Influence of Phase Composition on Stress-Corrosion Cracking of Ti-6Mo-5V-3Al-2Fe-2Zr Alloy in 3.5% NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Phase Composition and Microstructure
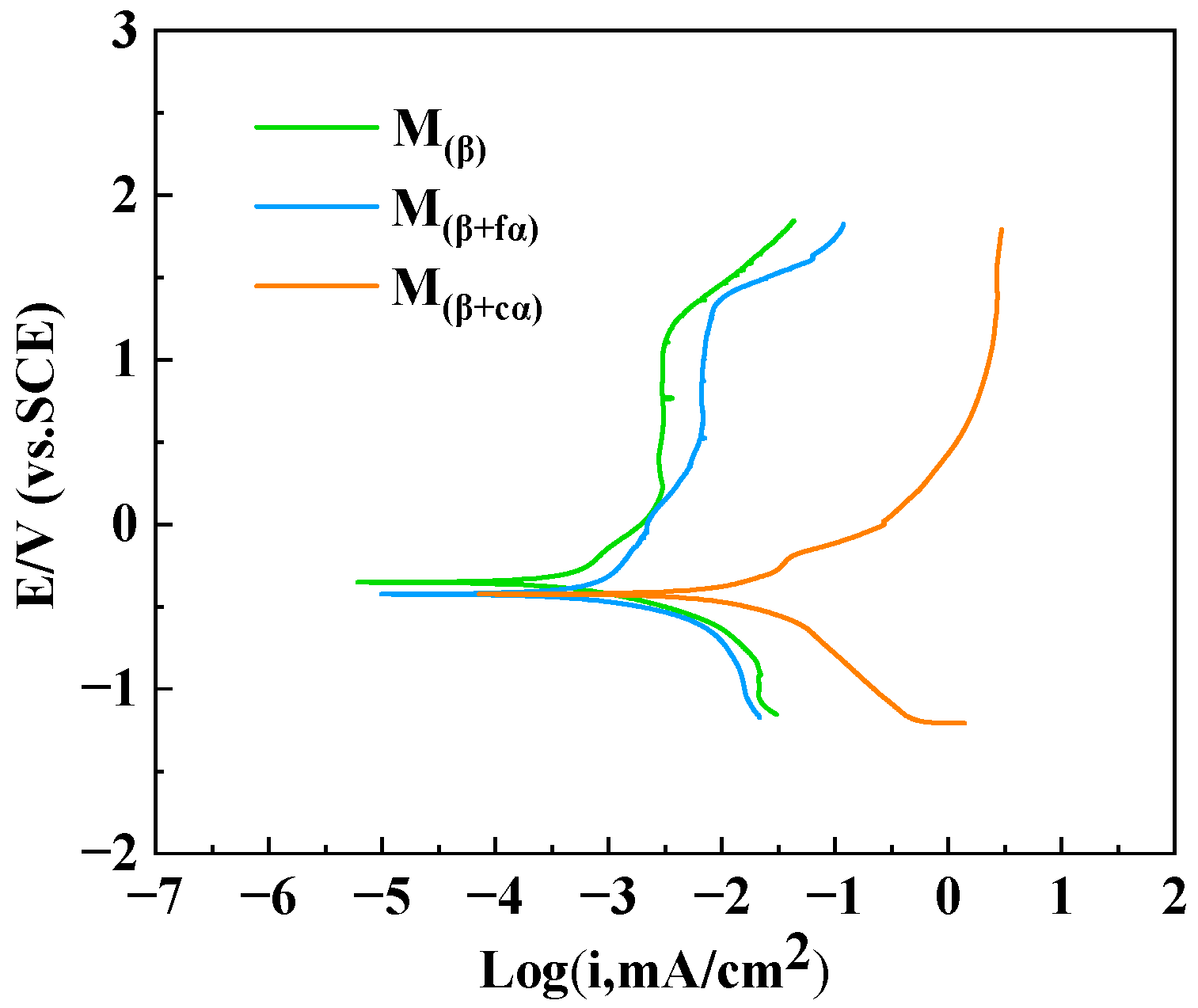
3.2. Polarization Curves and EIS Analysis
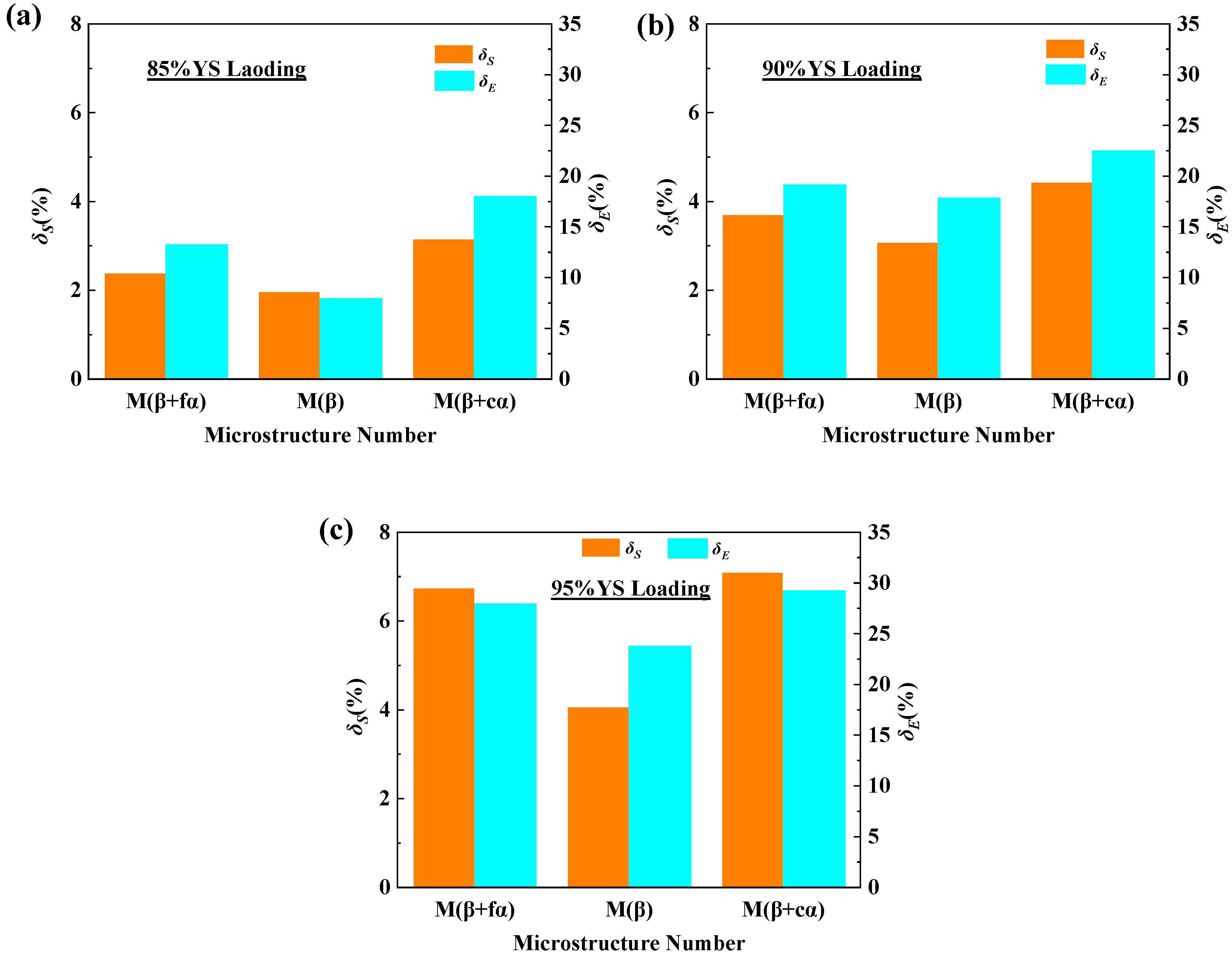
3.3. Constant-Stress Loading Test Results
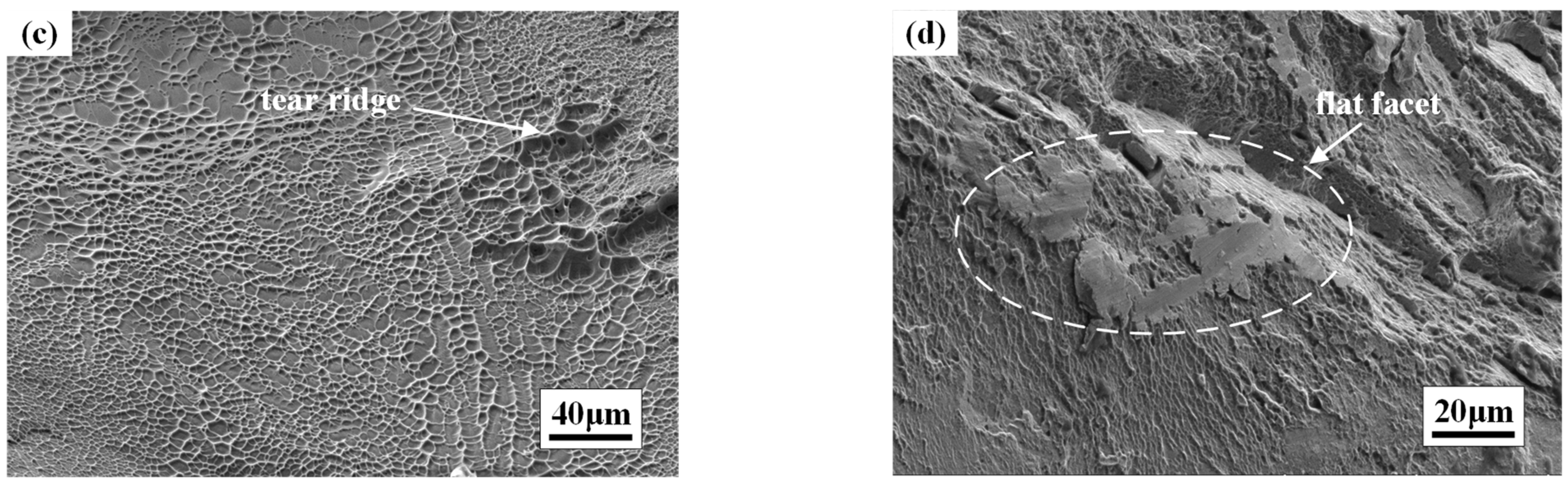
3.4. Fracture Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, S.K.; Song, G.L.; Li, Z.X.; Wang, H.; Zheng, D.; Cao, F.; Horynova, M.; Dargusch, M.S.; Zhou, L. A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Wake, H.; Takahashi, H.; Takimoto, T.; Takayanagi, H.; Ozawa, K.; Kadoi, H.; Okochi, M.; Matsunaga, T. Development of an electrochemical antifouling system for seawater cooling pipelines of power plants using titanium. Biotechnol. Bioeng. 2006, 95, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Deng, L.; Tian, W.H.; Che, L.Z.; Li, Y. Deduction of a quadratic velocity field and its application to rolling force of extra-thick plate. Comput. Math. Appl. 2022, 109, 58–73. [Google Scholar] [CrossRef]
- Yang, R.; Ma, Y.J.; Lei, J.F.; Hu, Q.; Huang, S. Toughening High Strength Titanium Alloys Through Fine Tuning Phase Composition and Refining Microstructure. Acta Metall. Sin. 2021, 57, 1455–1470. [Google Scholar] [CrossRef]
- Prakash Kolli, R.; Devaraj, A. A review of metastable beta titanium alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.Y.; Sun, Z.J.; Yang, S.L.; Jiang, P.; Liao, Z. Stress corrosion characteristics of electron beam welded titanium alloys joints in NaCl solution. Mater. Charact. 2022, 192, 112126. [Google Scholar] [CrossRef]
- Dong, Y.C.; Huang, S.; Wang, Y.Y.; Zhang, B.; Alexandrov, I.; Chang, H.; Dan, Z.; Ma, L.; Zhou, L. Stress corrosion cracking of TC4 ELI alloy with different microstructure in 3.5% NaCl solution. Mater. Charact. 2022, 194, 112357. [Google Scholar] [CrossRef]
- Ahn, S.; Park, J.; Jeong, D.; Sung, H.; Kwon, Y.; Kim, S. Effect of Cooling Rate on SCC Susceptibility of β-Processed Ti-6Al-4V Alloy in 0.6M NaCl Solution. Met. Mater. Int. 2018, 24, 327–336. [Google Scholar] [CrossRef]
- Chi, G.F.; Jiang, B.; Yi, D.Q.; Yang, L.; Pan, S.; Liu, H.; Feng, C.; Mao, W. Effect of lamellar α on the stress corrosion cracking of Ti-6Al-4V alloy in simulated oilfield brine. Mater. Charact. 2021, 174, 111000. [Google Scholar] [CrossRef]
- Sun, Y.W.; Feng, T.; Wang, Y.Z.; Yang, Q.; Jiao, A.Z. Stress-corrosion cracking susceptibility of tungsten-inert-gas-welded TC4 titanium alloy joints under low strain rates. Sens. Mater. 2019, 2, 491–499. [Google Scholar] [CrossRef]
- Li, Y.; Pei, Z.B.; Zaman, B.; Zhang, Y.; Yuan, H.; Cao, B. Effects of plastic deformations on the electrochemical and stress corrosion cracking behaviors of TC2 titanium alloy in simulated seawater. Mater. Res. Express 2018, 5, 116516. [Google Scholar] [CrossRef]
- Junior, P.C.P.S.; Correa, E.O. Effect of Duplex Aging Heat Treatment on the Stress Corrosion Cracking Behavior of Ti-6Al-4V α+β Titanium Alloy in Methanol. Mater. Res. 2021, 24, e20200456. [Google Scholar] [CrossRef]
- Pazhanivel, B.; Sathiya, P.; Muthuraman, K.; Sozhan, G. Influence of NaCl environment on stress corrosion cracking of additive manufactured Ti-6Al-4V alloy. Eng. Fail. Anal. 2021, 127, 105515. [Google Scholar] [CrossRef]
- Ciszak, C.; Popa, I.; Brossard, J.M.; Monceau, D.; Chevalier, S. NaCl induced corrosion of Ti-6Al-4V alloy at high temperature. Corros. Sci. 2016, 110, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Atapour, M.; Pilchak, A.L.; Shamanian, M.; Fathi, M. Corrosion behavior of Ti-8Al-1Mo-1V alloy compared to Ti-6Al-4V. Mater. Des. 2011, 32, 1692–1696. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hao, M.Y.; Li, D.; Li, P.; Liang, Q.; Wang, D.; Zheng, Y.; Sun, Q.; Wang, Y. Enhanced mechanical properties of Ti-5Al-5Mo-5V-3Cr-1Zr by bimodal lamellar precipitate microstructures via two-step aging. Mater. Sci. Eng. A 2022, 829, 142117. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Kuroda, S.; Motohashi, M.; Hiroto, T.; Ishida, A.; Murakami, H.; Itsumi, Y. Correlation between solution treatment temperature, microStructure and yield strength of forged Ti-17 alloys. Crystals 2021, 11, 625. [Google Scholar] [CrossRef]
- Liu, M.T.; Tian, Y.; Wang, Y.; Wang, K.; Zhang, K.; Lu, S. Critical conditions for dynamic recrystallization of S280 ultra-high-strength stainless steel based on work hardening rate. Metals 2022, 12, 1123. [Google Scholar] [CrossRef]
- Zhu, W.G.; Tan, C.S.; Xiao, R.Y.; Sun, Q.; Sun, J. Slip behavior of Bi-modal structure in a metastable β titanium alloy during tensile deformation. J. Mater. Sci. Technol. 2020, 57, 188–196. [Google Scholar] [CrossRef]
- Ji, P.F.; Li, B.; Liu, S.G.; Zhang, X.; Chen, B.; Ma, M.; Liu, R. Controlling the corrosion behavior of Ti-Zr alloy by tuning the α/β phase volume fraction and morphology of β phase. J. Alloys Compd. 2020, 825, 154153. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, C.; Liu, D.; Zhou, G.; Zhang, S.; Chen, L. Effects of secondary α phase on strength and ductility of Ti-6Mo-5V-3Al-2Fe-2Zr alloy. Rare Met. Mater. Eng. 2022, 51, 2137–2143. Available online: http://www.rmme.ac.cn/rmme/ch/reader/view_abstract.aspx?file_no=20210471&flag=1 (accessed on 6 Deceember 2022).
- Zhou, B.; Yang, L.; Yang, S.B.; Bai, D.; Olugbade, O.; Huang, G. Stress corrosion behavior of 6082 aluminum alloy. Mater. Corros. 2020, 71, 1194–1205. [Google Scholar] [CrossRef]
- Dos Santos, E.A.; Giorgetti, V.; Júnior, C.A.; Marcomini, J.B.; Sordi, V.L.; Rovere, C.A. Stress corrosion cracking and corrosion fatigue analysis of API X70 steel exposed to a circulating ethanol environment. Int. J. Press. Vessel. Pip. 2022, 200, 104846. [Google Scholar] [CrossRef]
- Nishimura, R.; Shirono, J.; Jonokuchi, A. Hydrogen-induced cracking of pure titanium in sulphuric acid and hydrochloric acid solutions using constant load method. Corros. Sci. 2008, 50, 2691–2697. [Google Scholar] [CrossRef]
- Shih, D.S.; Robertson, I.M.; Birnbaum, H.K. Hydrogen embrittlement of α titanium: In situ TEM studies. Acta Mater. 1988, 36, 111–124. [Google Scholar] [CrossRef]
- Cao, S.; Samuel Lim, C.V.; Hinton, B.; Wu, X. Effects of microtexture and Ti3Al (α2) precipitates on stress-corrosion cracking properties of a Ti-8Al-1Mo-1V alloy. Corros. Sci. 2017, 116, 22–33. [Google Scholar] [CrossRef]
- Yu, S. Corrosion resistance of titanium alloys. In ASM Handbook; Cramer, S.D., Covino, B.S., Eds.; ASM International: Geauga, OH, USA, 2003. [Google Scholar]
- Liu, R.; Cui, Y.; Liu, L.; Zhang, B.; Wang, F. A primary study of the effect of hydrostatic pressure on stress corrosion cracking of Ti-6Al-4V alloy in 3.5% NaCl solution. Corros. Sci. 2020, 165, 108402. [Google Scholar] [CrossRef]
- Lynch, S.P. Environmentally assisted cracking: Overview of evidence for an adsorption-induced localised-slip process. Acta Metall. 1988, 36, 2639–2661. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, S.M.; Lim, C.V.S.; Zhou, X.; Chen, X.; Hinton, B.R.; Boyer, R.R.; Williams, J.C.; Wu, X. The mechanism of aqueous stress-corrosion cracking of α + β titanium alloys. Corros. Sci. 2017, 125, 29–39. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, C.; Zhou, G.; Zhang, S.; Chen, L. Dependence of strength and ductility on secondary α phase in a novel metastable-β titanium alloy. J. Mater. Res. Technol. 2022, 18, 25257–25266. [Google Scholar] [CrossRef]
- Leon, A.; Levy, G.K.; Ron, T.; Shirizly, A.; Aghion, E. The effect of strain rate on stress corrosion performance of Ti6Al4V alloy produced by additive manufacturing process. J. Mater. Res. Technol. 2020, 9, 4097-4015. [Google Scholar] [CrossRef]
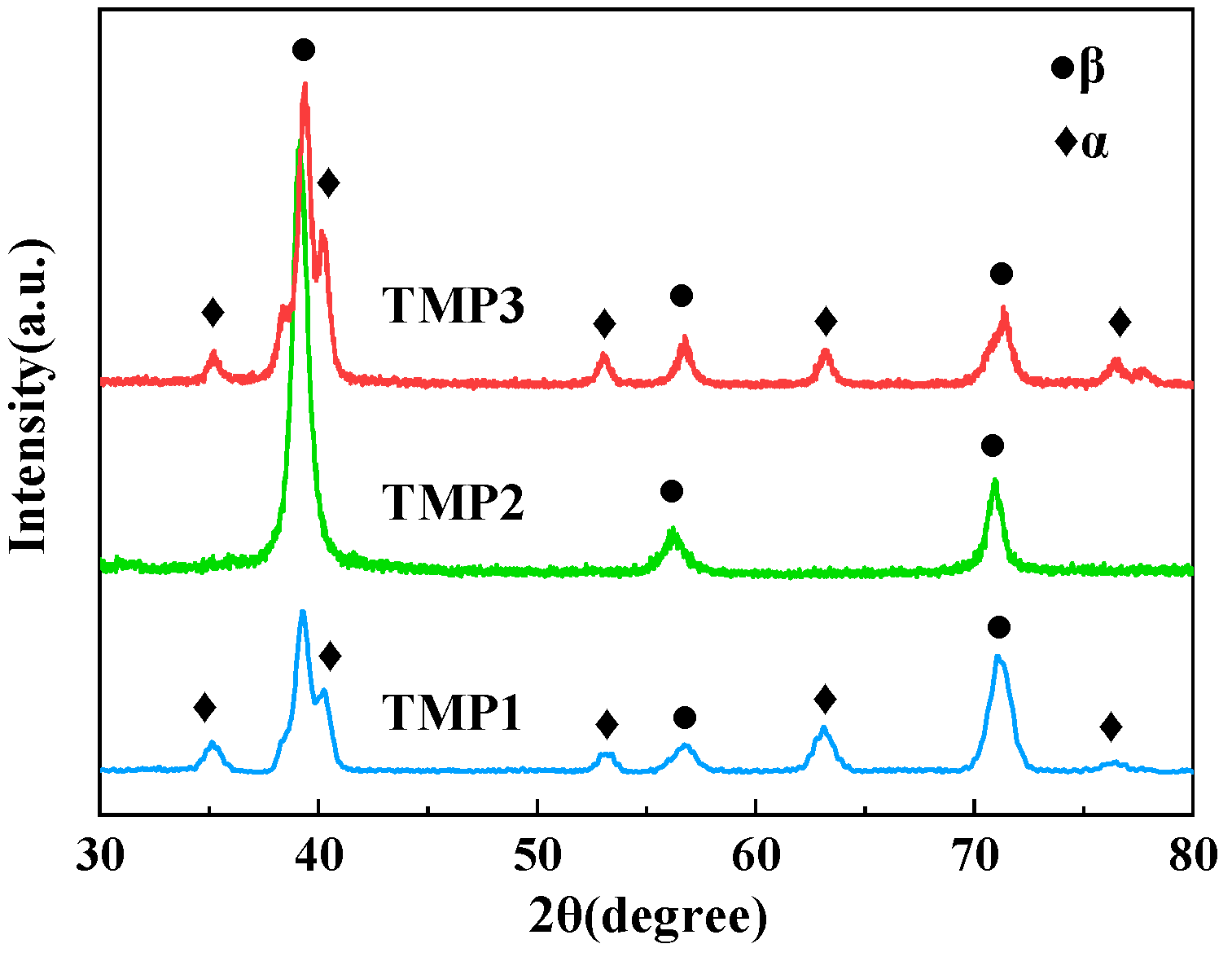



| TMP Number | Thermal Mechanical Processing | Phase Composition | Microstructure Number |
|---|---|---|---|
| TMP1 | Forged in α + β phase zone | β phase and fine α phase | M(β+fα) |
| TMP2 | Forged and solution-treated in β phase zone | Single β phase | M(β) |
| TMP3 | Forged in α + β phase zone and isothermal-treated at high temperature of 680 °C for 8 h | β phase and coarsened α phase | M(β+cα) |
| Microstructure Number | Ecorr (mV) | Icorr (μA/cm2) |
|---|---|---|
| M(β+fα) | −391 | 0.727 |
| M(β) | −352 | 0.359 |
| M(β+cα) | −447 | 1.015 |
| Microstructure Number | Constant Stress | UTS (MPa) | St. dev (MPa) | EL (%) | St. dev (%) |
|---|---|---|---|---|---|
| M(β+fα) | / | 1215 | 8.2 | 6.8 | 0.2 |
| 85% YS | 1186 | 8.3 | 5.9 | 0.2 | |
| 90% YS | 1170 | 7.7 | 5.5 | 0.3 | |
| 95% YS | 1133 | 7.2 | 4.9 | 0.2 | |
| M(β) | / | 812 | 6.1 | 10.1 | 0.8 |
| 85% YS | 796 | 5.8 | 9.3 | 0.6 | |
| 90% YS | 787 | 5.6 | 8.3 | 0.4 | |
| 95% YS | 779 | 5.7 | 7.7 | 0.3 | |
| M(β+cα) | / | 1014 | 7.8 | 8.9 | 0.6 |
| 85% YS | 982 | 7.4 | 7.3 | 0.2 | |
| 90% YS | 969 | 6.8 | 6.9 | 0.3 | |
| 95% YS | 942 | 6.6 | 6.3 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, J.; Zhou, G.; Yu, X.; Wang, C.; Gao, J. Influence of Phase Composition on Stress-Corrosion Cracking of Ti-6Mo-5V-3Al-2Fe-2Zr Alloy in 3.5% NaCl Solution. Crystals 2022, 12, 1794. https://doi.org/10.3390/cryst12121794
Zhang H, Sun J, Zhou G, Yu X, Wang C, Gao J. Influence of Phase Composition on Stress-Corrosion Cracking of Ti-6Mo-5V-3Al-2Fe-2Zr Alloy in 3.5% NaCl Solution. Crystals. 2022; 12(12):1794. https://doi.org/10.3390/cryst12121794
Chicago/Turabian StyleZhang, Haoyu, Jie Sun, Ge Zhou, Xiaoling Yu, Chuan Wang, and Jian Gao. 2022. "Influence of Phase Composition on Stress-Corrosion Cracking of Ti-6Mo-5V-3Al-2Fe-2Zr Alloy in 3.5% NaCl Solution" Crystals 12, no. 12: 1794. https://doi.org/10.3390/cryst12121794
APA StyleZhang, H., Sun, J., Zhou, G., Yu, X., Wang, C., & Gao, J. (2022). Influence of Phase Composition on Stress-Corrosion Cracking of Ti-6Mo-5V-3Al-2Fe-2Zr Alloy in 3.5% NaCl Solution. Crystals, 12(12), 1794. https://doi.org/10.3390/cryst12121794







