1.6 ± 0.2 | |||
| Co0.5 Zn0.5Fe2O4 NPs | 167.5–335.2 Oe | 197–280 | 83 ± 2 | 0.9–1.1 | [38] |
| MNPs | 167-335 Oe | 265 | 87 ± 3 | 0.96–1.04 | [39] |
| BMNPs | 47 ± 4 | 0.50–0.52 | [39] | ||
| Mg0.5Zn0.5Gd0.02Fe1.98O4 NPs | 5 kA/m | 600 | 33 ± 3 | 0.2–0.3 | [40] |
| CuFe2O4 NPs | 13–19 kA/m | 120 | 43 ± 2 | 1.04–1.23 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarova, A.; Kozlovskiy, A.L.; Rusakov, V.S.; Egizbek, K.B.; Fadeev, M.S.; Prmantayeva, B.A.; Chudoba, D.; Zdorovets, M.V.; Kadyrzhanov, K.K. Study of the Applicability of Magnetic Iron-Containing Nanoparticles in Hyperthermia and Determination of Their Resistance to Degradation Processes. Crystals 2022, 12, 1816. https://doi.org/10.3390/cryst12121816
Nazarova A, Kozlovskiy AL, Rusakov VS, Egizbek KB, Fadeev MS, Prmantayeva BA, Chudoba D, Zdorovets MV, Kadyrzhanov KK. Study of the Applicability of Magnetic Iron-Containing Nanoparticles in Hyperthermia and Determination of Their Resistance to Degradation Processes. Crystals. 2022; 12(12):1816. https://doi.org/10.3390/cryst12121816
Chicago/Turabian StyleNazarova, Assel, Artem L. Kozlovskiy, Vyacheslav S. Rusakov, Kamila B. Egizbek, Maxim S. Fadeev, Bekzat A. Prmantayeva, Dorota Chudoba, Maxim V. Zdorovets, and Kayrat K. Kadyrzhanov. 2022. "Study of the Applicability of Magnetic Iron-Containing Nanoparticles in Hyperthermia and Determination of Their Resistance to Degradation Processes" Crystals 12, no. 12: 1816. https://doi.org/10.3390/cryst12121816
APA StyleNazarova, A., Kozlovskiy, A. L., Rusakov, V. S., Egizbek, K. B., Fadeev, M. S., Prmantayeva, B. A., Chudoba, D., Zdorovets, M. V., & Kadyrzhanov, K. K. (2022). Study of the Applicability of Magnetic Iron-Containing Nanoparticles in Hyperthermia and Determination of Their Resistance to Degradation Processes. Crystals, 12(12), 1816. https://doi.org/10.3390/cryst12121816









