1. Introduction
A further exploration of the Arctic, Siberia and Canada requires the development of methods of protecting constructions, communication lines, facilities and machinery from corrosion, pollution and ice. One of the passive protection methods that have no energy cost is the use of hydrophobic and superhydrophobic coatings. On the one hand, they possess strong water-repellent properties and the capability of self-cleaning, yet on the other hand, they can fulfill the role of anti-icing coatings. Due to the small size of the contact angle area between the drop and the surface, the heat exchange with a cold surface is significantly slowed—the drop has time to slip down from the coating and does not freeze.
In addition, anti-icing properties can appear as a result of a delayed drop freeze due to the barrierless mechanism of drop formation, typical for superhydrophobic surfaces, as well as the shift of the triple point of water toward low temperatures and low ice adhesion to the hydrophobic surface [
1].
There are several approaches to creating water-repellent coatings used separately or in combination with each other. The first one is the chemical approach which consists of applying a hydrophobic substance, usually teflon. It is the most simple and practical method, but it cannot be used to obtain a wetting angle exceeding 122 degrees. Later, the lotus effect [
2] was discovered. This method allows to achieve high values of the contact angle, but such surfaces are generally mechanically unstable. Finally, using the SLIPS (slippery liquid-infused porous surfaces) effect [
3] was suggested, which involves impregnating the porous surface with a water-repellent lubricant. The lubricant can also be an anti-icing agent. Although this method has the advantage of a low slip start angle of the drop, the coating slowly loses its properties due to the lubricant evaporating, being carried over by liquids and to the shear stress.
To reproduce these effects, the use of nanoparticles or a relief with nano-roughness of the surface is typical. Works in this field are being conducted and are quite extensively demonstrated in reviews [
4,
5,
6,
7,
8].
In work [
9], we suggested an approach that involved obtaining the coating from ground xerogel glued to the protected surface. It was suggested to produce the xerogel from carbon nanotubes (CNTs), which could eventually allow to combine all three main approaches. A high aspect ratio of CNTs would allow the reproduction of the lotus effect, and the high sorptive capacity of the xerogel would enable the use of a lubricant to impregnate it with an anti-icing agent or a water-repellent compound for the prompt repair of damaged spots. Moreover, CNTs themselves are hydrophobic and do not require any additional chemical processing. The approach was developed in work [
10], where the creation of xerogel involved the use of onion-like carbons (OLCs), obtained through a cheap and manufacturable method. It was suggested to replace gluing the xerogel with sputtering nanoparticles in the solvent and forming xerogel on the protected surface itself which raises the manufacturability of the coating preparation significantly but reveals the necessity of giving the surface an initial roughness of a specific degree. Anti-icing and sorptive properties of the suggested coating have been studied.
This work provides a further development of these ideas. The possibility of obtaining a double-layer hybrid coating based on carbon nanotubes (heating layer) and onion-like carbons (protective layer) has been researched. Such a combination is necessary for overcoming the main drawback of superhydrophobic coatings in the role of anti-icing ones—at temperatures of 20 °C and lower, most of them lose the capability of slowing down the ice formation. In their turn, heating coatings have a high energy cost. Their combination looks promising for practical use in extreme arctic conditions.
The suggested method for producing a superhydrophobic coating based on carbon nanoparticles is competitive with most approaches that also include carbon nanotubes, based on its features.
Additionally, the recommended application method—sticking prepared xerogel particles with a certain morphology to the prepared surface—is quite distinctive from others and has a nuanced outcome.
Growing a nanotube forest is a complicated job that requires specialized equipment, high temperatures, and a limited supply of resources. Another challenging task is moving the finished “CNT forest” to the protected surface. Additionally, vertically oriented CNTs hardly ever come into contact with one another, which lowers the coating’s desirable conductivity and sorption levels.
The CNT array also loses its superhydrophobic or anti-icing capabilities when moisture or frost condenses on the side surface.
Although applying a different method that involves spraying CNT dispersions into a polymer is simple, their good conductivity is only ensured at large CNT concentrations.
With our method, you can work with any kind of surface (you just need to pick an adhesive layer that adheres to it well enough), and you do not need particular conditions for CNT growing. The method’s complexity is not noticeably greater than those based on applying CNT dispersions, but it enables the achievement of better conductivity and sorption capacities.
The aerogel is reinforced by CNTs, and it has a high sorption capacity on its own. However, higher conductivity is promised by xerogel manufactured only from carbon nanoparticles.
2. Materials and Methods
2.1. Installation Description
Unlike our previous works, the coating was applied using a software-controlled automated machine and not manually. This decreased the role of the human factor, raised the statistical reliability of the obtained data and reduced the number of defects on the coating. The sputtering device consisted of the flask where the nanoparticles were subjected to deagglomeration through a cavitation effect caused by an ultrasonic dispenser with an effective power of 0.5 kW. The dispersion took approximately 10 min, and afterward, the obtained sol was put in an aerograph affixed to the programmable coordinate machine. By slowly moving the aerograph over the sample, we applied the sol with nanoparticles uniformly and smoothly to the sample.
2.2. Experiment
As carbon nanoparticles, we chose Taunit-MD CNTs made by NanoTechCenter LLC [
11], Tuball CNTs made by the OCSiAl [
12] company and OLC with a size of 20–40 nm. The synthesis method is described at length in work [
13].
The choice of CNT models was determined by their high aspect ratio, as it is necessary to achieve good conductivity. The heating layer was obtained through smearing the CNT agglomerates over an adhesive surface, which, in this case, was double-sided tape. The thickness of the heating layer equaled 0.1 mm in the case of Taunit-M and 0.2 mm in the case of Tuball.
The hydrophobic layer was obtained through two methods. The first one involves OLC sol sputtering onto the heating layer. Two options were researched—direct application and application onto a thin layer pad made of polyvinylchloride (PVC). Before sputtering, the PVC was processed using a polishing machine in order to achieve roughness of the relief; otherwise the air stream from the aerograph blew away the applied nanoparticles. The surface density of the OLC layer equaled 3.5 × 10−5 g·cm−2 and was determined through changes in the mass of the known area sample caused by the application of seven coating layers. The layers were sputtered one at a time after the previous layer dried up. If the experiment description does not specify otherwise, the application parameters of the hydrophobic layer are as follows: PVC roughness—28 µm, OLC concentration—2 mg per mL, solvent—hexane. If necessary, the layer pad is glued to the heating layer or affixed mechanically.
Changes in the roughness of the surface were conducted using an INTEGRA II scanning microscope in the tapping mode (in
Figure 1) and analyzed using a VEGA TESCAN 3 scanning electron microscope (in
Figure 2).
Hydrophobic properties of the samples were measured with an EasyDrop installation through the tangential method, and the surface resistance, with an RM3542 laboratory ohmmeter.
3. Results and Discussion
3.1. Experimental Results
Table 1 presents the data on hydrophobic and electrophysical properties of double-layer samples, obtained through sputtering without the PVC layer pad.
A superhydrophobic surface is considered to be [
14,
15] a surface on which the wetting contact angle is less than 150 degrees, and the slip start angle is less than 5 degrees.
In the case of Taunit-MD, achieving a superhydrophobic state happens similarly to the case of sputtering a single-layer OLC coating onto a metal [
10] and after applying 7–8 OLC layers. This once again confirms the conclusion from the previous work: in order to achieve a hydrophobic state, it is necessary to cover the whole surface relief with a uniform layer of nanoobjects, as the spots that are not covered during sputtering create defects that increase the range of properties of samples due to the natural roughness. Surface resistance of the sample equaled 80 Ω*cm.
While using the Tuball CNTs as a heating layer, the previously known [
10] dependence of hydrophobic properties on the number of OLC layers does not happen. A wide range of slip start angle values, the lack of its improvement upon further layer application at a nearly constant contact angle speaks of the formation of a large number of defects and surface irregularities. They can be caused both by a high level of contamination of Tuball CNTs with amorphous carbon and by a higher aspect ratio of this CNT model—it enables the formation of thicker and larger agglomerates that lead to the emergence of an excessively developed relief and numerous surface defects. However, surface resistance of the heating layer in this case equaled 5 Ω*cm, which also stemmed from a high aspect ratio.
The data on sputtering OLCs onto the protective PVC layer are presented separately, as, in that case, the heating layer has no impact on the hydrophobic one. This allows to study the emergence mechanism of hydrophobic structures in more detail.
Other things being equal, the relief that carbon nanoparticles form during agglomeration is determined by the following factors: solvent polarity, evaporation rate, aspect ratio of nanoparticles and their concentration. We start with a search for an optimal environment for dispersion and nanoparticle application. For this purpose, we checked widespread solvents with a high evaporation rate: hexane, acetone, ethanol. The results are shown in
Table 2.
The best results were achieved using a non-polar hexane solvent. In works [
9,
11], it was demonstrated that more polar solvents enable the emergence of a surface with a more developed morphology and empower the lotus effect. In this OLC experiment, the different behavior is caused by a low aspect ratio: unlike the nanotubes, they cannot intertwine and form a specific nanorelief in the shape of globules [
16]. Nevertheless, these globules can emerge as small agglomerates that were not crushed during dispersion, which is caused by the use of a non-polar solvent.
Unlike work [
9], the xerogel processed with acetone and ethanol does not seem to have a slip start angle of the drop, despite an evident hydrophobicity of the surface. We cannot explain this phenomenon at the moment.
Another key feature that affects the nanoparticle agglomeration is their concentration in the environment (
Table 3). The agglomeration rate of the nanoparticles grows non-linearly with the concentration.
Upon increasing the OLC concentration in a mixture, the rate of achieving a superhydrophobic state grows. Upon increasing the concentration beyond 200 mg per 50 mL, the contact angle already reached 150 degrees on the first layer, and the slip start angle of the drop fell below 10 degrees. However, at such a concentration, we already observed an irregularity of the sputtering and a larger number of the defective samples caused by the formation of agglomerates in the aerograph stream itself. Further increases in the concentration are pointless. A careful study of the presented dependencies once again highlights the importance of using a bulk substrate for sputtering. Any relief irregularities “cast a shadow” for the aerograph stream and force the application of more OLC layers because that is where defects appear.
Researching the influence of the roughness of the substrate (
Table 4) gave the same results qualitatively and quantitatively as in our previous work, so it is not worth spending much time on their discussion. Nevertheless, in order to understand the peculiarities of obtaining a double-layer coating, we should explain the formation mechanism of a superhydrophobic relief taking place during the coating layer application.
Drops start to slip from the sample when all irregularities are covered with nanoparticles. This explains the majority of the coating defects on samples with high roughness as well as the wide range of their characteristics. As the relief recesses on the sample are filled with nanoparticles (as a result of applying another layer), the relief approaches the optimum (at the micro-scale, the surface relief is defined by roughness elements covered with OLCs, and at the nano-scale, by the size of the OLC particles themselves). However, after a certain layer, the contact angle starts to decrease, which reflects a full overlap of the rough surface with a smooth layer of OLCs and is explained by the lack of multimodality (irregularities disappeared at the micro-scale) in the size distribution of relief elements.
Hydrophobic properties of samples obtained with the second method were not researched, as they were extensively covered in a previous work [
10].
3.2. Anti-Icing Properties
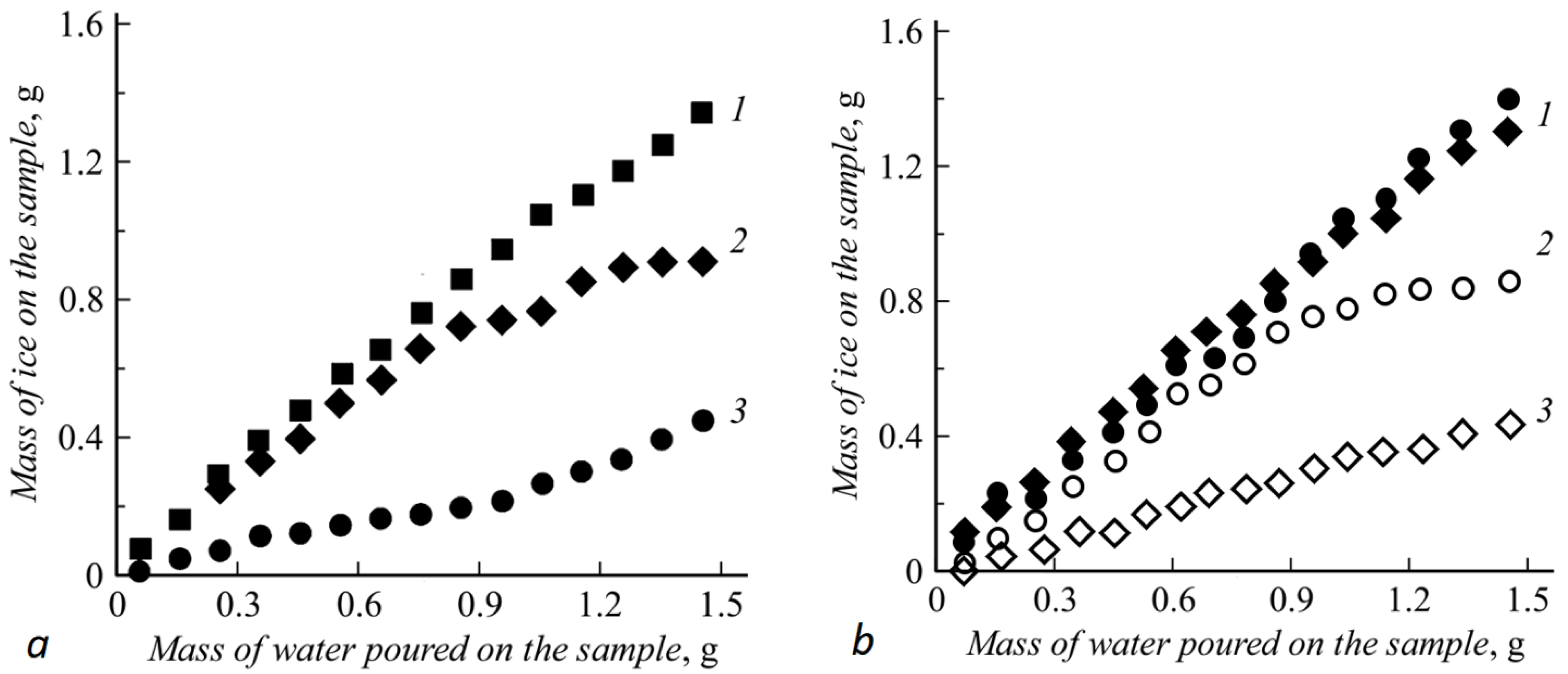
Samples (steel, a superhydrophobic layer, achieved through sputtering, a layer, achieved through gluing the xerogel powder and a double-layer sample) were affixed to the Peltier element in turn. Through the element, the samples were cooled down to the temperatures of −10 °C and −30 °C. A series of drops with a total volume of 0.1 ± 0.05 mL of water was dripped on the samples through a microdispenser. The drops slipped from the surface of the samples into a special basin, and their mass was measured. The cycle was repeated several times, and the weight of the water in the basin helped determine the mass of the liquid frozen on the surface of the sample. The experiment was repeated three times, and the dependence of the icing mass on the total drop mass was averaged. The results are presented in
Figure 3.
Anti-icing properties of a separate superhydrophobic layer are no different from the ones we obtained previously in work [
10] because the composition and the achievement technique are similar. We will not describe them in detail. At −10 °C, the superhydrophobic layer applied through sputtering turned out to be non-resistant to condensation of frost on the surface and in pores. As a result, falling drops linger on the ice crystals and become welded to them. Nevertheless, with a further increase in the icing mass, the ice separates from the sample surface and falls into the basin. The coating applied through the second method demonstrates a much lower ice accumulation rate, yet after defrosting, loses a large portion of its hydrophobic properties (contact angle decreases by 30–40 degrees).
At the temperature of −30 °C, almost all of the liquid poured onto the hydrophobic layer becomes welded to it. Only introducing the heating layer (U = 10 V, I = 1 A, the surface temperature increases till −10 °C) allows the return of the anti-icing properties into a working state corresponding to a higher temperature.
4. Conclusions
A simple method of obtaining a superhydrophobic coating from CNT and OLC nanoparticles, synthesized through a cheap and manufacturable method, was suggested. The coating possesses the properties of a superhydrophobic one. During the experiment, we managed to discover the optimal sputtering conditions and obtained superhydrophobic surfaces with a contact angle of more than 155 degrees with the slip start angle of 1.2 ± 0.5 degrees.
We suggested a method of creating hydrophobic surfaces from nanoparticles in works [
9,
10], but we have only now received practical confirmation of the possibility of the effective implementation of a duplex protective coating with a heating layer and a hydrophobic layer based on the proposed principles. The main difficulty is the necessity of creating a substrate for the hydrophobic layer of a specific roughness level. Both the protected object and the heating layer could serve as a substrate. In the case of the heating layer, the roughness was created by CNT agglomerates.
The local relief of the hydrophobic layer demonstrates a vivid hierarchical multiscale nature. The largest irregularities are defined by the relief of the substrate or the heating layer (28 ± 5 µm); average irregularities, by OLC particle agglomerates (0.9 ± 0.4 µm); and small irregularities, by the size of the OLC particles themselves (31.1 ± 10 nm) [
10]. In order to achieve the maximum water-repellent effect, it is necessary to choose the nanoparticle spraying conditions in a certain way so that these irregularities are close to the optimal size. The substrate relief can be prepared before applying the hydrophobic layer, and the size of the nanoparticle agglomerates is regulated by the degree of solvent polarity and the concentration of nanoparticles [
9,
10]. The size of the nanoparticles themselves is defined by the synthesis conditions.
The variation of the OLC concentration and the choice of solvent allows us to select the spraying conditions if it is impossible to form the required microrelief on the protected object. It is also possible to modernize the procedure of applying the heating layer to achieve the formation of the optimal relief in order to apply the hydrophobic layer, for example, trying to use the stamp when the heating layer is dried. We assume that it will help make the relief as smooth as possible and minimize the number of defects. Work in this field will be continued.
Superhydrophobic properties appear due to a considerable relief of nanoparticle agglomerates that reproduce the lotus effect and the non-polarity of C–C chemical bonds [
16]. The drop does not wet the surface of OLC agglomerates and stays in the Cassie state. The classic Cassie–Baxter model [
17] shows that minimizing the area of contact between the drop and the surface leads to an increase in the contact angle. Models that are more modern [
18] achieve similar results, taking the nanorelief into account only on the contact line of the three phases at the edge of the drop, but they are incapable of providing accurate qualitative assessments.
For practical application of the suggested coating, an increase in its mechanical durability is desirable. We assume that the simplest way of achieving it is to complement the xerogel with a polymer that would glue the nanoparticles together by covering them with a thin layer. Such procedures are well established for xerogel and are fairly common [
19].
Anti-icing properties of the coating were enhanced compared to the previous works. The majority of superhydrophobic coating lose their anti-icing properties at low temperatures because of the fact that their specific relief becomes covered with frost. A heating layer can nullify this effect.