Rapid Growth of High-Quality Rutile TiO2 Single Crystals through a Laser Floating Zone Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
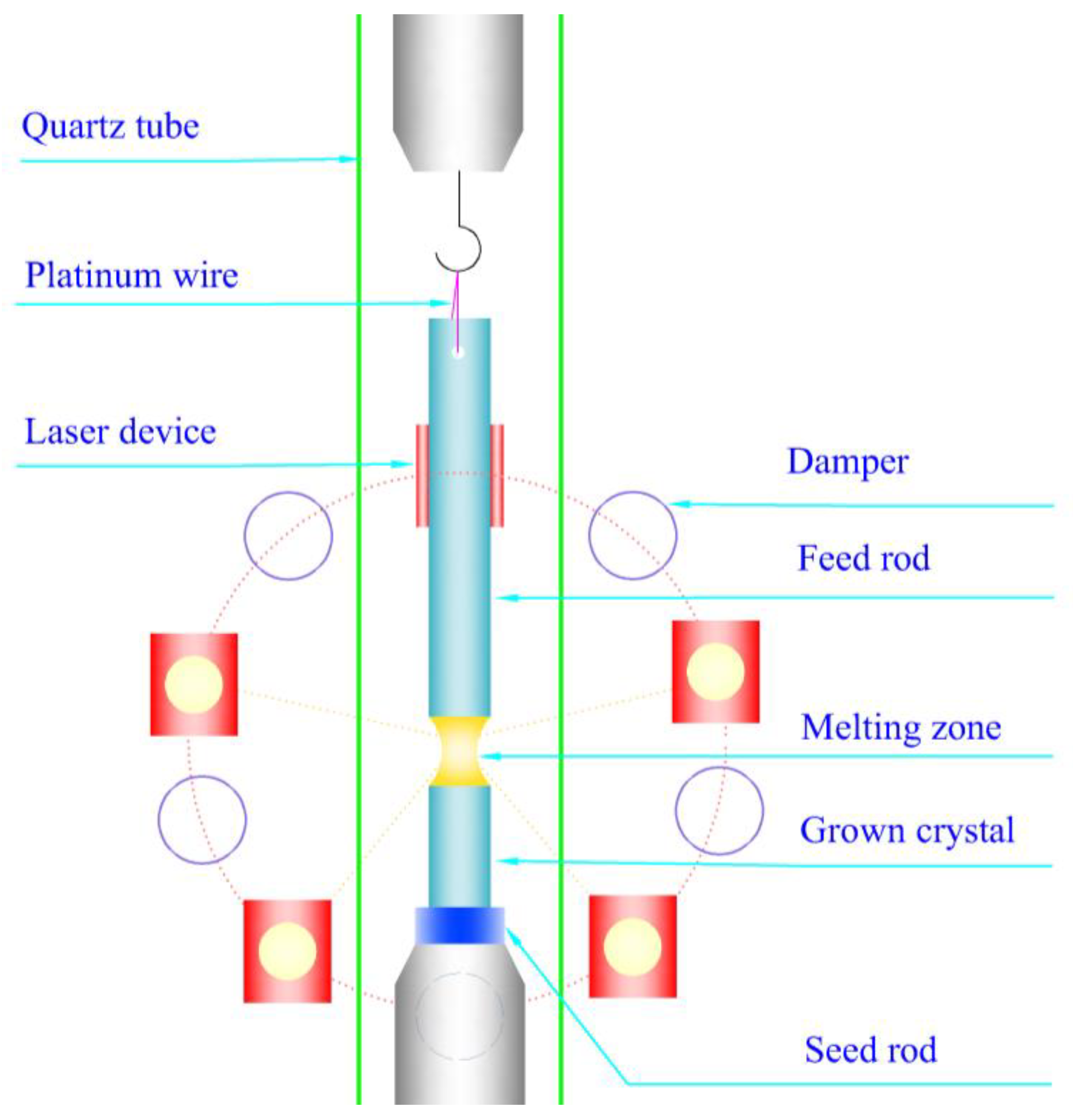
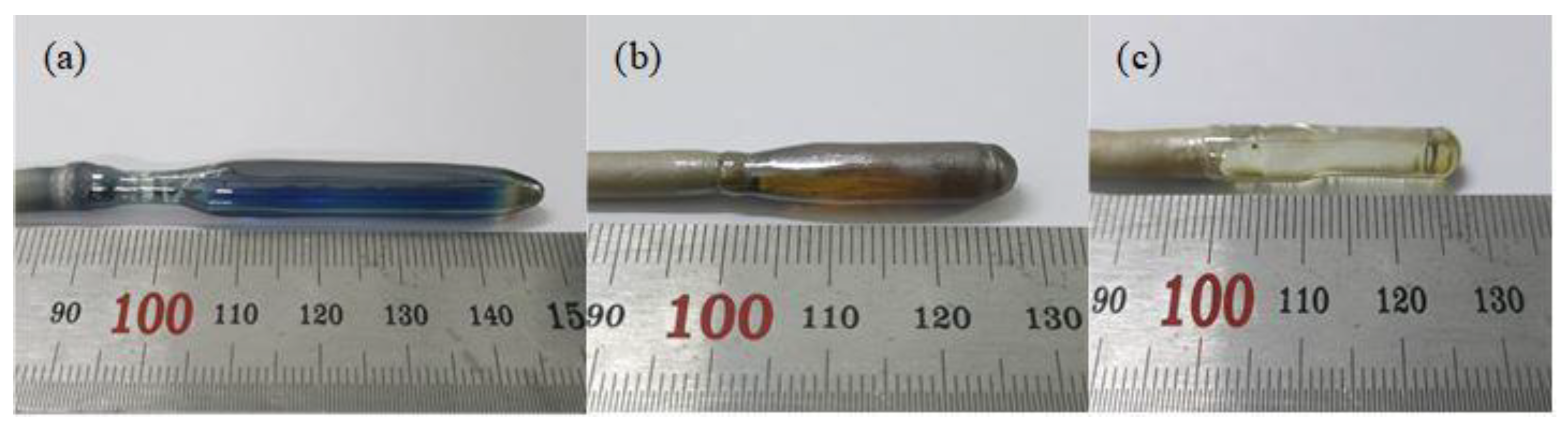
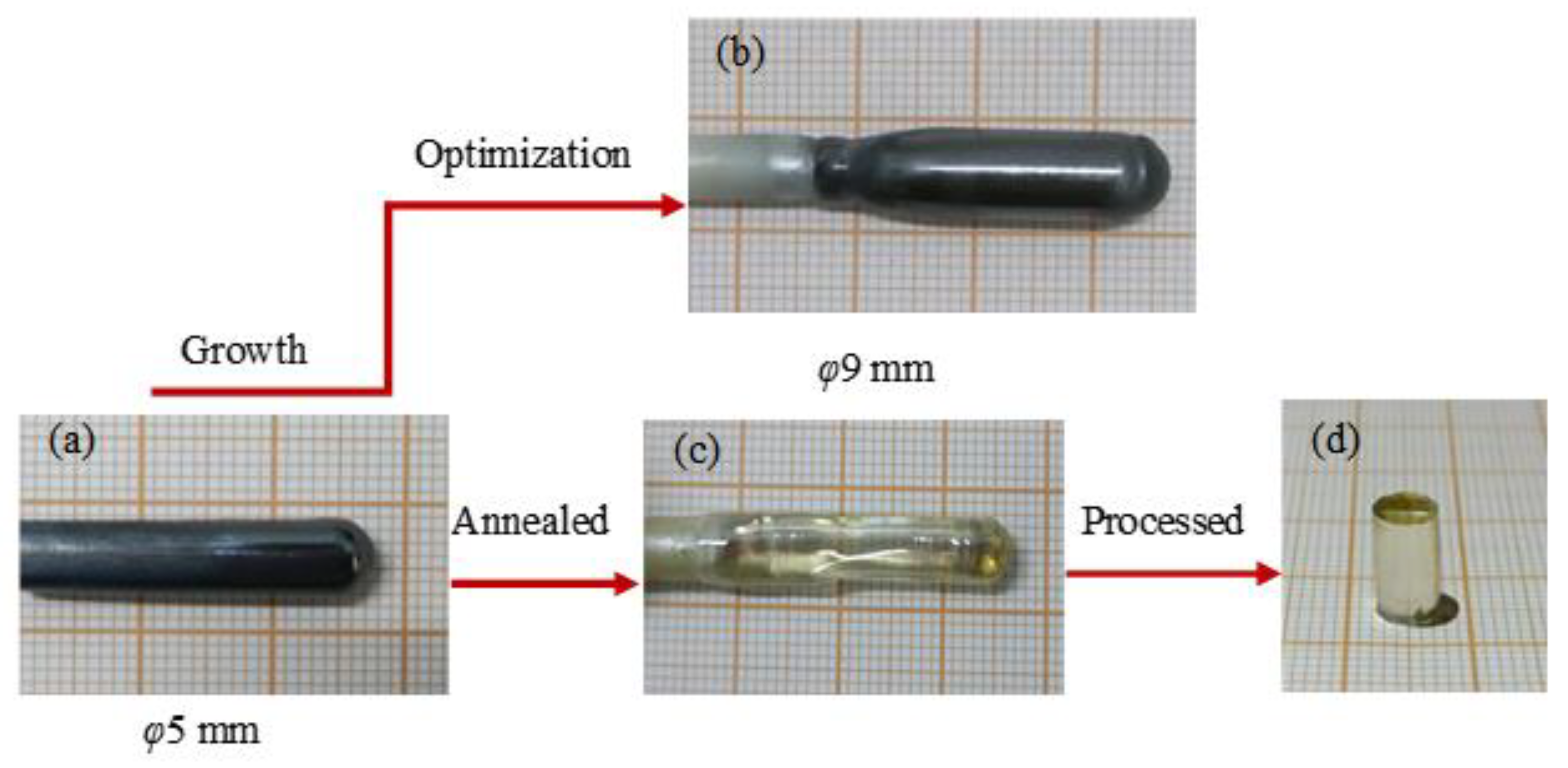
3.1. Crystal Growth
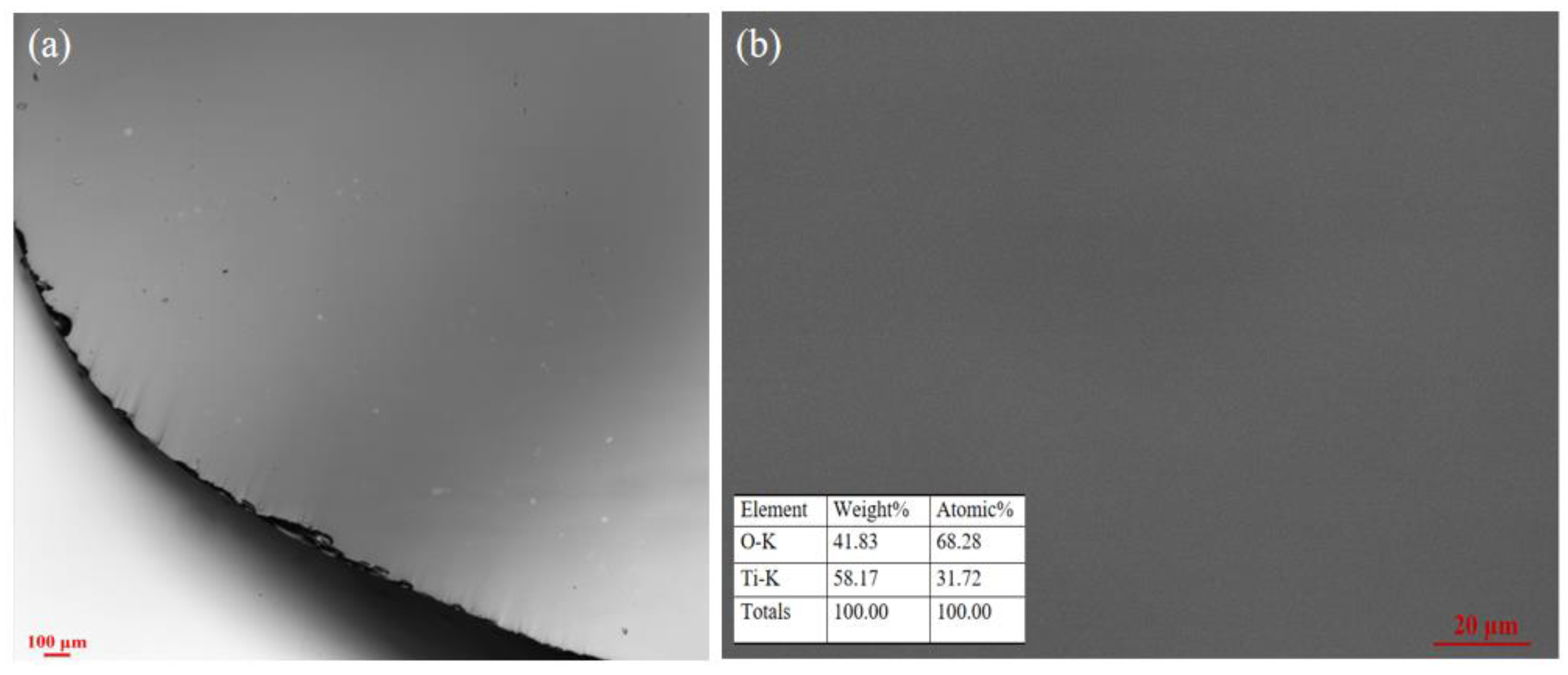
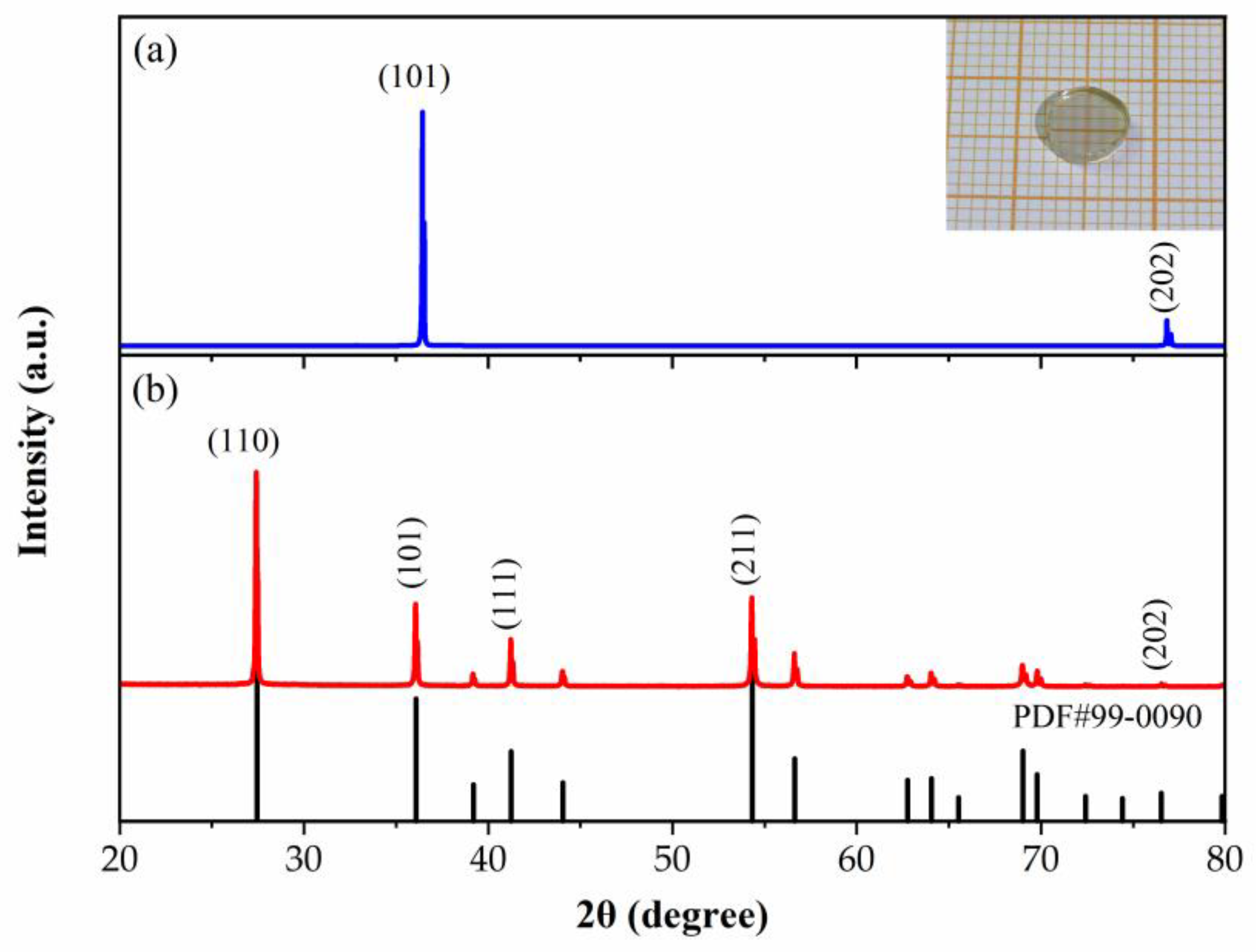
3.2. Crystal Structure
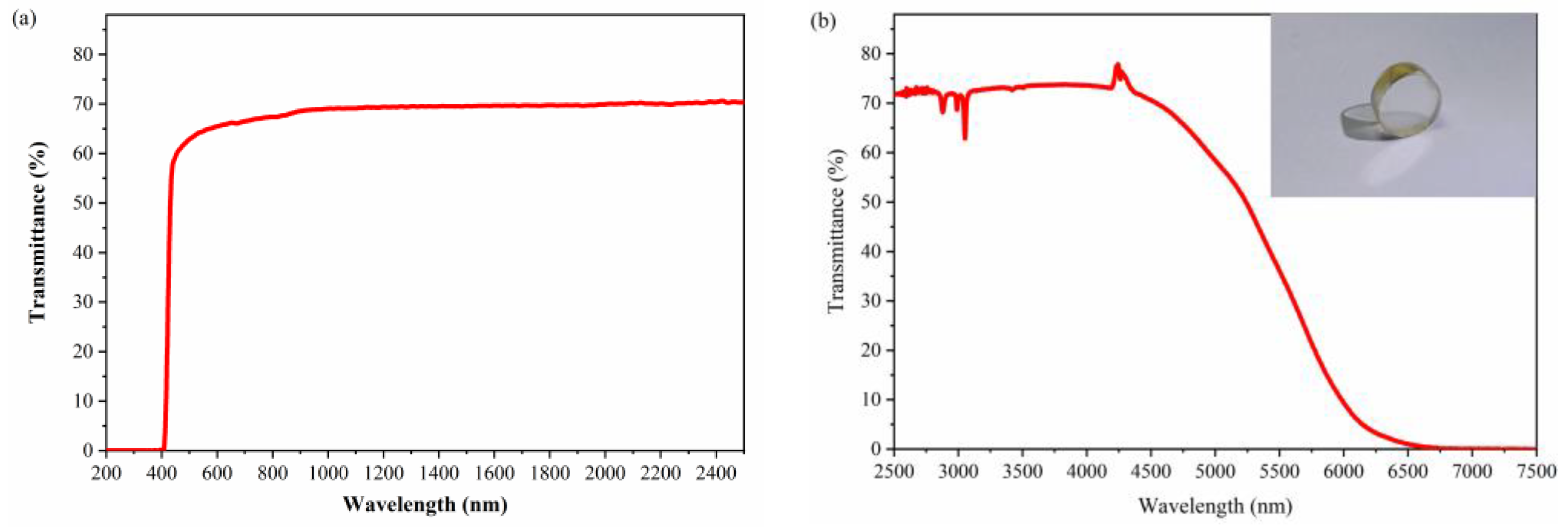
3.3. Optical Properties
3.3.1. Transmission Spectrum
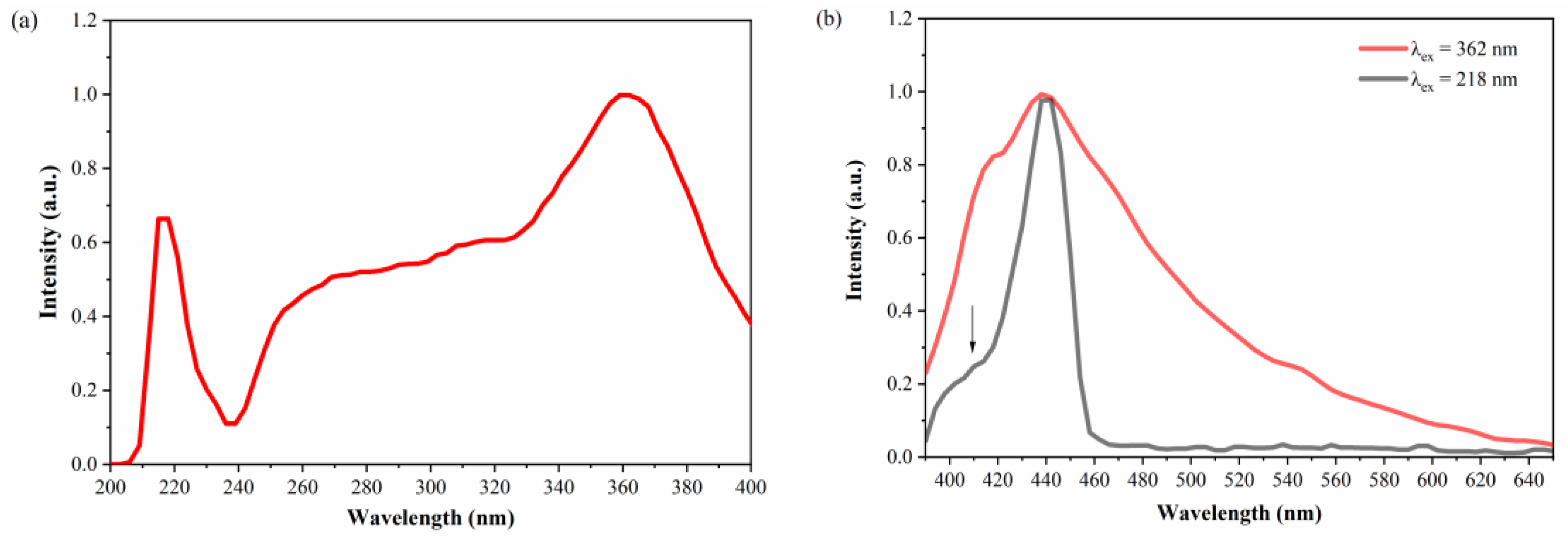
3.3.2. Fluorescence Spectra
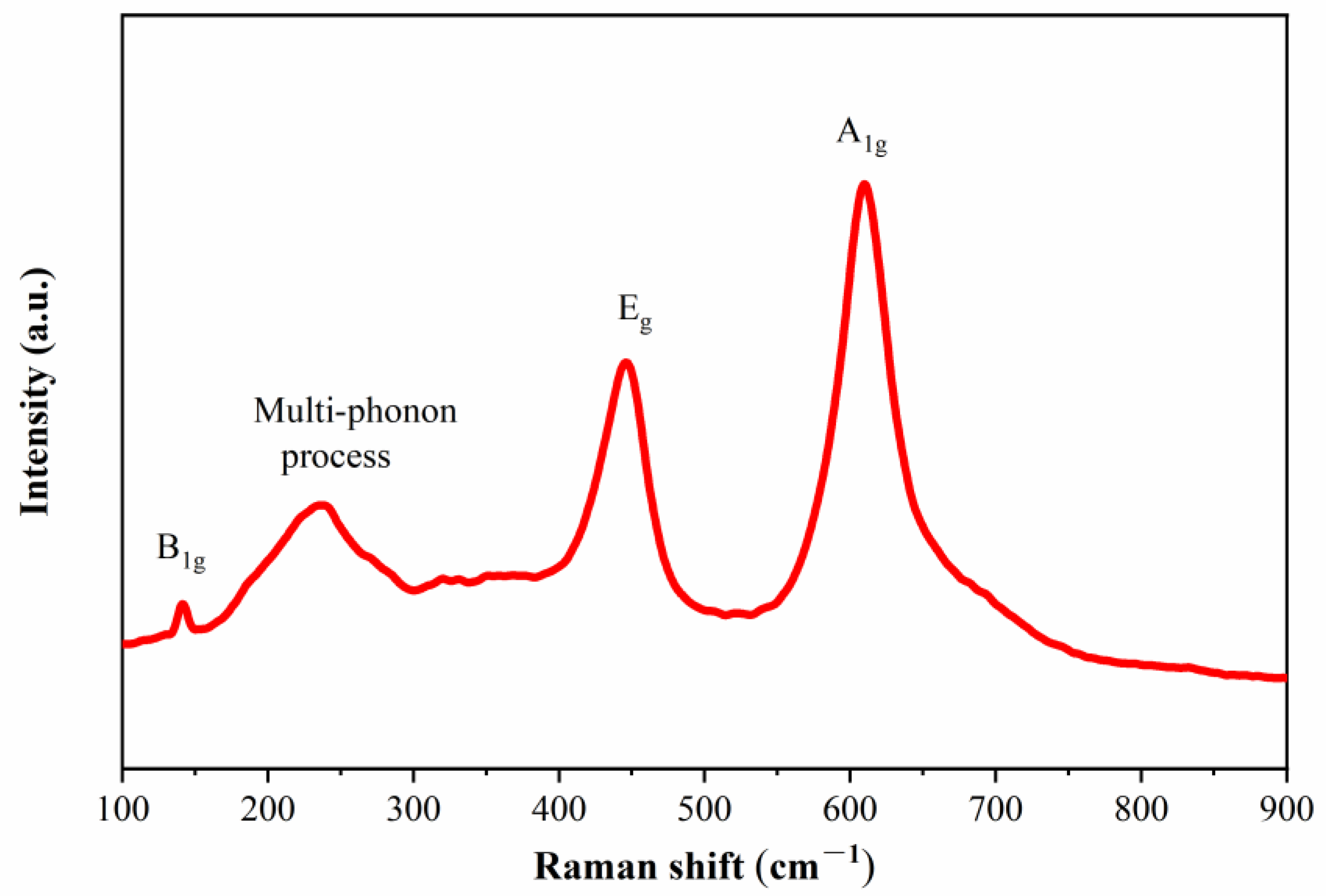
3.3.3. Raman Spectra
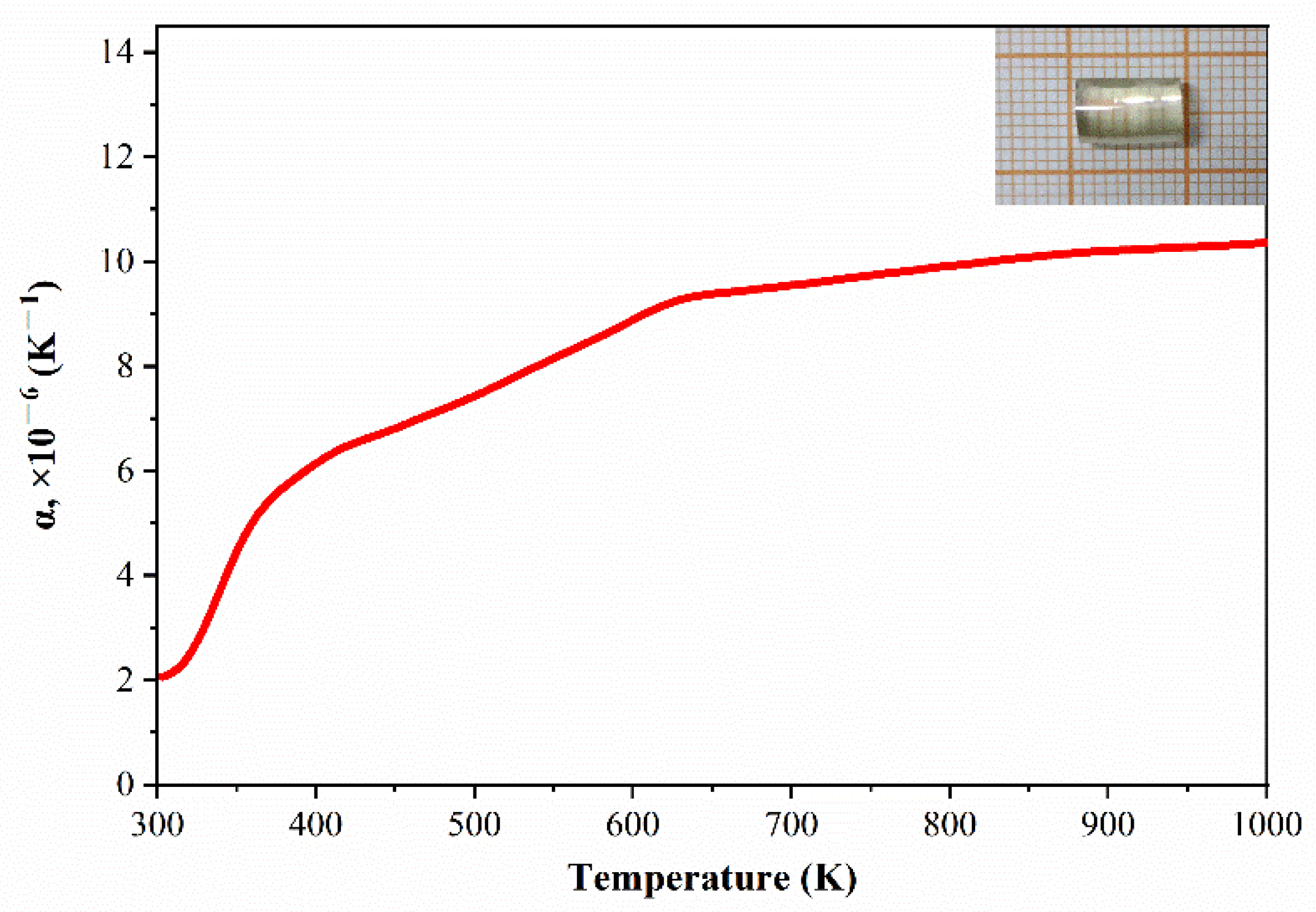
3.4. Thermal Properties
3.5. Laser Damage Threshold (LDT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Liu, X.D.; Bi, X.G.; Ma, Z.X.; Li, J.S.; Sun, X.D. Verneuil growth of TiO2 (rutile) crystals of large size and low dislocation density at low gas flow rate. Ceram. Int. 2022, 48, 30240–30248. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, K.H.; Tanaka, I.; Shim, K.B. Characteristics of rutile single crystals grown under two different oxygen partial pressures. J. Cryst. Growth 2004, 268, 103–107. [Google Scholar] [CrossRef]
- Higuchi, M.; Hatta, K.; Takahashi, J.; Kodaira, K.; Kaneda, H.; Saito, J. Floating-zone growth of rutile single crystals inclined at 48 degrees to the c-axis. J. Cryst. Growth 2000, 208, 501–507. [Google Scholar] [CrossRef]
- Ha, S.; Tang, Y.; van Oene, M.M.; Janissen, R.; Dries, R.M.; Solano, B.; Adam, A.J.L.; Dekker, N.H. Single-Crystal Rutile TiO2 Nanocylinders are Highly Effective Transducers of Optical Force and Torque. ACS Photonics 2019, 6, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, M.; Togi, T.; Kodaira, K. Growth of rutile single crystals by the pulling-down method. J. Cryst. Growth 1999, 203, 450–453. [Google Scholar] [CrossRef]
- Koohpayeh, S.M.; Fort, D.; Abell, J.S. Some observations on the growth of rutile single crystals using the optical floating zone method. J. Cryst. Growth 2005, 282, 190–198. [Google Scholar] [CrossRef]
- Park, J.K.; Shim, K.B.; Auh, K.H.; Tanaka, I. The growth of TiO2 (rutile) single crystals using the FZ method under high oxygen pressure. J. Cryst. Growth 2002, 237, 730–734. [Google Scholar] [CrossRef]
- Kalb, J.; Dorman, J.A.; Siroky, S.; Schmidt-Mende, L. Controlling the Spatial Direction of Hydrothermally Grown Rutile TiO2 Nanocrystals by the Orientation of Seed Crystals. Crystals 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Issar, S.; Mahapatro, A.K. Hydrothermally grown rutile titanium dioxide nanostructures with various morphologies. Mater. Sci. Semicond. Process 2019, 104, 104676. [Google Scholar] [CrossRef]
- Feng, Q.G.; Cai, H.D.; Lin, H.Y.; Qin, S.Y.; Liu, Z.; Ma, D.C.; Ye, Y.Y. Synthesis and structural characteristics of high surface area TiO2 aerogels by ultrasonic-assisted sol-gel method. Nanotechnology 2018, 29, 075702. [Google Scholar]
- Matrosov, V.N. Conditions for Obtaining High-Quality Crystals by the Czochralski Method. Crystallogr. Rep. 2019, 64, 174–176. [Google Scholar] [CrossRef]
- Machida, H.; Fukuda, T. Difficulties encountered during the Czochralski growth of TiO2 single crystals. J. Cryst. Growth 1991, 112, 835–837. [Google Scholar] [CrossRef]
- Pastor, A.C.; Pastor, R.C.; Soffer, B.H. Steady state studies in the conventional Verneuil process-the gas-liquid and the liquid-solid interfaces. Mater. Res. Bull. 1966, 1, 205–210. [Google Scholar] [CrossRef]
- Grzymkowski, R.; Mochnacki, B.; Suchy, J. Numerical simulation of the process of production of single crystals by the Verneuil method. J. Cryst. Growth 1985, 73, 529–536. [Google Scholar] [CrossRef]
- Feigelson, R.S. Crystal growth History: Theory and melt growth processes. J. Cryst. Growth 2022, 594, 126800. [Google Scholar] [CrossRef]
- Sarker, M.A.R.; Watauchi, S.; Nagao, M.; Watanabe, T.; Shindo, I.; Tanaka, I. Reduced Etch Pit Density of Rutile (TiO2) Single Crystals by Growth Using a Tilting-Mirror-Type Infrared Heating Image Furnace. Cryst. Growth Des. 2010, 10, 3929–3930. [Google Scholar] [CrossRef]
- Watauchi, S.; Sarker, M.A.R.; Nagao, M.; Tanaka, I.; Watanabe, T.; Shindo, I. Crystal growth of rutile by tilting-mirror-type floating zone method. J. Cryst. Growth 2012, 360, 105–110. [Google Scholar] [CrossRef]
- Kotani, T.; Tuller, H.L. Growth of TiO2 single crystals and bicrystals by the laser-heated floating-zone method. J. Am. Ceram. Soc. 1998, 81, 592–596. [Google Scholar] [CrossRef]
- Hossain, M.M.; Watauchi, S.; Nagao, M.; Tanaka, I. Effects of growth parameters on silicon molten zone formed by infrared convergent-heating floating zone method. J. Cryst. Growth 2017, 459, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.A.R.; Watauchi, S.; Nagao, M.; Watanabe, T.; Shindo, I.; Tanaka, I. Effects of the diameter of rutile (TiO2) single crystals grown using tilting-mirror-type infrared heating image furnace on solid-liquid interface and etch pit density. J. Cryst. Growth 2011, 317, 135–138. [Google Scholar] [CrossRef]
- Lu, T.C.; Wu, S.Y.; Lin, L.B.; Zheng, W.C. Defects in the reduced rutile single crystal. Physica B 2001, 304, 147–151. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Harris, C.X.; Wallenmeyer, P.; Murowchick, J.; Chen, X.B. Asymmetric Lattice Vibrational Characteristics of Rutile TiO2 as Revealed by Laser Power Dependent Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.B.; Chen, H.N.; Fan, J.H.; Wang, X.Y.; Ma, X.P. Correlation between the radius of acceptor ion and the dielectric properties of co-doped TiO2 ceramics. Ceram. Int. 2019, 45, 14625–14633. [Google Scholar] [CrossRef]
- Wittawat, R.; Rittipun, R.; Jarasfah, M.; Nattaporn, B. Synthesis of ZnO/TiO2 spherical particles for blue light screening by ultrasonic spray pyrolysis. Mater. Today Commun. 2020, 24, 101126. [Google Scholar] [CrossRef]
- Pathak, G.; Pandey, S.; Katiyar, R.; Srivastava, A.; Dabrowski, R.; Garbat, K.; Manohar, R. Analysis of photoluminescence, UV absorbance, optical band gap and threshold voltage of TiO2 nanoparticles dispersed in high birefringence nematic liquid crystal towards its application in display and photovoltaic devices. J. Lumin. 2017, 192, 33–39. [Google Scholar] [CrossRef]
- Munnix, S.; Schmeits, M. Electronic structure of ideal TiO2(110), TiO2(001), and TiO2(100) surfaces. Phys. Rev. B 1984, 30, 2202–2211. [Google Scholar] [CrossRef]
- Betsch, R.J.; Park, H.L.; White, W.B. Raman spectra of stoichiometric and defect rutile. Mater. Res. Bull. 1991, 26, 613–622. [Google Scholar] [CrossRef]
- Ma, H.L.; Yang, J.Y.; Dai, Y.; Zhang, Y.B.; Lu, B.; Ma, G.H. Raman study of phase transformation of TiO2 rutile single crystal irradiated by infrared femtosecond laser. Appl. Surf. Sci. 2007, 253, 7497–7500. [Google Scholar] [CrossRef]
- Kangarlou, H.; Abdollahi, A. Thermodynamic Properties of Rutile (TiO2) Within the Phonon Calculations. Int. J. Thermophys. 2016, 37, 110. [Google Scholar] [CrossRef]
- Yu, J.X.; Fu, M.; Ji, G.F.; Chen, X.R. Phase transition and thermodynamic properties of TiO2 from first-principles calculations. Chin. Phys. B 2009, 18, 269–274. [Google Scholar]
- Yao, J.K.; Xu, C.; Qiang, Z.W.; Ma, J.Y.; Lv, G.N.; Wang, Y.Z.; He, H.B.; Shao, J.D. Effects of deposition temperatures on laser damage threshold of TiO2/SiO2 high reflectors. Surf. Eng. 2008, 24, 63–65. [Google Scholar] [CrossRef]
- Das, S.K.; Dufft, D.; Rosenfeld, A.; Bonse, J.; Bock, M.; Grunwald, R. Femtosecond-laser-induced quasiperiodic nanostructures on TiO2 surfaces. J. Appl. Phys. 2009, 105, 084912. [Google Scholar] [CrossRef]
- Museur, L.; Tsibidis, G.D.; Manousaki, A.; Anglos, D.; Kanaev, A. Surface structuring of rutile TiO2 (100) and (001) single crystals with femtosecond pulsed laser irradiation. J. Opt. Soc. Am. B-Opt. Phys. 2018, 35, 2600–2607. [Google Scholar] [CrossRef]

| Material | Polycrystalline Material without Seed Crystals |
|---|---|
| Crystal dimension | Φ 9 × 25 mm3 |
| Growth orientation | (101) |
| Growth rate | 6 mm/h |
| Rotation | 20 rpm |
| Zone length | ~7 mm |
| Atmosphere | Argon + 0.1 MPa Oxygen (70%) |
| Transmission range (transmittance) | 410–6560 nm |
| Absorption peak position | 218, 362 nm |
| Emission peak position | 403, 438 nm |
| Thermal expansion coefficient | 9.92 × 10−6/K |
| Laser damage threshold | 1.44 GW/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Ma, S.; Hu, Z.; Wang, J.; Wang, J.; Wu, Y. Rapid Growth of High-Quality Rutile TiO2 Single Crystals through a Laser Floating Zone Method. Crystals 2022, 12, 1789. https://doi.org/10.3390/cryst12121789
Wu J, Ma S, Hu Z, Wang J, Wang J, Wu Y. Rapid Growth of High-Quality Rutile TiO2 Single Crystals through a Laser Floating Zone Method. Crystals. 2022; 12(12):1789. https://doi.org/10.3390/cryst12121789
Chicago/Turabian StyleWu, Jialing, Shihui Ma, Zhanggui Hu, Jiajia Wang, Jiyang Wang, and Yicheng Wu. 2022. "Rapid Growth of High-Quality Rutile TiO2 Single Crystals through a Laser Floating Zone Method" Crystals 12, no. 12: 1789. https://doi.org/10.3390/cryst12121789
APA StyleWu, J., Ma, S., Hu, Z., Wang, J., Wang, J., & Wu, Y. (2022). Rapid Growth of High-Quality Rutile TiO2 Single Crystals through a Laser Floating Zone Method. Crystals, 12(12), 1789. https://doi.org/10.3390/cryst12121789




