Facile One-Pot Hydrothermal Synthesis of Hierarchical MoS2/α-MnS Nanocomposites with Good Cycling Performance as Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MoS2/α-MnS
2.3. Electrochemical Measurements
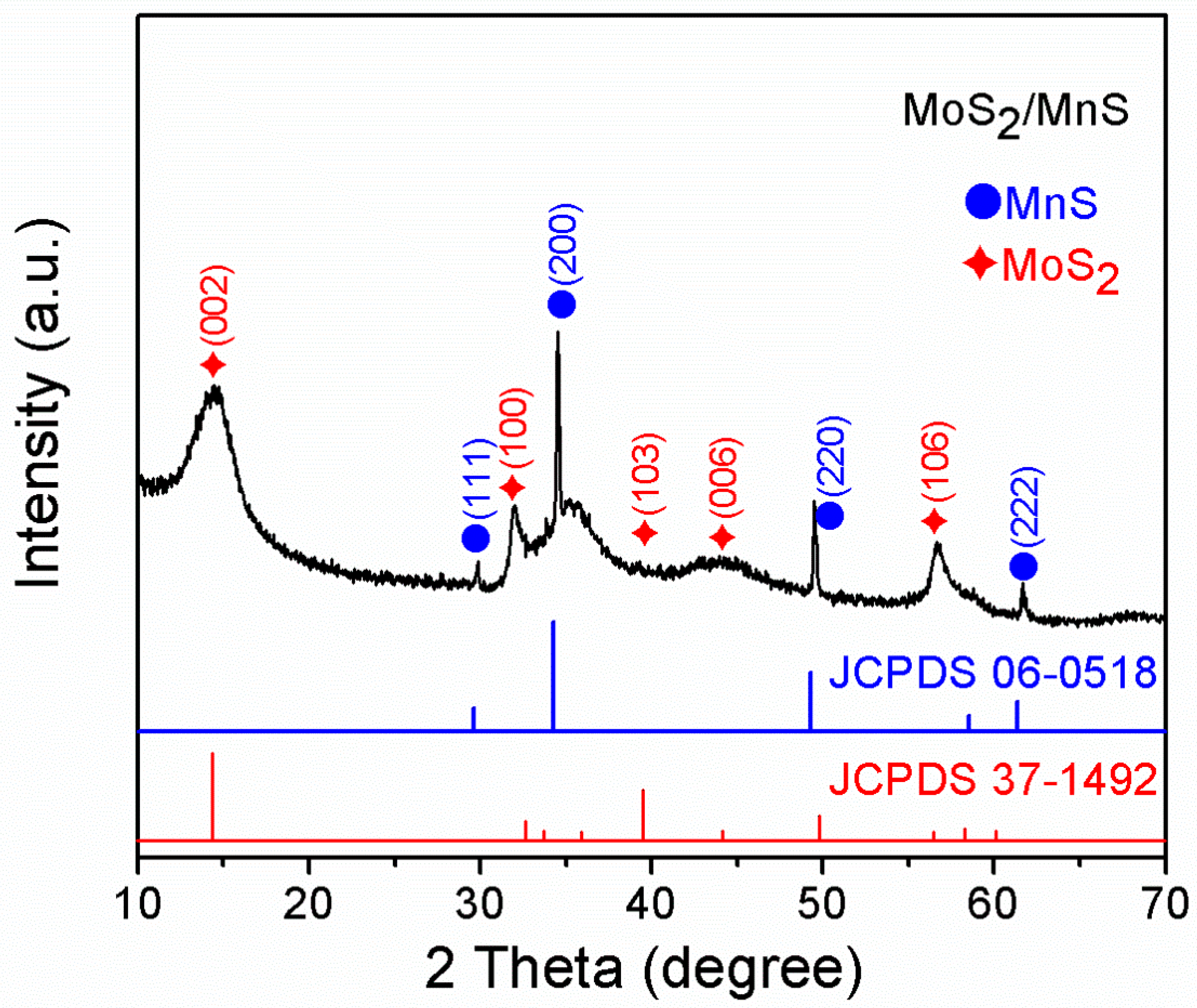
2.4. Materials Characterization
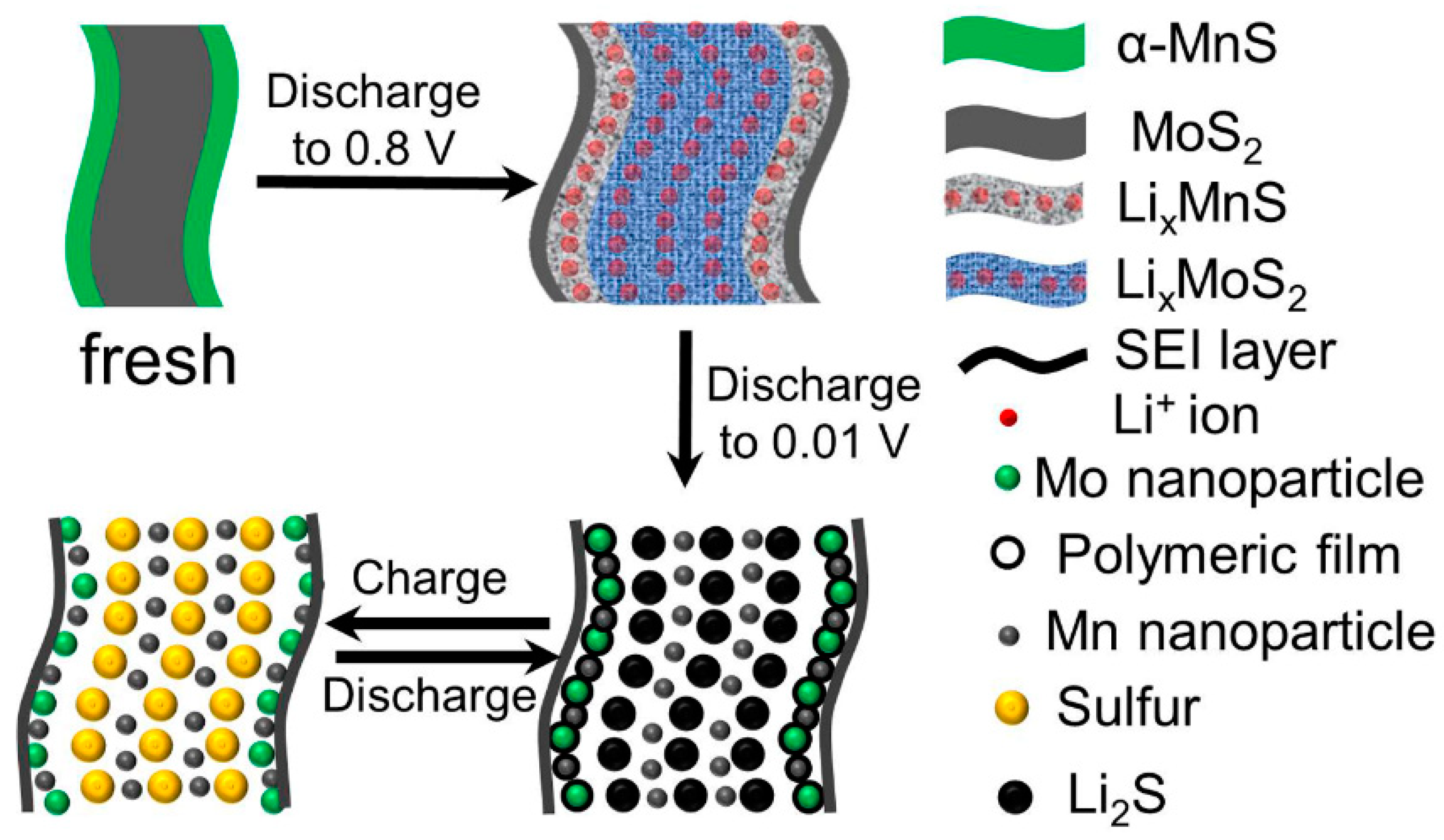
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mo, R.W.; Li, F.; Tan, X.Y.; Xu, P.C.; Tao, R.; Shen, G.R.; Lu, X.; Liu, F.; Shen, L.; Xu, B.; et al. High-quality mesoporous graphene particles as high-energy and fast-charging anodes for lithium-ion batteries. Nat. Commun. 2019, 10, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Chung, S.H.; Zu, C. Lithium-sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Goodenough, J.B. Lithium insertion into transition-metal monosulfides: Tuning the position of the metal 4s band. J. Phys. Chem. C 2008, 112, 15060–15064. [Google Scholar] [CrossRef]
- Yan, J.M.; Huang, H.Z.; Zhang, J.; Liu, Z.J.; Yang, Y. A study of novel anode material CoS2 for lithium ion battery. J. Power Sources 2005, 146, 264–269. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; Angelis De, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Yamakawa, N.; Jiang, M.; Grey, C.P. Investigation of the conversion reaction mechanisms for binary copper (II) compounds by solid-state NMR spectroscopy and X-ray diffraction. Chem. Mater. 2009, 21, 3162–3176. [Google Scholar] [CrossRef]
- Liao, F.; Światowska, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Zanna, S.; Marcus, P. Electrochemical lithiation and passivation mechanisms of iron monosulfide thin film as negative electrode material for lithium-ion batteries studied by surface analytical techniques. Appl. Surf. Sci. 2013, 283, 888–899. [Google Scholar] [CrossRef]
- Rui, X.H.; Tan, H.T.; Yan, Q.Y. Nanostructured metal sulfides for energy storage. Nanoscale 2014, 6, 9889–9924. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Feng, X.; Zhang, N.; Seok, J.; Abruña, H.D. Understanding conversion-type electrodes for lithium rechargeable batteries. Acc. Chem. Res. 2018, 51, 273–281. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Kim, Y.; Cho, J. Nanostructured transition metal sulfides for lithium ion batteries: Progress and challenges. Nano Today 2014, 9, 604–630. [Google Scholar] [CrossRef]
- Li, X.F.; Shen, J.F.; Li, N.; Ye, M.N. Fabrication of γ-MnS/rGO composite by facile one-pot solvothermal approach for supercapacitor applications. J. Power Sources 2015, 282, 194–201. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.J.; Yang, X.Q.; Xun, S.D.; Liu, G.; Koech, P.K.; Liu, J.; Lemmon, J.P. Electrochemically induced high capacity displacement reaction of PEO/MoS2/graphene nanocomposites with lithium. Adv. Funct. Mater. 2011, 21, 2840–2846. [Google Scholar] [CrossRef]
- Fang, X.P.; Yu, X.Q.; Liao, S.F.; Shi, Y.F.; Hu, Y.S.; Wang, Z.X.; Stucky, G.D.; Chen, L.Q. Lithium storage performance in ordered mesoporous MoS2 electrode material. Microporous Mesoporous Mater. 2012, 151, 418–423. [Google Scholar] [CrossRef]
- Zhang, D.A.; Wang, Q.; Wang, Q.; Sun, J.; Xing, L.L.; Xue, X.Y. High capacity and cyclability of hierarchical MoS2/SnO2 nanocomposites as the cathode of lithium-sulfur battery. Electrochim. Acta 2015, 173, 476–482. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.D.; Xu, H.Y.; Qian, Y.T.; Yang, J. Enhanced lithium storage performances of hierarchical hollow MoS2 nanoparticles assembled from nanosheets. ACS Appl. Mater. Interfaces 2013, 5, 1003–1008. [Google Scholar] [CrossRef]
- Liu, H.; Su, D.W.; Zhou, R.F.; Sun, B.; Wang, G.X.; Qiao, S.Z. Highly ordered mesoporous MoS2 with expanded spacing of the (002) crystal plane for ultrafast lithium ion storage. Adv. Energy Mater. 2012, 2, 970–975. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, C.; Yang, X.Y.; Xiao, G.J.; Zou, B.; Yang, J.W.; Wang, C.L. Studies on intrinsic phase-dependent electrochemical properties of MnS nanocrystals as anodes for lithium-ion batteries. J. Power Sources 2017, 338, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Tarascon, J.M. Rationalization of the low-potential reactivity of 3d-metal-based inorganic compounds toward Li. J. Electrochem. Soc. 2002, 149, 1212–1217. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Zhang, X.W.; Bustillo, K.; Niu, K.Y.; Gammer, C.; Xu, J.; Zheng, H.M. In situ study of lithiation and delithiation of MoS2 nanosheets using electrochemical liquid cell transmission electron microscopy. Nano Lett. 2015, 15, 5214–5220. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, W.X.; Li, Z.; Hu, X.L.; Yuan, L.X.; Huang, Y.H. Coral-like α-MnS composites with N-doped carbon as anode materials for high-performance lithium-ion batteries. J. Mater. Chem. 2012, 22, 24026–24033. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Li, D.; Guo, P.Q.; Liu, D.Q.; Shang, X.N.; Lv, M.Z.; He, D.Y. Nanoscale alpha-MnS crystallites grown on N-S co-doped rGO as a long-life and high-capacity anode material of Li-ion batteries. Appl. Surf. Sci. 2017, 416, 858–867. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.L.; Wei, Y.Z.; Song, X.Y.; Fub, Y.B.; Battaglia, V.S. A ZnS nanocrystal/reduced graphene oxide composite anode with enhanced electrochemical performances for lithium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 30630–30642. [Google Scholar] [CrossRef]
- Qu, B.H.; Ji, G.; Ding, B.; Lu, M.H.; Chen, W.X.; Lee, J.Y. Origin of the increased Li+-storage capacity of stacked SnS2/graphene nanocomposite. ChemElectroChem 2015, 2, 1138–1143. [Google Scholar] [CrossRef]
- Zhang, J.T.; Yu, L.; Lou, X.W. Embedding CoS2 nanoparticles in N-doped carbon nanotube hollow frameworks for enhanced lithium storage properties. Nano Res. 2017, 10, 4298–4304. [Google Scholar] [CrossRef]
- Xia, H.C.; Li, K.X.; Guo, Y.Y.; Guo, J.H.; Xu, Q.; Zhang, J.N. CoS2 nanodots trapped within the graphitic structured N-doped carbon spheres with efficient performances for lithium storage. J. Mater. Chem. A 2018, 6, 7148–7154. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Yan, D.; Xu, H.Y.; Yang, J.; Qian, Y.T. Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: A high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale 2015, 7, 3520–3525. [Google Scholar] [CrossRef]
- Yang, X.; Chan, C.Y.; Xue, H.T.; Xu, J.; Tang, Y.B.; Wang, Q.; Wong, T.L.; Lee, C.S. One-pot synthesis of graphene/In2S3 nanoparticle composites for stable rechargeable lithium ion battery. Crystengcomm 2013, 15, 6578–6584. [Google Scholar] [CrossRef]
- Tan, R.; Yang, J.L.; Hu, J.T.; Wang, K.; Zhao, Y.; Pan, F. Core-shell nano-FeS2@N-doped graphene as an advanced cathode material for rechargeable Li-ion batteries. Chem. Commun. 2015, 52, 986–989. [Google Scholar] [CrossRef]
- Zhu, J.L.; Li, Y.Y.; Kang, S.; Shen, P.K. One-step synthesis of Ni3S2 nanoparticles wrapped with in situ generated nitrogen-self-doped graphene sheets with highly improved electrochemical properties in Li-ion batteries. J. Mater. Chem. A 2014, 2, 3142–3147. [Google Scholar] [CrossRef]
- Cao, X.; Li, H.; He, J.; Kang, L.; Jiang, R.; Shi, F.; Xu, H.; Lei, Z.; Liu, Z. Preparation and formation process of α-MnS@MoS2 microcubes with hierarchical core/shell structure. J. Colloid Interface Sci. 2017, 507, 18–26. [Google Scholar] [CrossRef]
- Chen, D.Z.; Quan, H.Y.; Luo, X.B.; Luo, S.L. 3-D graphene cross-linked with mesoporous MnS clusters with high lithium storage capability. Scr. Mater. 2014, 76, 1–4. [Google Scholar] [CrossRef]
- Wang, D.D.; Cai, D.P.; Qu, B.H.; Wang, T.H. Rational combination of α-MnS/rGO nanocomposites for high-performance lithium-ion batteries. CrystEngComm 2016, 18, 6200–6204. [Google Scholar] [CrossRef]
- Yu, J.; Tang, H. Solvothermal synthesis of novel flower-like manganese sulfide particles. J. Phys. Chem. Solids 2008, 69, 1342–1345. [Google Scholar] [CrossRef]
- Biswas, S.; Kar, S.; Chaudhuri, S. Solvothermal synthesis of α-MnS single crystals. J. Cryst. Growth 2005, 284, 129–135. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dollé, M.; Tarascon, J.M. On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J. Electrochem. Soc. 2002, 149, A627–A634. [Google Scholar] [CrossRef]
- Kilibarda, G.; Szabó, D.V.; Schlabach, S.; Winkler, V.; Bruns, M.; Hanemann, T. Investigation of the degradation of SnO2 electrodes for use in Li-ion cells. J. Power Sources 2013, 233, 139–147. [Google Scholar] [CrossRef]
- Yang, L.C.; Wang, S.N.; Mao, J.J.; Deng, J.W.; Gao, Q.S.; Tang, Y.; Schmidt, O.G. Hierarchical MoS2/polyaniline nanowires with excellent electrochemical performance for lithium-ion batteries. Adv. Mater. 2013, 25, 1180–1184. [Google Scholar] [CrossRef]
- Sen, U.K.; Mitra, S. High-rate and high-energy-density lithium-ion battery anode containing 2D MoS2 nanowall and cellulose binder. ACS Appl. Mater. Interfaces 2013, 5, 1240–1247. [Google Scholar] [CrossRef]
- Xu, D.; Jiao, R.R.; Sun, Y.W.; Sun, D.Z.; Zhang, X.X.; Zeng, S.Y.; Di, Y.Y. L-cysteine-assisted synthesis of urchin-Like γ-MnS and its lithium storage properties. Nanoscale Res. Lett. 2016, 11, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.P.; Yin, Z.Y.; Zhu, J.X.; Huang, X.; Wu, X.J.; Zeng, Z.Y.; Yan, Q.Y.; Zhang, H. A general method for the large-scale synthesis of uniform ultrathin metal sulphide nanocrystals. Nat. Commun. 2012, 3, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.W.; Chen, C.H.; Zhang, J.; Ren, F. Novel electrochemical milling method to fabricate copper nanoparticles and nanofibers. Chem. Mater. 2005, 17, 5242–5245. [Google Scholar] [CrossRef]
- Hassan, M.F.; Guo, Z.P.; Chen, Z.X.; Liu, H.K. α-Fe2O3 as an anode material with capacity rise and high rate capability for lithium-ion batteries. Mater. Res. Bull. 2011, 46, 858–864. [Google Scholar] [CrossRef]
- Xing, L.L.; He, B.; Nie, Y.X.; Deng, P.; Cui, C.X.; Xue, X.Y. SnO2–MnO2–SnO2 sandwich-structured nanotubes as high-performance anodes of lithium ion battery. Mater. Lett. 2013, 105, 169–172. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.-A.; Zhao, X.; Zhu, L.; Wang, Q. Facile One-Pot Hydrothermal Synthesis of Hierarchical MoS2/α-MnS Nanocomposites with Good Cycling Performance as Anode Materials for Lithium-Ion Batteries. Crystals 2022, 12, 1763. https://doi.org/10.3390/cryst12121763
Zhang D-A, Zhao X, Zhu L, Wang Q. Facile One-Pot Hydrothermal Synthesis of Hierarchical MoS2/α-MnS Nanocomposites with Good Cycling Performance as Anode Materials for Lithium-Ion Batteries. Crystals. 2022; 12(12):1763. https://doi.org/10.3390/cryst12121763
Chicago/Turabian StyleZhang, De-An, Xishan Zhao, Lin Zhu, and Qi Wang. 2022. "Facile One-Pot Hydrothermal Synthesis of Hierarchical MoS2/α-MnS Nanocomposites with Good Cycling Performance as Anode Materials for Lithium-Ion Batteries" Crystals 12, no. 12: 1763. https://doi.org/10.3390/cryst12121763
APA StyleZhang, D.-A., Zhao, X., Zhu, L., & Wang, Q. (2022). Facile One-Pot Hydrothermal Synthesis of Hierarchical MoS2/α-MnS Nanocomposites with Good Cycling Performance as Anode Materials for Lithium-Ion Batteries. Crystals, 12(12), 1763. https://doi.org/10.3390/cryst12121763





