Current Trends in Green Solvents: Biocompatible Ionic Liquids
Abstract
1. Introduction
2. Synthesis and Properties
3. Applications of Bio-ILs
3.1. Catalysis
3.2. Biomedical Applications
3.2.1. Bio-ILs as Skin Permeability and Bioavailability Enhancers for Transdermal and Oral Drug Administration
3.2.2. Improvement in Drug Solubility with the Presence of Bio-ILs
3.2.3. Bio-ILs Derived from Active Pharmaceutical Ingredients (API-ILs)
3.2.4. Bio-ILs Used in Various Biomedical Applications
3.3. Separation Processes
3.4. Lubricants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAILs | Amino Acid-based Ionic Liquids |
| ABS | Aqueous Biphasic System |
| Abt | Abietate |
| Ace | Acetate |
| Adi | Adipate |
| Ala | Alanine |
| APIs | Active Pharmaceutical Ingredients |
| Arg | Arginine |
| Asc | Ascorbate |
| Asp | Aspartate |
| Asp2 | Aspartate II |
| ATR | Attenuated total reflectance |
| BA | bromoacetate |
| BAC | Benzalkonium chloride |
| BDP | 1,1′-(1,4-butanediyl)bis(1-H-pyrrolidinium) |
| Ben | 4-hydroxybenzoate |
| Benz | Benzoate |
| Bic | Bicarbonate |
| Bmim | 1-butyl-3-methylimidazolium |
| But | Butyrate |
| C3C | Coumarine-3-carboxylate |
| C3mim | 1-allyl-3-methylimidazolium |
| C5mim | 3-methyl-1-pentylimidazolium |
| C6mim | 1-hexyl-3-methylimidazolium |
| C8mim | 1-octyl-3-methylimidazolium |
| C10mim | 1-decyl-3-methylimidazolium |
| Caf | Caffeate |
| Cap | Caproate |
| Capl | Caprylate |
| Capr | Capriate |
| Ch-AA-ILs | Choline- and Amino Acid-based Ionic Liquids |
| ChILs | Choline-based Ionic Liquids |
| Ch | Cholinium |
| Cip | Ciprofloxacin |
| Cit | Citrate |
| COF | Coefficient of Friction |
| Cou | p-Coumarate |
| CRC | Curcumin |
| CTAC | Cetyltrimethylammonium chloride |
| Cyc | Cyclohexane carboxylate |
| Cys | Cysteinate |
| DBN | 1,5-diazabicyclo[4.3.0]-5-nonene |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DDBS | dodecylbenzenesulfonate |
| DFT | Density Functional Theory |
| D-Gal | A-Galactouronate |
| DHB | 2,5-Dihydroxybenzoate |
| DLS | Dynamic Light Scattering |
| DMBA | Butyldimethylamine |
| Doc | Deoxycholate |
| DSC | Differential Scanning Calorimetry |
| EDX | Energy-Dispersive X-ray |
| EEG | Ethoxylate oleyl ether glycolate |
| Fer | Ferulate |
| For | Formate |
| FQ | Fluoroquinolones |
| FTIR | Fourier Transform Infrared Spectroscopy |
| Fu | Fumarate |
| GA3 | Gibberellate |
| Gal | Gallate |
| Gen | Gentisate |
| Ger | Geranate |
| Glc | Glucuronate |
| Glm | L-Glutamate |
| Gln | Glutaminate |
| Glu | Glucose |
| Glut | Glutarate |
| Gly | Glycinate |
| HCAs | Hydrocinnamic Acids |
| Hex | Hexanoate |
| His | Histidine |
| HPLC | High-Performance Liquid Chromatography |
| Ibu | Ibuprofenate |
| iBut | iso-Butyrate |
| IFT | Interfacial Tension |
| Iso | Isonicotinate |
| KH | Henry’s law constant |
| LAA | Lipoaminoacids |
| Lac | Lactate |
| Lau | Laurate |
| LC50 | Lethal concentration that kills 50% of the tested organism |
| Leu | Leucine |
| Lev | Levulinate |
| Lid | Lidocaine |
| LLE | Liquid-Liquid Equilibrium |
| LSar | Lauryl sarcosinate |
| Lut | Luteonin |
| Lys | Lysinate |
| LYZ | Lysozyme |
| MAG | Monoacylglycerol |
| Mal | Malonate |
| Mali | Malate |
| MD | Molecular Dynamic |
| MEP | Molecular Electrostatic Potential |
| Met | L-methioninate |
| MIC | Minimun Inhibitory Concentration |
| MRSA | Methicillin-Resistant S.Aureus |
| MS | Mass Spectrometry |
| NCI | Non-Covalent Interactions |
| NMR | Nuclear Magnetic Resonance |
| Nor | Norfloxacin |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| Oct | Octanoate |
| Ole | Oleate |
| OSILs | Organic Salts and Ionic Liquids |
| P4444 | Tetrabutylphosphonium |
| P666,14 | Trihexyl(tetradecyl)phosphonium |
| PEEP-ILs | Proline Ethyl Ester Phenolate Ionic Liquids |
| Phe | Phenylalaninate |
| PILs | Protic Ionic Liquids |
| PLM | Polarized Light Microscopy |
| PP | Polyphosphate |
| Prc | Procaine |
| Proc | Protocatechuate |
| ProEt | L-proline ethyl ester |
| PS | 1,3-propanesultone |
| Qui | D-Quinate |
| ROS | Reactive Oxygen Species |
| RSN | Relative Solubility Numbers |
| RTILs | Room Temperature Ionic Liquids |
| Sac | Saccharinate |
| SAILs | Surface-Active Ionic Liquids |
| Sal | Salicylate |
| Sar | Sarcosinate |
| SAXS | Small-Angle X-ray Scattering |
| SC | Stratum Corneum |
| SEDDS | Self-Emulsifying Drug Delivery Systems |
| Ser | Serine |
| Sin | Sinapinate |
| Sor | Sorbate |
| SRF | Sorafenib |
| Suc | Succinate |
| Syr | Syringate |
| TBA | Tetrabutylammonium |
| Td | Degradation Temperature |
| TEM | Transmission Electron Microscopy |
| TEWL | Transepidermal Water Loss |
| Tg | Glass transition Temperature |
| TGA | Thermogravimetric Analysis |
| Theob | Theobrominate |
| Theop | Theophyllinate |
| TLC | Thin Layer Chromatography |
| Tm | Melting Temperature |
| TMA | Tetramethylammonium |
| TMG | 1,1,3,3-Tetramethylguanidine |
| Tol | Tolfenamic acid |
| Tr | Tretinoin |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| UPLC | Ultra Performance Liquid Chromatography |
| Ur | Urate |
| UV | Ultraviolet Visible Spectroscopy |
| Val | Vanillate |
| VOCs | Volatile Organic Compounds |
| WAXS | Wide-Angle X-ray Scattering |
| Xan | Xanthinate |
| XO | Xanthine Oxidase |
| XRD | X-Ray Diffraction Analysis |
References
- Wu, X.; Zhang, H.; He, S.; Yu, Q.; Lu, Y.; Wu, W.; Ding, N.; Zhu, Q.; Chen, Z.; Ma, Y.; et al. Improving dermal delivery of hyaluronic acid by ionic liquids for attenuating skin dehydration. Int. J. Biol. Macromol. 2020, 150, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.R.F.; Veloso, T.; Schaeffer, N.; Pereira, J.L.; Ventura, S.P.M.; Rizzi, C.; Sirieix, J.; Passos, H.; Coutinho, A.P. Synthesis of Purine-Based Ionic Liquids and Their Applications. Molecules 2021, 26, 6958. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, K.; Pernak, J. L-Carnitine-Based Bio-Ionic Liquids as Antioxidants. ChemistrySelect 2021, 6, 1994–2001. [Google Scholar] [CrossRef]
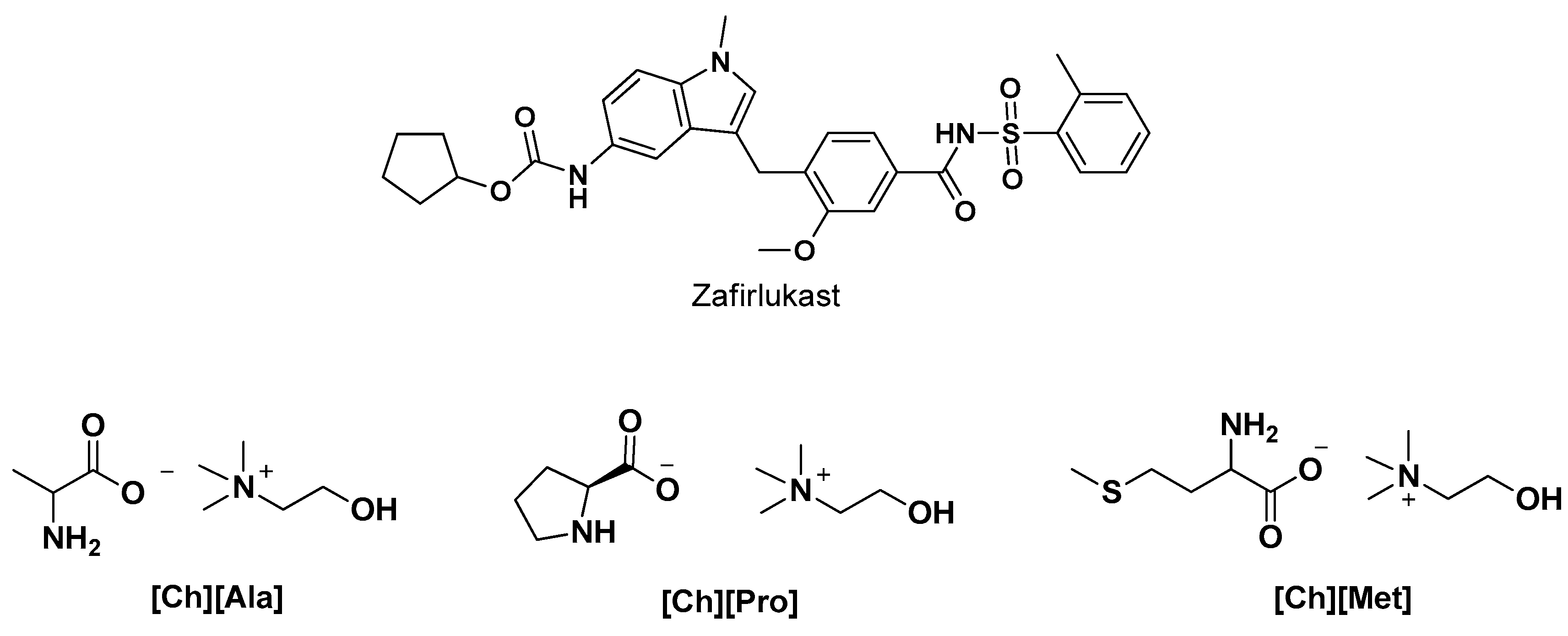
- Gaikwad, N.; Kudal, S.; Avachat, A.M. Choline-Amino Acid-Derived Bio-ionic Liquids for Solubility Enhancement of Zafirlukast. AAPS PharmSciTech 2022, 23, 146. [Google Scholar] [CrossRef]
- Shimul, I.; Moshikur, R.; Minamihata, K.; Moniruzzaman, M. Amino Acid Ester based Phenolic Ionic Liquids as a Potential Solvent for the Bioactive Compound Luteolin: Synthesis, Characterization, and Food Preservation Activity. J. Mol. Liq. 2022, 349, 118103. [Google Scholar] [CrossRef]
- Santiago, R.; Díaz, I.; González-Miquel, M.; Navarro, P.; Palomar, J. Assessment of bio-ionic liquids as promising solvents in industrial separation processes: Computational screening using COSMO-RS method. Fluid Phase Equilibria 2022, 560, 113495. [Google Scholar] [CrossRef]
- Mustahil, N.A.; Baharuddin, S.H.; Abdullah, A.A.; Reddy, A.V.B.; Abdul Mutalib, M.I.; Moniruzzaman, M. Synthesis, characterization, ecotoxicity and biodegradability evaluations of novel biocompatible surface active lauroyl sarcosinate ionic liquids. Chemosphere 2019, 229, 349–357. [Google Scholar] [CrossRef]
- Foulet, A.; Ghanem, O.B.; El-Harbawi, M.; Lévêque, J.M.; Mutalib, M.I.A.; Yin, C.Y. Understanding the physical properties, toxicities and anti-microbial activities of choline-amino acid-based salts: Low-toxic variants of ionic liquids. J. Mol. Liq. 2016, 221, 133–138. [Google Scholar] [CrossRef]
- Sivapragasam, M.; Moniruzzaman, M.; Goto, M. An Overview on the Toxicological Properties of Ionic Liquids toward Microorganisms. Biotechnol. J. 2020, 15, 1900073. [Google Scholar] [CrossRef]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Więcek, P.; Guzik, M.; Chrobok, A. Combining amino acids and carbohydrates into readily biodegradable, task specific ionic liquids. RSC Adv. 2020, 10, 18355–18359. [Google Scholar] [CrossRef] [PubMed]
- Mena, I.F.; Diaz, E.; Palomar, J.; Rodriguez, J.J.; Mohedano, A.F. Cation and anion effect on the biodegradability and toxicity of imidazolium– and choline–based ionic liquids. Chemosphere 2020, 240, 124947. [Google Scholar] [CrossRef] [PubMed]
- Tzani, A.; Skarpalezos, D.; Papadopoulos, A.; Aravopoulou, D.; Kleidas, I.; Ioannou, E.; Voutsas, E.; Kyritsis, A.; Detsi, A. Synthesis of novel non-toxic naphthenic and benzoic acid ionic liquids. Structure-properties relationship and evaluation of their biodegradability potential. J. Mol. Liq. 2019, 296, 111927. [Google Scholar] [CrossRef]
- Le Donne, A.; Bodo, E. Cholinium amino acid-based ionic liquids. Biophys. Rev. 2021, 13, 147–160. [Google Scholar] [CrossRef]
- Md Moshikur, R.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Kirchhecker, S.; Esposito, D. Amino acid based ionic liquids: A green and sustainable perspective. Curr. Opin. Green Sustain. Chem. 2016, 2, 28–33. [Google Scholar] [CrossRef]
- Tzani, A.; Vaitsis, C.; Kritsi, E.; Smiljkovic, M.; Sokovic, M.; Zoumpoulakis, P.; Detsi, A. Green synthesis of bis-(β-dicarbonyl)-methane derivatives and biological evaluation as putative anticandidial agents. J. Mol. Struct. 2020, 1216, 128276. [Google Scholar] [CrossRef]
- Tzani, A.; Douka, A.; Papadopoulos, A.; Pavlatou, E.A.; Voutsas, E.; Detsi, A. Synthesis of biscoumarins using recyclable and biodegradable task-specific ionic liquids. ACS Sustain. Chem. Eng. 2013, 1, 1180–1185. [Google Scholar] [CrossRef]
- Honarmand, M.; Tzani, A.; Detsi, A. Synthesis of novel multi-OH functionalized ionic liquid and its application as dual catalyst-solvent for the one-pot synthesis 4 H -pyrans. J. Mol. Liq. 2019, 290, 111358. [Google Scholar] [CrossRef]
- Honarmand, M.; Tzani, A.; Detsi, A. A biodegradable and recyclable ionic liquid for the one-pot synthesis of 2-amino-3-cyano-4 H -pyrans. J. Iran. Chem. Soc. 2019, 16, 571–581. [Google Scholar] [CrossRef]
- Sheldon, R.A. Biocatalysis in ionic liquids: State-of-the-union. Green Chem. 2021, 23, 8406–8427. [Google Scholar] [CrossRef]
- Papadopoulou, A.A.; Tzani, A.; Alivertis, D.; Katsoura, M.H.; Polydera, A.C.; Detsi, A.; Stamatis, H. Hydroxyl ammonium ionic liquids as media for biocatalytic oxidations. Green Chem. 2016, 18, 1147–1158. [Google Scholar] [CrossRef]
- Papadopoulou, A.A.; Tzani, A.; Polydera, A.C.; Katapodis, P.; Voutsas, E.; Detsi, A.; Stamatis, H. Green biotransformations catalysed by enzyme-inorganic hybrid nanoflowers in environmentally friendly ionic solvents. Environ. Sci. Pollut. Res. 2018, 25, 26707–26714. [Google Scholar] [CrossRef]
- Tzani, A.; Koutsoukos, S.; Koukouzelis, D.; Detsi, A. Synthesis and characterization of silver nanoparticles using biodegradable protic ionic liquids. J. Mol. Liq. 2017, 243, 212–218. [Google Scholar] [CrossRef]
- Olleik, H.; Yahiaoui, S.; Roulier, B.; Courvoisier-Dezord, E.; Perrier, J.; Pérès, B.; Hijazi, A.; Baydoun, E.; Raymond, J.; Boumendjel, A.; et al. Aurone derivatives as promising antibacterial agents against resistant Gram-positive pathogens. Eur. J. Med. Chem. 2019, 165, 133–141. [Google Scholar] [CrossRef]
- Eden, S.; Tanner, E.L. Improved nanoformulation and bio-functionalization of linear-dendritic block copolymers with biocompatible ionic liquids. Nanoscale 2022, 14, 6021–6036. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Rogers, R.D.; Gurau, G. Is “choline and geranate” an ionic liquid or deep eutectic solvent system? Proc. Natl. Acad. Sci. USA 2018, 115, E10999. [Google Scholar] [CrossRef]
- Ibsen, K.N.; Ma, H.; Banerjee, A.; Tanner, E.E.L.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE). ACS Biomater. Sci. Eng. 2018, 4, 2370–2379. [Google Scholar] [CrossRef]
- Kelley, S.P.; Narita, A.; Holbrey, J.D.; Green, K.D.; Reichert, W.M.; Rogers, R.D. Understanding the effects of ionicity in salts, solvates, co-crystals, ionic co-crystals, and ionic liquids, rather than nomenclature, is critical to understanding their behavior. Cryst. Growth Des. 2013, 13, 965–975. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Definitions of ionic liquids and deep eutectic solvents. Proc. Natl. Acad. Sci. USA 2018, 115, E11000–E11001. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Foltran, S.; Favier, I.; Pla, D. Palladium nanoparticles stabilized by novel choline-based ionic liquids in glycerol applied in hydrogenation reactions. Catal. Today 2020, 346, 69–75. [Google Scholar] [CrossRef]
- Ordo, J.; Garg, G.; Masdeu-bulto, A.M.; Farfa, N.; Medina-gonza, Y. Palladium Nanoparticles in Glycerol/Ionic Liquid/Carbon Dioxide Medium as Hydrogenation Catalysts. ACS Appl. Nano Mater. 2020, 3, 12240–12249. [Google Scholar] [CrossRef]
- Mu, L.; Cao, D.; Zhuang, W.; Yu, Q.; Cai, M.; Shi, Y. Stable Dispersed Zeolitic Imidazolate Framework/Graphene Oxide Nanocomposites in Ionic Liquids Resulting in High Lubricating Performance. Adv. Mater. Interfaces 2020, 7, 1902194. [Google Scholar] [CrossRef]
- Leu, M.; Campbell, P.; Mudring, A. Green Chemistry Letters and Reviews Synthesis of luminescent semiconductor nanoparticles in ionic liquids—The importance of the ionic liquid in the formation of quantum dots. Green Chem. Lett. Rev. 2021, 14, 128–136. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Samoylenko, D.E.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Metal nanoparticles in ionic liquids: Synthesis and catalytic applications. Coord. Chem. Rev. 2021, 445, 213982. [Google Scholar] [CrossRef]
- Balischewski, C.; Choi, H.; Behrens, K.; Beqiraj, A. Metal Sulfide Nanoparticle Synthesis with Ionic Liquids—State of the Art and Future Perspectives. ChemistryOpen 2021, 10, 272–295. [Google Scholar] [CrossRef]
- Li, X.; Ma, N.; Zhang, L.; Ling, G.; Zhang, P. Applications of choline-based ionic liquids in drug delivery. Int. J. Pharm. 2022, 612, 121366. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with l-valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Synthesis and antibacterial activity of ionic liquids and organic salts based on penicillin g and amoxicillin hydrolysate derivatives against resistant bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef]
- Lai, A.; Leong, N.; Zheng, D.; Ford, L.; Nguyen, T.H.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. Biocompatible Cationic Lipoamino Acids as Counterions for Oral Administration of API-Ionic Liquids. Pharm. Res. 2022, 39, 2405–2419. [Google Scholar] [CrossRef]
- Fahri, F.; Bacha, K.; Chiki, F.F.; Mbakidi, J.P.; Panda, S.; Bouquillon, S.; Fourmentin, S. Air pollution: New bio-based ionic liquids absorb both hydrophobic and hydrophilic volatile organic compounds with high efficiency. Environ. Chem. Lett. 2020, 18, 1403–1411. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, D.; Sequeira, R.A.; Talsaniya, R.K.; Maru, D.A.; Moradiya, K.; Prasad, K. Syntheses and characterization of few bio-ionic liquids comprising of cholinium cation and plant derived carboxylic acids as anions. J. Indian Chem. Soc. 2021, 98, 100205. [Google Scholar] [CrossRef]
- Takeda, J.; Iwao, Y.; Karashima, M.; Yamamoto, K.; Ikeda, Y. Structural Evaluation of the Choline and Geranic Acid/Water Complex by SAXS and NMR Analyses. ACS Biomater. Sci. Eng. 2021, 7, 595–604. [Google Scholar] [CrossRef]
- Miao, S.; Atkin, R.; Warr, G.G. Amphiphilic nanostructure in choline carboxylate and amino acid ionic liquids and solutions. Phys. Chem. Chem. Phys. 2020, 22, 3490–3498. [Google Scholar] [CrossRef]
- Dhattarwal, H.S.; Kashyap, H.K. Unique and generic structural features of cholinium amino acid-based biocompatible ionic liquids. Phys. Chem. Chem. Phys. 2021, 23, 10662–10669. [Google Scholar] [CrossRef]
- Daso, R.E.; Mitchell, S.M.; Lebedenko, C.G.; Heise, R.M.; Banerjee, I.A. Exploring the interactions of ionic liquids with bio-organic amphiphiles using computational approaches. ACS Omega 2021, 6, 32460–32474. [Google Scholar] [CrossRef]
- Uddin, S.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Lipid based biocompatible ionic liquids: Synthesis, characterization and biocompatibility evaluation. Chem. Commun. 2020, 56, 13756–13759. [Google Scholar] [CrossRef]
- Patil, K.R.; Surwade, A.D.; Rajput, P.J.; Shaikh, V.R. Investigations of solute—Solvent interactions in aqueous solutions of amino acids ionic liquids having the common nitrate as anion at different temperatures. J. Mol. Liq. 2021, 329, 115546. [Google Scholar] [CrossRef]
- Panić, J.; Tot, A.; Janković, N.; Drid, P.; Gadžurić, S.; Vraneš, M. Physicochemical and structural properties of lidocaine-based ionic liquids with anti-inflammatory anions. RSC Adv. 2020, 10, 14089–14098. [Google Scholar] [CrossRef]
- Sun, S.; Lv, Y.; Wang, G.; Chen, X. Soybean oil-based monoacylglycerol synthesis using bio-compatible amino acid ionic liquid as a catalyst at low temperature. J. Mol. Liq. 2021, 340, 117231. [Google Scholar] [CrossRef]
- Weng, S.; Dong, J.; Ma, J.; Bai, J.; Liu, F.; Liu, M. Biocompatible anions-derived ionic liquids a sustainable media for CO2 conversion into quinazoline-2, 4 (1 H, 3 H) -diones under additive-free conditions. J. CO2 Util. 2022, 56, 101841. [Google Scholar] [CrossRef]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E.L. Recent Advances in Ionic Liquids in Biomedicine. Adv. Sci. 2021, 8, 2004819. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Z.; Gao, Y.; Pan, D.C.; Salinas, A.K.; Tanner, E.E.L.; Guo, J.; Mitragotri, S. Oral delivery of sorafenib through spontaneous formation of ionic liquid nanocomplexes. J. Control. Release 2020, 322, 602–609. [Google Scholar] [CrossRef]
- Ko, J.; Mandal, A.; Dhawan, S.; Mitragotri, S.; Joshi, N.; Shevachman, M. Clinical translation of choline and geranic acid deep eutectic solvent. Bioeng. Transl. Med. 2021, 6, e10191. [Google Scholar] [CrossRef]
- Sintra, E.; Abranches, D.O.; Benfica, J.; Soares, B.P.; Ventura, P.M.; Coutinho, J.A.P. Cholinium-based ionic liquids as bioinspired hydrotropes to tackle solubility challenges in drug formulation. Eur. J. Pharm. Biopharm 2021, 164, 86–92. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, N.; Wu, J.; Yin, T.; Jia, Y. Ionic liquids as effective additives to enhance the solubility and permeation for puerarin and ferulic acid. RSC Adv. 2022, 12, 3416–3422. [Google Scholar] [CrossRef]
- Bastos, J.C.; Vieira, N.S.M.; Gaspar, M.M.; Pereiro, A.B.; Araújo, J.M.M. Human Cytotoxicity, Hemolytic Activity, Anti-Inflammatory Activity and Aqueous Solubility of Ibuprofen-Based Ionic Liquids. Sustain. Chem. 2022, 3, 23. [Google Scholar] [CrossRef]
- Wu, X.; Xuan, J.; Yu, Q.; Wu, W.; Lu, Y.; Zhu, Q.; Chen, Z.; Qi, J. Converting Tretinoin into Ionic Liquids for Improving Aqueous Solubility and Permeability across Skin. Pharm. Res. 2022, 39, 2421–2430. [Google Scholar] [CrossRef]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial activities of highly bioavailable organic salts and ionic liquids from fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef]
- Panić, J.; Vraneš, M.; Mirtič, J.; Cerc Korošec, R.; Zupančič, Š.; Gadžurić, S.; Kristl, J.; Bešter-Rogač, M. Preparation and characterization of innovative electrospun nanofibers loaded with pharmaceutically applicable ionic liquids. Int. J. Pharm. 2022, 615, 121510. [Google Scholar] [CrossRef]
- Rita Pereira, A.; Gomes, I.B.; Simões, M. Choline-based ionic liquids for planktonic and biofilm growth control of Bacillus cereus and Pseudomonas fluorescens. J. Mol. Liq. 2022, 346, 117077. [Google Scholar] [CrossRef]
- Demurtas, M.; Onnis, V.; Zucca, P.; Rescigno, A.; Lachowicz, J.I.; De Villiers Engelbrecht, L.; Nieddu, M.; Ennas, G.; Scano, A.; Mocci, F.; et al. Cholinium-Based Ionic Liquids from Hydroxycinnamic Acids as New Promising Bioactive Agents: A Combined Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2021, 9, 2975–2986. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Singh, D.; Kesavan, A.K.; Kang, T.S. Antimicrobial Colloidal Complexes of Lysozyme with Bio-Based Surface Active Ionic Liquids in Aqueous Medium. J. Phys. Chem. B 2020, 124, 3791–3800. [Google Scholar] [CrossRef]
- Latini, G.; Signorile, M.; Rosso, F.; Fin, A.; Bordiga, S.; Bocchini, S.; Giordani, S.; Pirri, F.; Crocell, V. Efficient and reversible CO2 capture in bio-based ionic liquids solutions. J. CO2 Util. 2022, 55, 101815. [Google Scholar] [CrossRef]
- Kumar, A.; Alphons, R.; Kumar, T.; Prasad, K. Bio-ionic liquid promoted selective coagulation of κ -carrageenan from Kappaphycus alvarezii extract. Food Hydrocoll. 2021, 111, 106382. [Google Scholar]
- Liu, E.; Segato, F.; Prade, R.A.; Wilkins, M.R. Bioresource Technology Exploring lignin depolymerization by a bi-enzyme system containing aryl alcohol oxidase and lignin peroxidase in aqueous biocompatible ionic liquids. Bioresour. Technol. 2021, 338, 125564. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Muhammad, N.; Hallett, J.P. Production of Food-Grade Glucose from Rice and Wheat Residues Using a Biocompatible Ionic Liquid. ACS Sustain. Chem. Eng. 2021, 9, 8080–8089. [Google Scholar] [CrossRef]
- Pimienta, J.A.P.; Papa, G.; Sun, J.; Stavila, V.; Sanchez, A.; Gladden, J.M.; Simmons, B.A. One-pot ethanol production under optimized pretreatment conditions using agave bagasse at high solids loading with low-cost biocompatible. Green Chem. 2022, 24, 207–217. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Y. Controllable recovery and regeneration of bio-derived ionic liquid choline acetate for biomass processing via bipolar membrane electrodialysis-based methodology. Sep. Purif. Technol. 2022, 297, 121455. [Google Scholar] [CrossRef]
- Mohan, M.; Choudhary, H.; George, A.; Simmons, B.A.; Sale, K.; Gladden, J.M. Towards understanding of delignification of grassy and woody biomass in cholinium-based ionic. Green Chem. 2021, 23, 6020–6035. [Google Scholar] [CrossRef]
- Hawatulaila, S.; Adawiyah, N.; Vijaya, A.; Reddy, B.; Aini, A.; Ibrahim, M.; Mutalib, A.; Moniruzzaman, M. Chemosphere Development, formulation and optimization of a novel biocompatible ionic liquids dispersant for the effective oil spill remediation. Chemosphere 2020, 249, 126125. [Google Scholar] [CrossRef]
- Abdullah, M.M.S.; Al-Lohedan, H.A. Novel bio-based amphiphilic ionic liquids for the efficient demulsification of heavy crude oil emulsions. Molecules 2021, 26, 6119. [Google Scholar] [CrossRef]
- Sadanandan, A.M.; Khatri, P.K.; Saxena, R.C.; Jain, S.L. Guanidine based amino acid derived task speci fi c ionic liquids as noncorrosive lubricant additives for tribological performance. J. Mol. Liq. 2020, 313, 113527. [Google Scholar] [CrossRef]
- Reeves, C.J.; Kasar, A.K.; Menezes, P.L. Tribological Performance of environmental friendly ionic liquids for High-Temperature Applications. J. Clean. Prod. 2021, 279, 123666. [Google Scholar] [CrossRef]
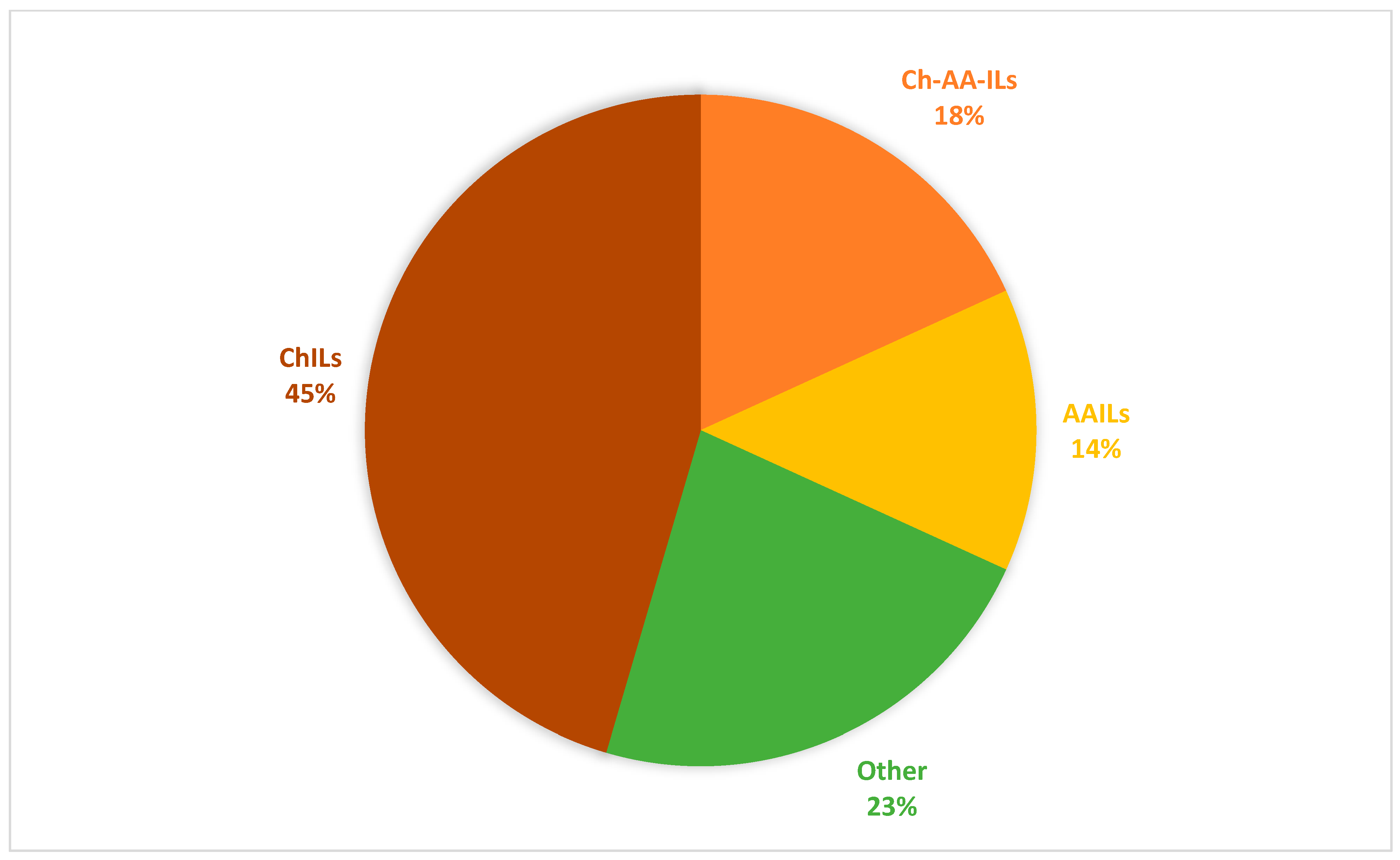
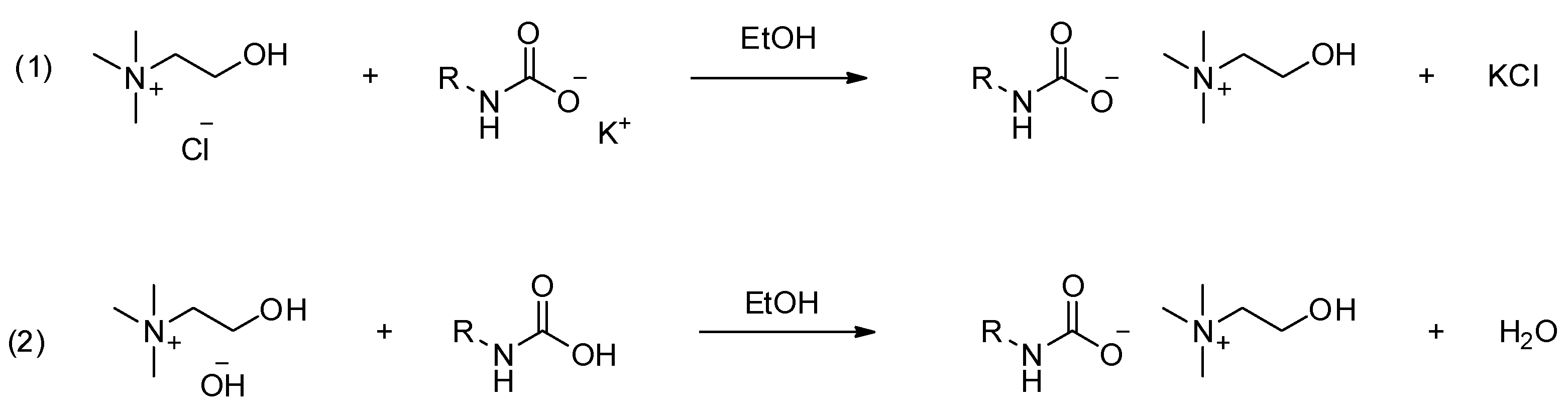
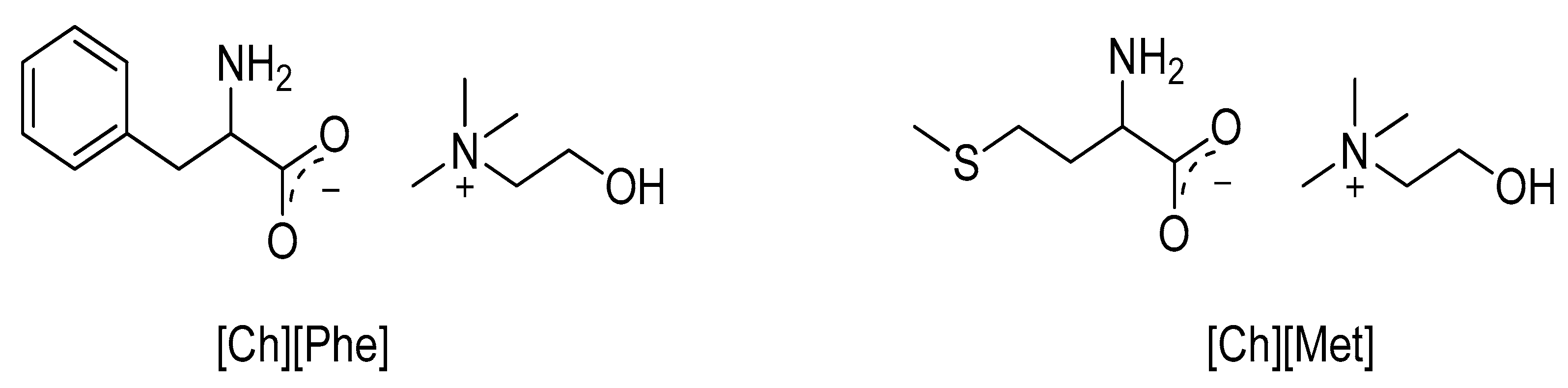
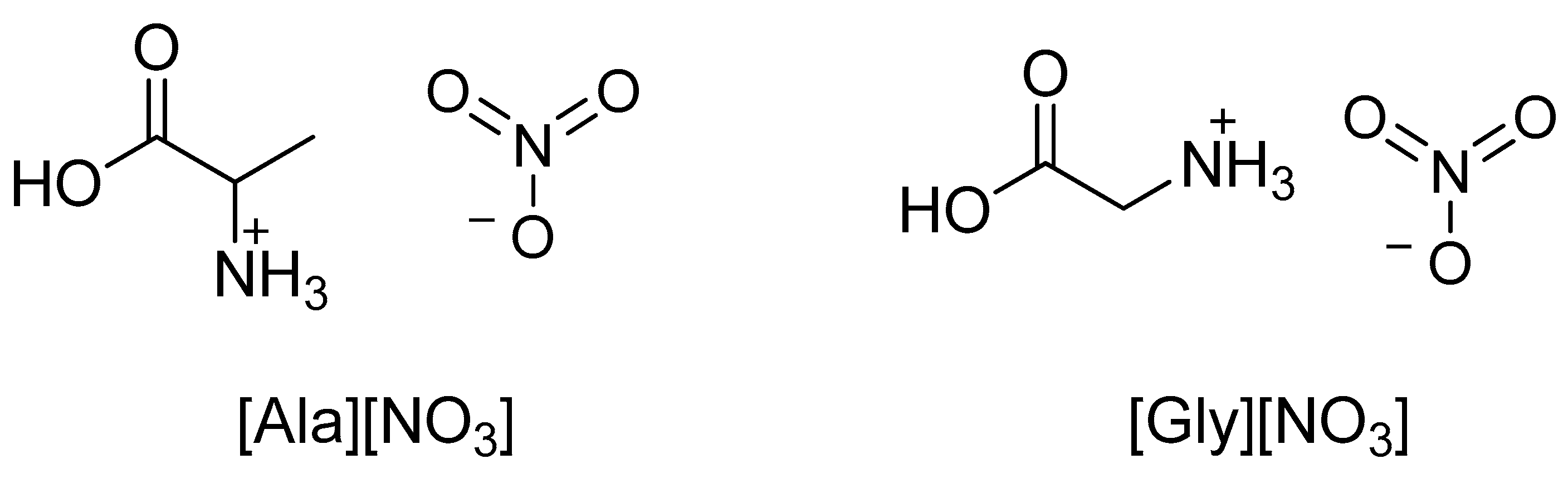
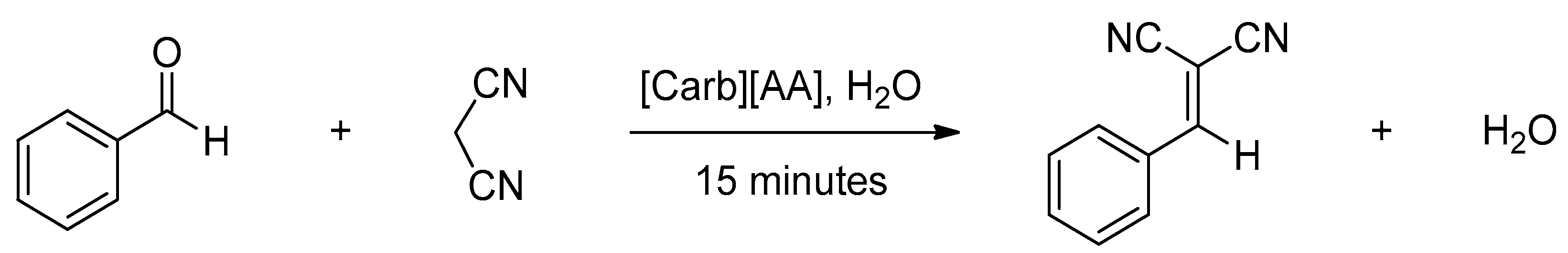
| IL | Synthesis | Physicocemical Properties | Ref. |
|---|---|---|---|
| [Ch][Abt] | Neutralization reaction | Tg * = −46.3 °C Tm = −20.5 °C Td = 314 °C η = 107 Pa·s (25 °C) | [43] |
| [Ch][Asc] | Tg = −70.1 °C Tm = 15.3 °C Td = 251 °C η = 1.18 Pa·s (25 °C) | ||
| [Ch][Caf] | Tg = − 35.72 °C Tm = 92.2 °C Td = 350 °C η = not measurable (25 °C) | ||
| [Ch][C3C] | Tg = −88.3 °C Tm = −28.0 °C Td = 261 °C η = 6290 Pa·s (25 °C) | ||
| [Ch][DHB] | Tg = −66.5 °C Tm = 40.7 °C Td = 279 °C η = not measurable (25 °C) | ||
| [Ch][Fer] | Tg = −96.6 °C Tm = 16.8 °C Td = 230 °C η = 10.10 Pa·s (25 °C) | ||
| [Ch][D-Gal] | Tg = −63.3 °C Tm = 17.4 °C Td = 245 °C η = 21.21 Pa·s (25 °C) | ||
| [Ch][GA3] | Tg = −97.7 °C Tm = −26.1 °C Td = 275 °C η = 1.56 Pa·s (25 °C) | ||
| [Ch][Glc] | Tg = −47.8 °C Tm = 13.1 °C Td = 240 °C η = 1.20 Pa·s (25 °C) | ||
| [Ch][Qui] | Tg = −76.3 °C Tm = 14.3 °C Td = 330 °C η = 0.23 Pa·s (25 °C) | ||
| [Ch][Sin] | Tg = −90.8 °C Tm = 13.6 °C Td = 210 °C η = 3.99 Pa·s (25 °C) | ||
| [Ch][Ger] | Salt metathesis reaction | η = 908 Pa·s (25 °C) | [44] |
| [Ch][Lac] | Neutralization reaction | - | [45] |
| [Ch][iBut] | - | ||
| [Ch][Asp] | - | ||
| [Ch][Asp2-] | - | ||
| [Ch][PHe] | - | ||
| [Ch][Lys] | - | ||
| [Ch][Phe] | Simulation | - | [46] |
| [Ch][Met] | - | ||
| [Ch][Gln] | - | ||
| [Ch][Glu] | - | ||
| [Ch][Gly] | - | ||
| [Ch][Cys] | - | ||
| [Ch][Bic] | Simulation | - | [47] |
| [Ch][Cit] | - | ||
| [Ch][Pho] | - | ||
| [Ch][Glc] | - | ||
| [Ch][Lev] | - | ||
| [Ch][Ser] | - | ||
| [Ch][Cl] | - | ||
| [Gbet][Bic] | - | ||
| [Gbet][Cit] | - | ||
| [Gbet][Pho] | - | ||
| [Gbet][Glc] | - | ||
| [Gbet][Lev] | - | ||
| [Gbet][Ser] | - | ||
| [Gbet][Cl] | - | ||
| [Ch][Bic] | - | ||
| [Ch][Cit] | - | ||
| [EDMPC][Lin] | Salt metathesis reaction | Tm = 20.8 °C | [48] |
| [EDMPC][Ole] | Tm = 24.3 °C | ||
| [EDMPC][Ste] | Tm = 54.9 °C | ||
| [Gly][NO3] | Neutralization reaction | d = 1023.902 kg/m3 (25 °C, ∼0.5 mol/kg H2O) | [49] |
| [Ala][NO3] | d = 1022.800 kg/m3 (25 °C, ∼0.5 mol/kg H2O) | ||
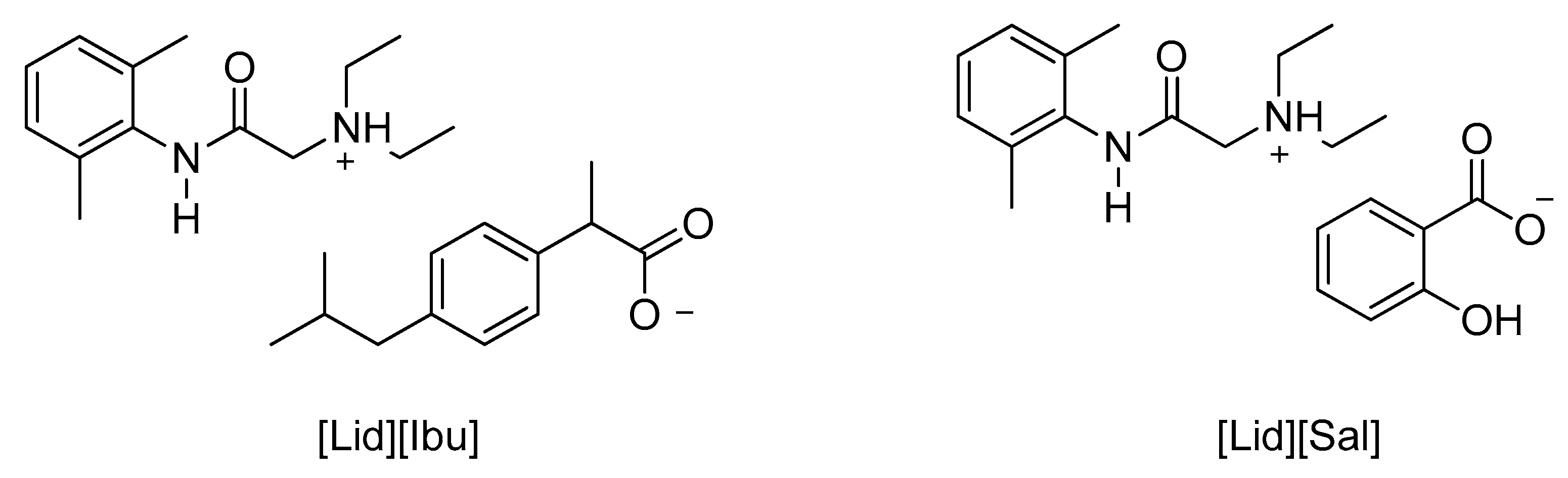
| [Lid][Ibu] | Neutralization reaction | [50] | |
| [Lid][Sal] |
| IL | Synthesis | Physicocemical Properties | Application | Ref. |
|---|---|---|---|---|
| [Glu][Gly] | Neutralization reaction | Tg * = –19 °C Td * = 198 °C η = 10,196 mPa·s (70 °C) | Organocatalysts in the Knoevenagel condensation reaction | [11] |
| [Glu][Ser] | Tg = −18 °C Td = 198 °C η = 28,645 mPa·s (70 °C) | |||
| [Glu][Leu] | Tg = 4 °C Td = 208 °C η = 359,841 mPa·s (70 °C) | |||
| [Glu][Arg] | Tg = −15 °C Td = 107 °C η = 247,626 mPa·s (70 °C) | |||
| [Glu][His] | Tg = −9 °C Td = 207 °C η = 106,930 mPa·s (70 °C) | |||
| [Glu][Trp] | Tg = 0 °C Td = 211 °C η = 408,284 mPa·s (70 °C) | |||
| [Glu][Tyr] | Tg = 6 °C Td = 209 η = 1,476,023 mPa·s (70 °C) | |||
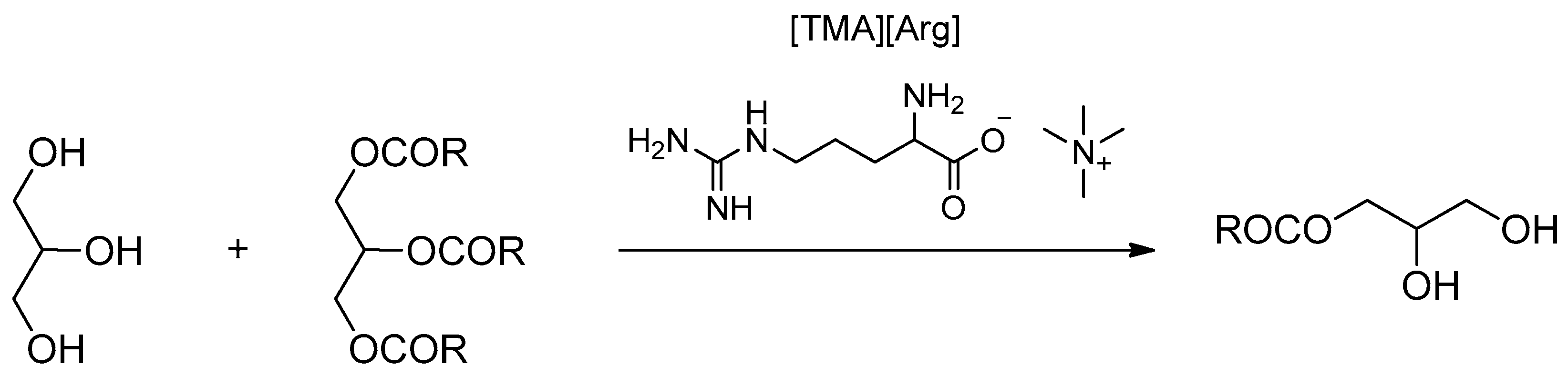
| [Ch][Arg] | Neutralization reaction | - | Catalysts for the synthesis of Soybean oil-based monoacylglycerol | [51] |
| [Ch][Lys] | - | |||
| [Ch][His] | - | |||
| [Ch][Trp] | - | |||
| [Ch][Glum] | - | |||
| [TMA][Arg] | - | |||
| [TBA][Arg] | - | |||
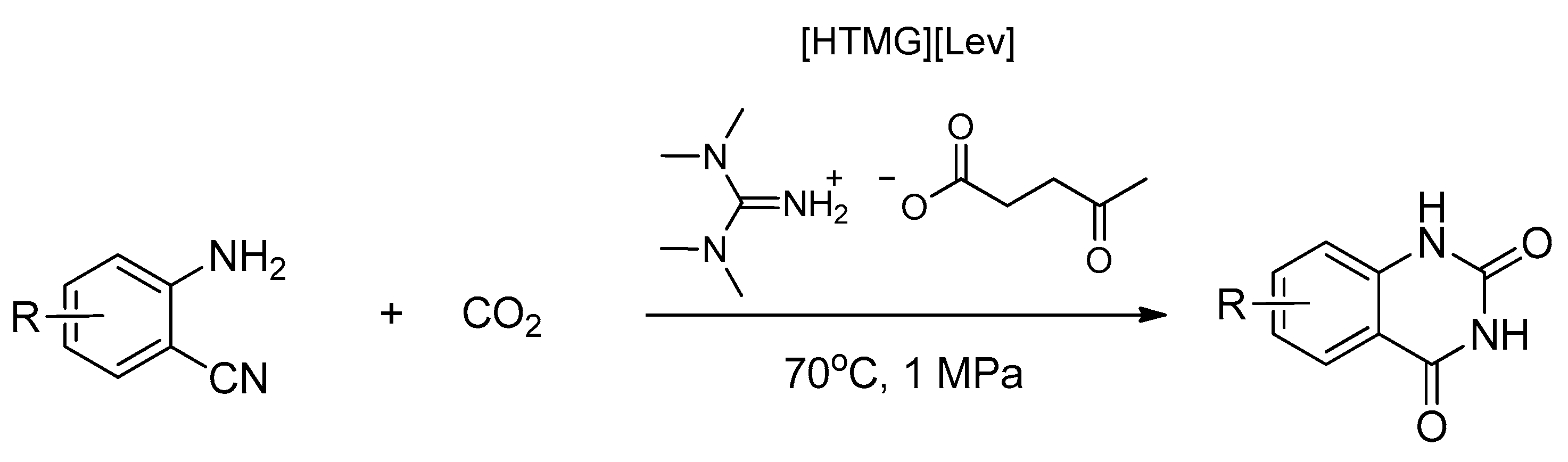
| [HTMG][Lae] | Neutralization reaction | - | Solvent-catalyst for CO2 conversion into quinazoline-2,4(1H,3H)-diones | [52] |
| [HDBU][Lae] | - | |||
| [HDBN][Lae] | - | |||
| [P4444][Lae] | - | |||
| [HTMG][Iso] | - | |||
| [HTMG][LA] | - | |||
| [HTMG][Imi] | - | |||
| [HTMG][Glut] | - |
| IL | Synthesis | Physicochemical Properties * | Application | Ref. |
|---|---|---|---|---|
| [Ch][LSar] | Salt metathesis reaction | - | Antimicrobial activity | [65] |
| [Ch][Doc] | - | |||
| [Ch][Ger] | Salt metathesis reaction | - | Oral delivery of sorafenib | [55] |
| [Ch][Mali] | Salt metathesis reaction | - | Dermal delivery of hyaluronic acid (In vitro skin penetration through porcine skin, in vivo skin protection effect and skin irritation tests) | [1] |
| [Ch][Sorb] | - | |||
| [Ch][Mal] | Tg * = −77.6 °C | |||
| [Ch][Suc] | - | |||
| [Ch][Lac] | - | |||
| [Ch][Ger] | Tg = 39.5 °C | |||
| [Ch][Cit] | Tg = −60.5 °C | |||
| [Ch][Ole] | - | |||
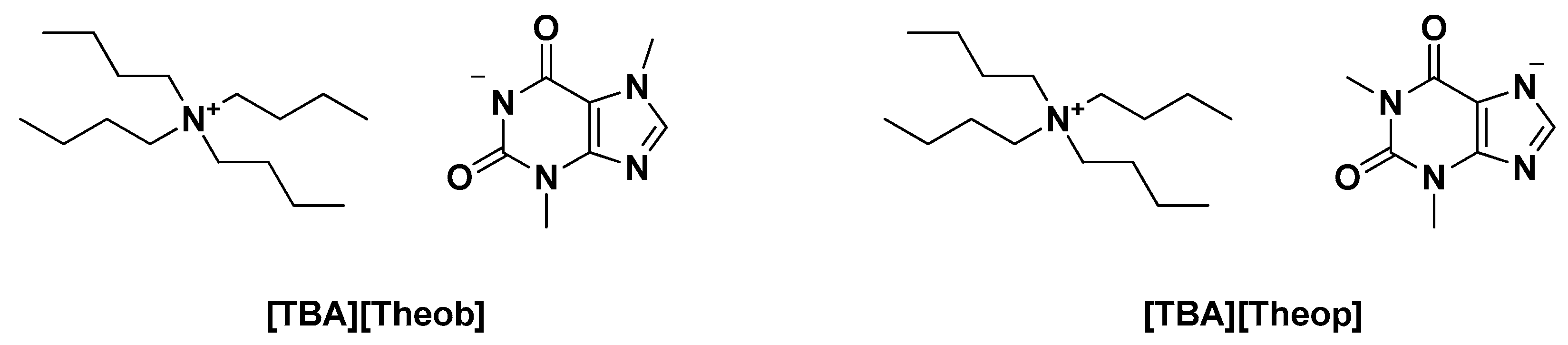
| [TBA][Theob] | Neutralization reaction | Tm * = 377.8 K Td * = 457 K ΔHm = 50,920 J mol−1 | Ecotoxicity against the microalgae Raphidocelis subcapitata, formation of aqueous biphasic systems, solubility enhancers | [2] |
| [TBA][Theop] | Tm = 370.6 K Td = 486 K ΔHm = 36,853 J mol−1 | |||
| [TBA][Xan] | Tm = 487 K Td = 495 K | |||
| [TBA][Ur] | Td = 505 K | |||
| [Car][Asc] | protonation reaction of a zwitterionic form of L-carnitine with a proper antioxidant acid | Tg = 38.1 °C Tonset5 * = 175 °C Tonset50 = 226 °C | Antioxidant activity (DPPH, ABTS, FRAP, CUPRAC, chelation of ferrous (II), inhibition of xanthine oxidase) | [3] |
| [Car][Proc] | Tg = 30.8 °C Tonset5 = 187 °C Tonset50 = 213 °C | |||
| [Car][Gen] | Tg = 9.7 °C Tonset5 = 205 °C Tonset50 = 231 °C | |||
| [Car][Gal] | Tg = 43.0oC Tonset5 = 186oC Tonset50 = 235oC | |||
| [Car][Syr] | Tg = 45.7 °C Tonset5 = 221 °C Tonset50 = 250 °C | |||
| [Car][Cou] | Tg = 27.9 °C Tonset5 = 189 °C Tonset50 = 294 °C | |||
| [Car][Caf] | Tg = 31.9 °C Tonset5 = 182 °C Tonset50 = 302 °C | |||
| [Car][Fer] | Tg = 21.6 °C Tonset5 = 163 °C Tonset50 = 202 °C | |||
| [Car][Sin] | Tg = 47.3 °C Tonset5 = 193 °C Tonset50 = 249 °C | |||
| [Ch][Val] | Neutralization reaction | - | Solubility of ibuprofen and naproxen | [57] |
| [Ch][Gal] | - | |||
| [Ch][Sal] | - | |||
| [Ch][Ger] | Salt metathesis reaction | Tg = −68 °C Conductivity = ∼1.3 mS cm−1 | Antimicrobiological activity, KLK5 inhibition, human cadaver skin permeation evaluation. Dermal toxicity in minipigs Cosmetic study (Clinical evaluation in human volunteers) | [56] |
| [Ch][Ala] | Not mentioned | - | Planktonic and biofilm growth control of Bacillus cereus and Pseudomonas fluorescens | [63] |
| [Ch][Gly] | - | |||
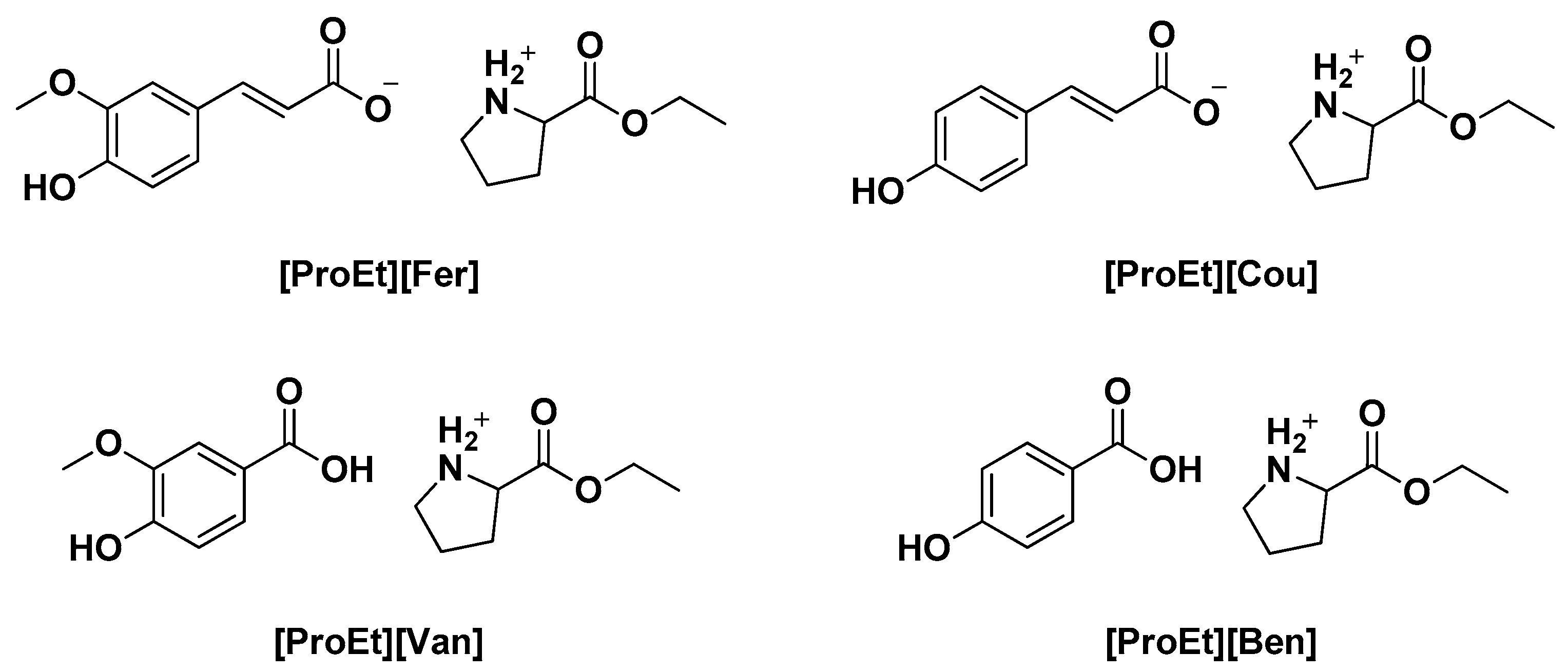
| [ProEt][Fer] | Neutralization reaction | Tg= 6.6 °C Tm = 52.7 °C Tonset = 113.6 °C | Solubility of luteonin in IL Food preservation test | [5] |
| [ProEt][Cou] | Tg = 1.5 °C Tm = 86.4 °C Tonset = 116.4 °C | |||
| [ProEt][Ben] | Tg = 4.2 °C Tm = 127 °C Tonset = 141.5 °C | |||
| [ProEt][Van] | Tg = 3.6 °C Tm = 110.0 °C Tonset = 138.4 °C | |||
| [Ch][Pro] | Salt metathesis reaction | Tg = −59 °C TODT * = 179 °C Td = 231 °C | Solubility enhancement of poorly water-soluble drug, Zafirlukast (ZFL) | [4] |
| [Ch][Ala] | Tg = −52 TODT = 145 °C Td = 206 | |||
| [Ch][Met] | Tg = −63.29 °C TODT = 168.5 °C Td = 281 °C | |||
| [Ch][Ser] | Neutralization reaction | - | Solubility οf APIs, Cytotoxicity, in vitro permeation behavior | [58] |
| [Ch][Ile] | - | |||
| [Ch][Ala] | - | |||
| [Ch][Gly] | - | |||
| [Ch][Lys] | - | |||
| [Ch][Asp] | - | |||
| [Ch][Ibu] | Neutralization reaction | Tm = 70.89 °C | Solubility studies, cytotoxicity assays, Hemolytic activity, protein albumin denaturation assay, cyclooxygenases (COX-1 and COX-2) inhibition Assays | [59] |
| [Ch][Tr] (2:1) | Salt metathesis reaction | Tg = 79.8 °C Td = 117.4 °C | Photostability studies, solubility studies, preparation of o/w emulsions, in vitro drug release studies, skin permeation test | [60] |
| [Ch][seco-Amx] | Neutralization, salt metathesis reaction | Tm = 143–144 °C | Antimicrobial activity | [40] |
| [Ch][seco-Pen] | Tm = 69–71 °C | |||
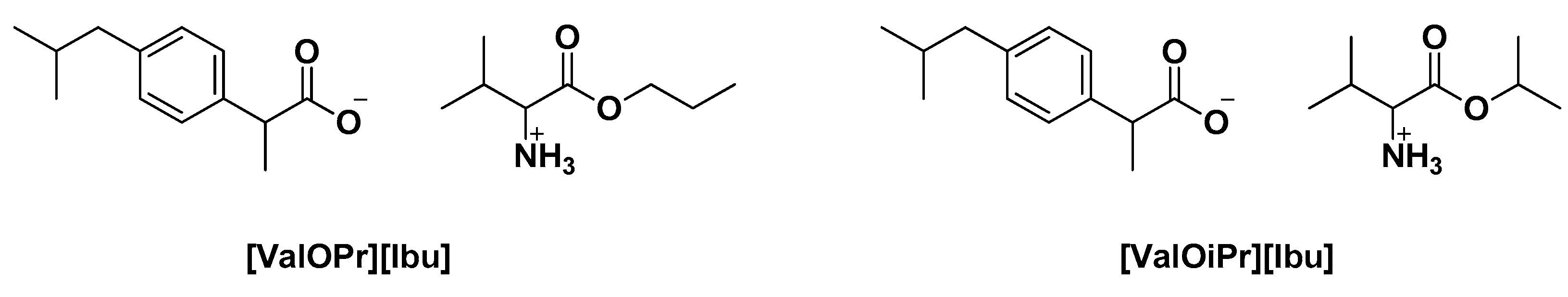
| [ValOEt][Ibu] | Neutralization reaction | Tm = 77.9 °C | Solubility studies, determination of partition coefficient Skin electrical impedance Skin permeation studies, accumulation in the skin | [39] |
| [ValOPr][Ibu] | Tm = 79.81 °C | |||
| [ValOiPr][Ibu] | Tm = 78.01 °C | |||
| [ValOBu][Ibu] | Tm = 76.80 °C | |||
| [ValOAm][Ibu] | Tm = 73.81 °C | |||
| [ValOHex][Ibu | Tm = 67.35 °C | |||
| [Ch][Cip] | Salt metathesis reaction | Tm = 111.2 °C | Solubility, critical micelle concentration, cytotoxicity and antimicrobial activity studies | [61] |
| [Ch][Nor] | Tg= 54.8 °C Tm = 94.5 °C | |||
| [Ch][Fer] | Neutralization reaction | Td = 105 °C | Protonation equilibria and solubility, antioxidant activity, cytotoxicity, DFT calculations | [64] |
| [Ch][Sin] | Td = 103 °C | |||
| [Ch][Caf] | Tm = 141 °C | |||
| Td = 148 °C | ||||
| [Ch][o-Coum] | Td = 180 °C | |||
| [Ch][m-Coum] | Td = 132 °C | |||
| [Ch][p-Coum] | Td = 118 °C | |||
| [Lid][Sal] | Neutralization reaction | Tg = 3.7 °C | Development of electrospun nanofibers loaded with ILs | [62] |
| Td = 170–250 °C | ||||
| n * = 5836 × 10−4 mPa s (25 °C) | ||||
| d * = 1.12741 g cm−3 (45 °C) | ||||
| k * = 0.4 × 104 mS cm−1 (40 °C) | ||||
| [Prc][Sal] | Tg = −21.6 °C, | |||
| Td = 200–270 °C | ||||
| d = 1.18324 g cm−3 (40 °C) | ||||
| η = 137.67 Pa s (50 °C) | ||||
| k = 3.51 μS cm−1 (55 °C) | ||||
| [Tol][DecAla] | Salt metathesis reaction | Tg = −16 °C | Solubility studies, in vitro lipolysis studies, in vitro digestibility, pharmacokinetic studies | [41] |
| Tm = 90–178 °C | ||||
| [Tol][DecPhe] | Tg = −6 °C | |||
| Tm = 88–174 °C |
| IL | Synthesis | Physicochemical Properties * | Application | Ref. |
|---|---|---|---|---|
| [Ch][Gly] | Neutralization reaction | - | Valorization of Chestnut Shell Waste | [69] |
| [Bmim][LSar] | Salt metathesis and neutralization reactions | - | Ιonic liquid dispersant for the effective oil spill remediation | [74] |
| [BDP][DDBS] | - | |||
| [TBA][Cit] | - | |||
| [TBA][PP] | - | |||
| [TBA][EEG] | - | |||
| [Chol-C6][Lev] | Salt metathesis reaction | Tg * = − 79.80 °C ENR * = 52.92 kcal/mol | Absorption of toluene, dichloromethane and methyl ethyl ketone. Determination of vapor–liquid partition coefficients | [42] |
| [Chol-C8][Lev] | Tg = − 82.59 °C ENR = 53.88 kcal/mol | |||
| [Chol-C6][Lac] | Tg = − 73.07 °C ENR = 52.54 kcal/mol | |||
| [Chol-C8][Lac] | Tg = − 68.69 °C ENR = 52.99 kcal/mol | |||
| GCP-IL | Neutralization reaction | - | Demulsification of Heavy Crude Oil Emulsions | [75] |
| GRB-IL | - | |||
| [Ch][Fu] | Salt metathesis reaction | - | Selective coagulation of κ-carrageenan from Kappaphycus alvarezii extract | [67] |
| [Ch][Adi] | - | |||
| [Ch][Cap] | - | |||
| [Ch][Capl] | - | |||
| [Ch][Capr] | - | |||
| [Ch][Lau] | - | |||
| [Ch][Ala] | Neutralization reaction | - | Pretreatment of corn stover and lignin depolymerization by a bi-enzyme system | [68] |
| [Ch][Gly] | ||||
| [Ch][Lys] | ||||
| [Ch][Lys] | Neutralization reaction | - | Delignification Rice and Wheat Residues for production of food-grade glucose | [70] |
| [DMBA][HSO4] | - | |||
| [Ch][Gly] | Salt metathesis reaction | η * = 1230 cP (25 °C) d * = 1.156 g/cm3 | CO2 capture | [66] |
| [Ch][Ala] | η = 720 cP (25 °C) d = 1.130 g/cm3 | |||
| [Ch][Ser] | η = 12,500 cP (25 °C) d = 1.201 g/cm3 | |||
| [Ch][Pro] | η = 9810 cP (25 °C) d = 1.138 g/cm3 | |||
| [Ch][Phe] | η = 55,300 cP (25 °C) d = 1.143 g/cm3 | |||
| [Ch][Sar] | η = 1058 cP (25 °C) d = 1.116 g/cm3 | |||
| [2-HEA][OAc] | Simulation | - | Optimization of agave bagasse pretreatment in a one-pot ethanol production process | [71] |
| [C2C1Im][OAc] | - | |||
| [Ch][For] | Simulation | - | COSMO–RS and MD simulations of lignin dissolution in ILs | [73] |
| [Ch][Ace] | d = 1.10 g/cm3 | |||
| [Ch][But] | d = 1.07 g/cm3 | |||
| [Ch][Hex] | d = 1.02 g/cm3 | |||
| [Ch][Oct] | - | |||
| [Ch][Lys] | d = 1.09 g/cm3 | |||
| [Ch][Ace] | Purchased | - | Delignification of sugar baggasse and recovery and regenerartion of IL | [72] |
| Cholinium + 50 anions (amino/carboxylic acids) | Simulation | - | COSMO RS Simulation for Gas absorption, liquid-liquid extraction | [6] |
| IL | Synthesis | Physicochemical Properties | Application | Ref. |
|---|---|---|---|---|
| [TMG][His] | Neutralization reaction | Td *= 302 °C Kinematic viscosity = 256.07 mm2 s−1 (40 °C) Kinematic viscosity = 19.780 mm2 s−1 (100 °C) Viscosity index = 88 d * = 1.1654 g/cm3 (40 °C) | Νoncorrosive lubricant additives for tribological performance | [76] |
| [TMG][Glm] | Td = 338 °C Kinematic viscosity = 282.10 mm2 s−1 (40 °C) Kinematic viscosity = 21.74 mm2 s−1 (100 °C) Viscosity index = 93 d = 1.1796 g/cm3 (40 °C) | |||
| [TMG][Asp] | Td = 304 °C Kinematic viscosity = 325.26 mm2 s−1 (40 °C) Kinematic viscosity = 23.244 mm2 s−1 (100 °C) Viscosity index = 89 d = 1.2063 g/cm3 (40 °C) | |||
| [C3mim][Tf2N] | Salt metathesis reaction | - | Potential high-temperature lubricants in steel-steel tribo-contacts | [77] |
| [C5mim][Tf2N] | Td = 446 °C | |||
| [C6mim][Tf2N] | Td = 448 °C | |||
| [C8mim][Tf2N] | Td = 450 °C | |||
| [C10mim][Tf2N] | Td = 451 °C | |||
| [C8mim][Sal] | - | |||
| [C10mim][Sac] | - | |||
| [P666,14][Cl] | - | |||
| [P666,14][Tf2N] | Td = 408 °C | |||
| [P666,14][Benz] | Td = 379°C | |||
| [P666,14][Sal] | Td = 371 °C | |||
| [P666,14][Sac] | Td = 410 °C | |||
| [P666,14][Cyc] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzani, A.; Karadendrou, M.-A.; Kalafateli, S.; Kakokefalou, V.; Detsi, A. Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals 2022, 12, 1776. https://doi.org/10.3390/cryst12121776
Tzani A, Karadendrou M-A, Kalafateli S, Kakokefalou V, Detsi A. Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals. 2022; 12(12):1776. https://doi.org/10.3390/cryst12121776
Chicago/Turabian StyleTzani, Andromachi, Maria-Anna Karadendrou, Styliani Kalafateli, Vasiliki Kakokefalou, and Anastasia Detsi. 2022. "Current Trends in Green Solvents: Biocompatible Ionic Liquids" Crystals 12, no. 12: 1776. https://doi.org/10.3390/cryst12121776
APA StyleTzani, A., Karadendrou, M.-A., Kalafateli, S., Kakokefalou, V., & Detsi, A. (2022). Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals, 12(12), 1776. https://doi.org/10.3390/cryst12121776








