Improvement of Hydrogenation and Dehydrogenation Kinetics of As-Cast AZ91 Magnesium Alloy via Twin Parallel Channel Angular Extrusion Processing
Abstract
1. Introduction
2. Materials and Methods
- For each test, 0.22 g powder was placed in the reactor and washed with hydrogen gas, followed by vacuuming to 10−2 mbar. The washing process was repeated three times.
- Afterward, it was placed under a highly enhanced vacuum condition with a pressure of 10−5 mbar. Then, the temperature was increased up to 150 °C. As the temperature increased, followed by the increase in the pressure, the vacuum pump worked for one hour to achieve the pressure of 10−5 mbar at 150 °C.
- Similar procedure was repeated as the temperature increased from 150 °C to 250 °C and from 250 °C to 350 °C, respectively, and the pressure was maintained at 10−5 mbar at the end of each temperature-rise step.
- Afterwards, hydrogen gas was injected into the sample at 350 °C under 30 bar pressure, and kept for 15 h.
- In the last stage, the sample powder was placed again under a vacuum of 10−5 mbar at 350 °C.
3. Results
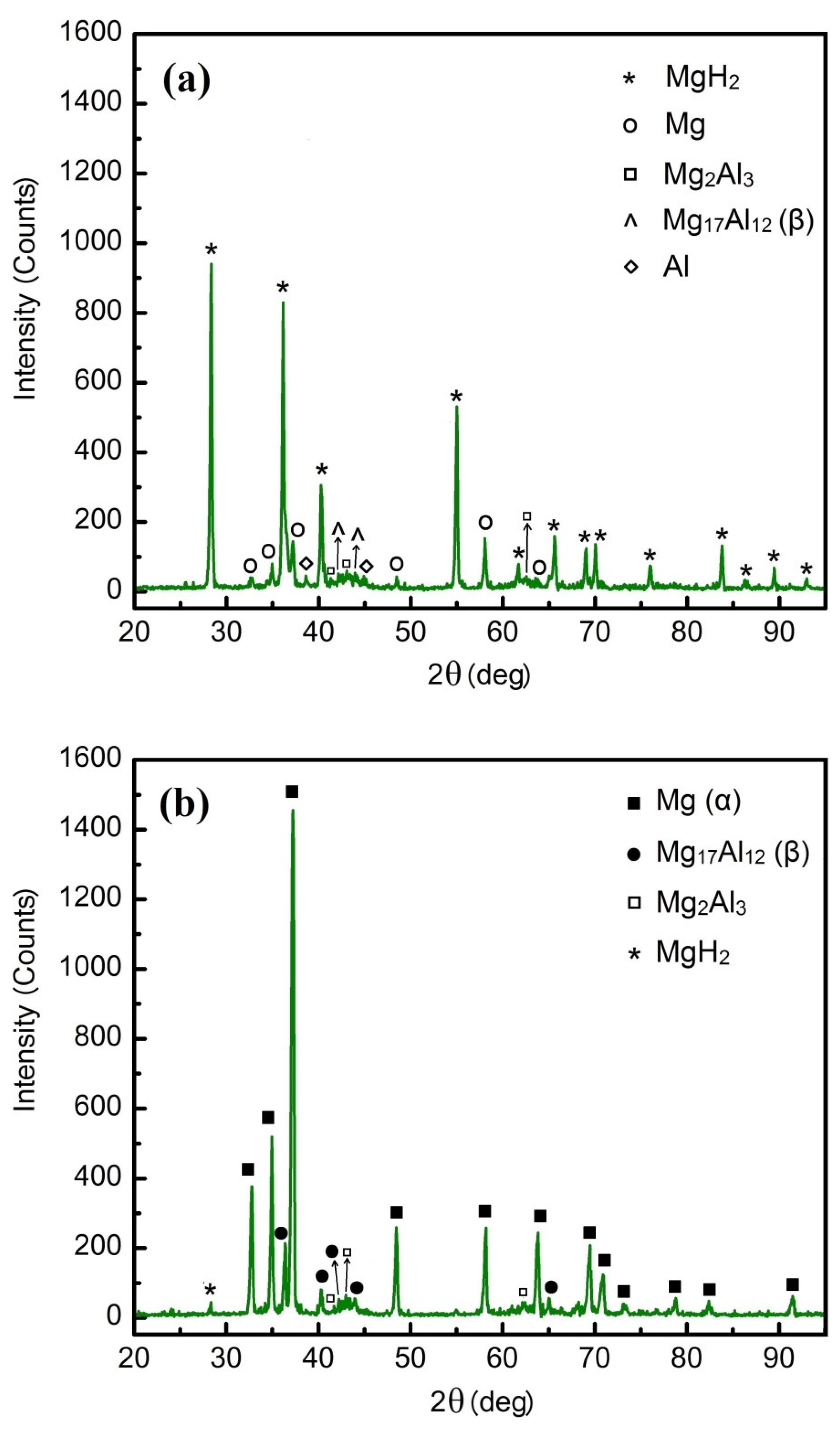
3.1. Microstructure Evolution
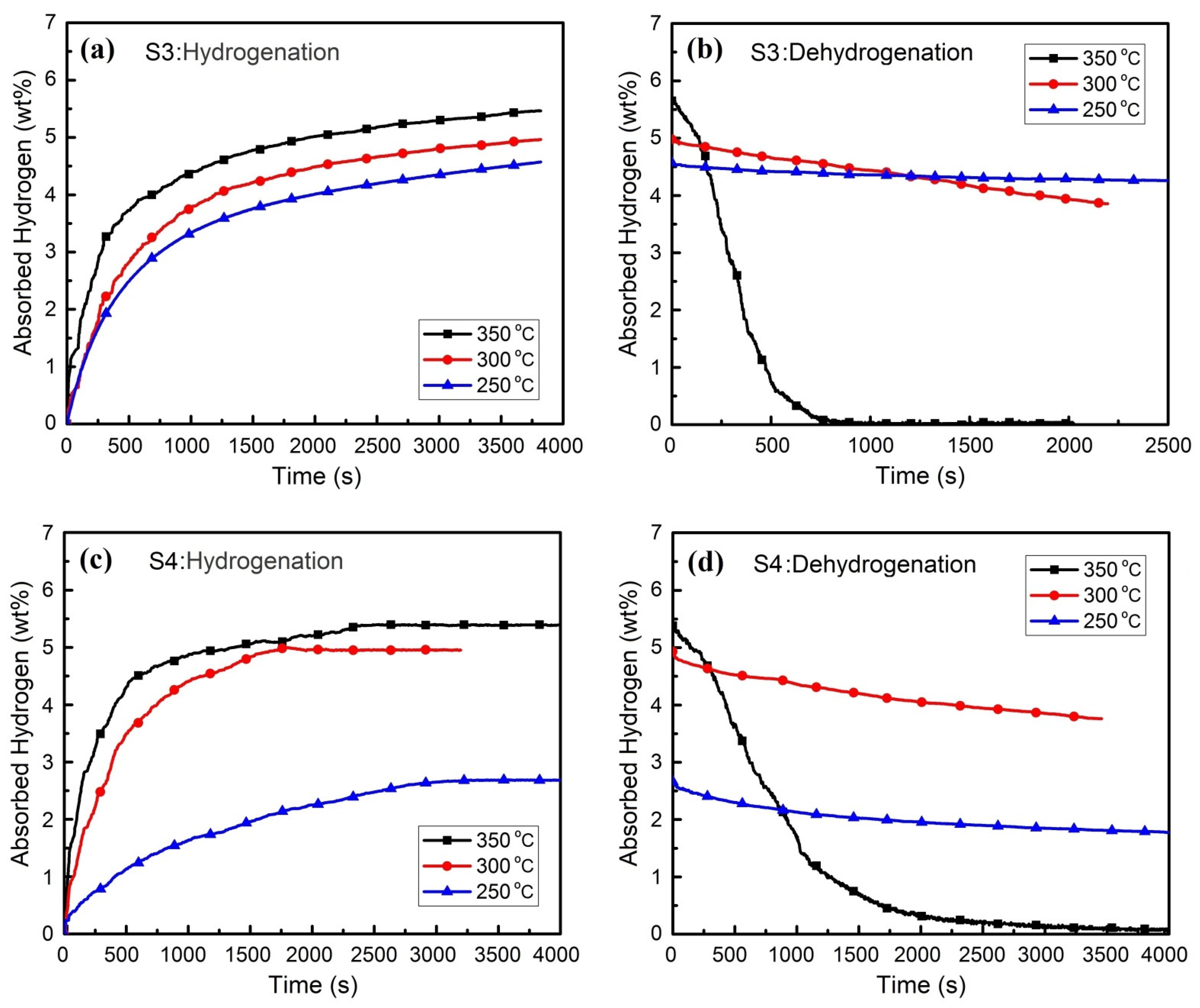
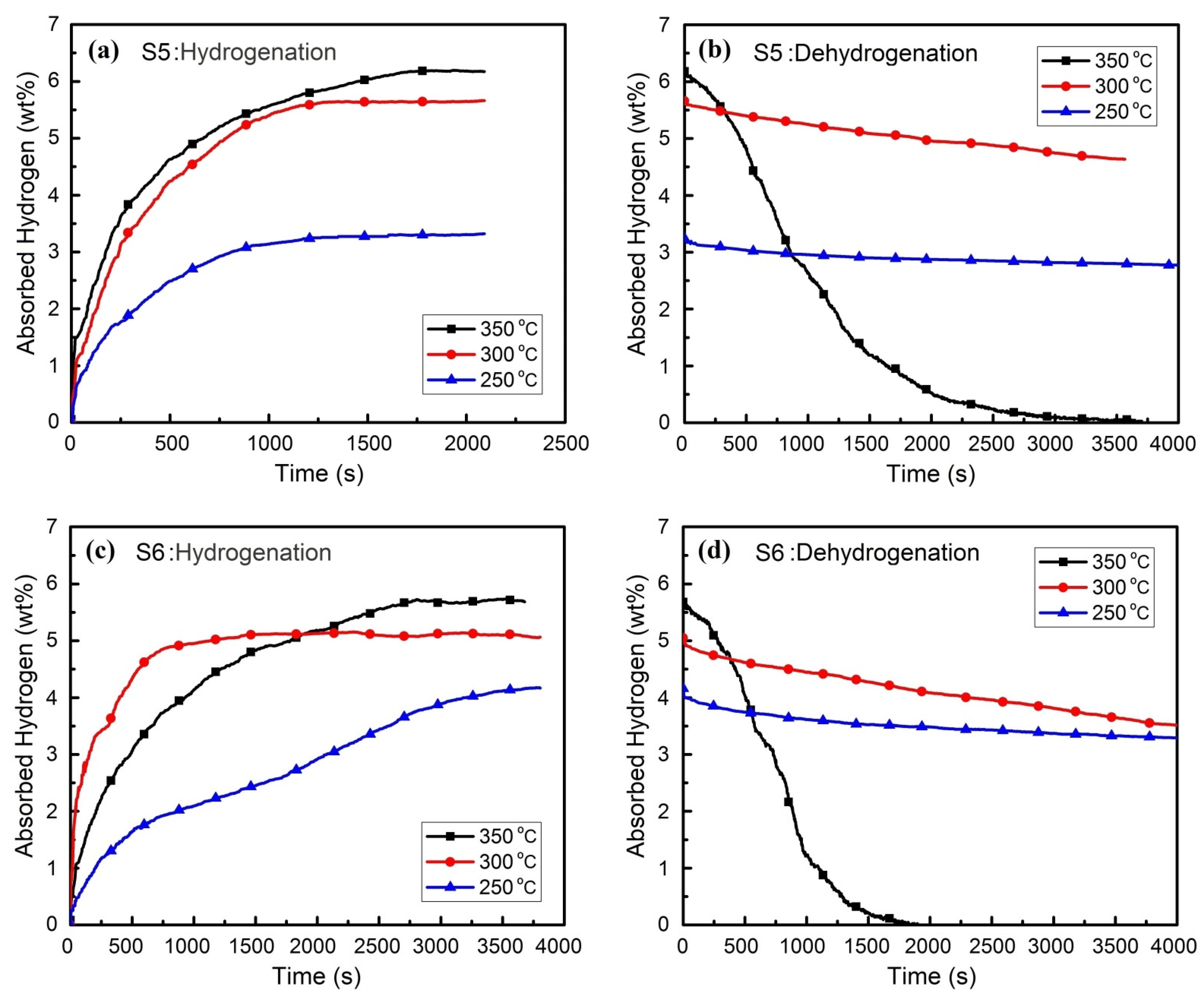
3.2. Hydrogen Absorption and Desorption Properties
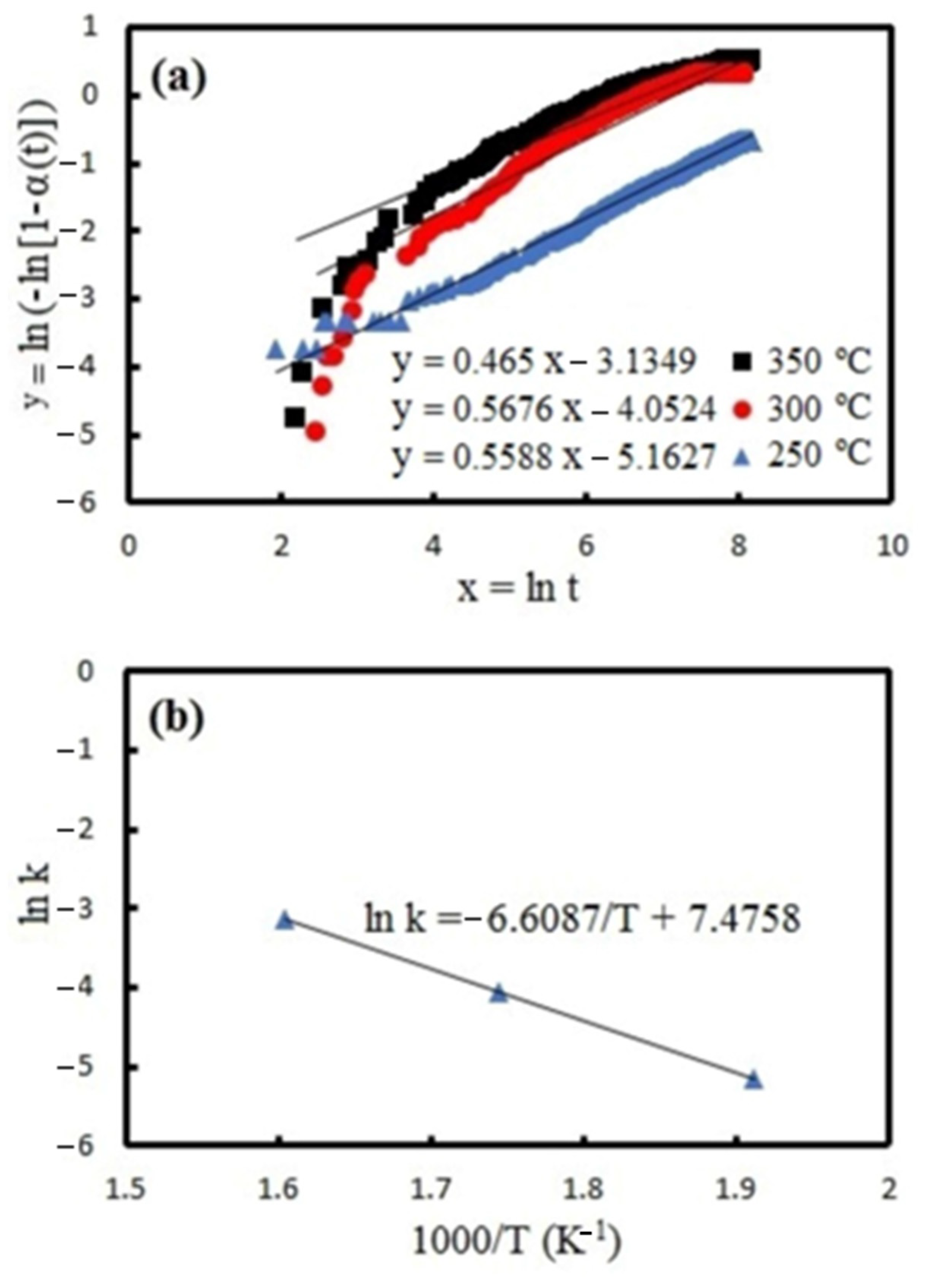
4. Discussion
5. Conclusions
- The maximum storage capacity of 6.1 wt.% is obtained at a time span below 2000 s.
- The activation energy for hydrogenation is reduced from 82.9 kJ/mol to the range of 27–54.9 kJ/mol. The results show that the TPCAE processing is capable of reducing the activation energy to lower than 30 kJ/mol.
- The complete dehydrogenation is done at 350 °C in a time range of 1000 s up to maximum 2500 s.
Author Contributions
Funding
Conflicts of Interest
References
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage; Springer Science & Business Media: New York, NY, USA, 2009; pp. 3–5. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 265–270. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Song, M.; Lee, J.Y. A study of the hydriding kinetics of Mg-(10–20 w/o) LaNi5. Int. J. Hydrogen Energy 1983, 8, 363–367. [Google Scholar] [CrossRef]
- Soyama, J.; Triques, M.R.M.; Leiva, D.R.; Junior, A.M.J.; da Silva, E.P.; Pinto, H.C.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J. Hydrogen storage in heavily deformed ZK60 alloy modified with 2.5 wt.% Mm addition. Int. J. Hydrogen Energy 2016, 41, 4177–4184. [Google Scholar] [CrossRef]
- Huang, S.-J.; Chiu, C.; Chou, T.-Y.; Rabkin, E. Effect of equal channel angular pressing (ECAP) on hydrogen storage properties of commercial magnesium alloy AZ61. Int. J. Hydrogen Energy 2018, 43, 4371–4380. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y. Influence of Al doping on stability of Mg1− xTix and their hydrides. Acta Phys. Sin. 2013, 62, 138801. [Google Scholar] [CrossRef]
- Au, M. Hydrogen storage properties of magnesium based nanostructured composite materials. Mater. Sci. Eng. B 2005, 117, 37–44. [Google Scholar] [CrossRef]
- Leiva, D.R.; Fruchart, D.; Bacia, M.; Girard, G.; Skryabina, N.; Villela, A.C.; Miraglia, S.; Santos, D.S.; Botta, W.J. Mg alloy for hydrogen storage processed by SPD. Int. J. Mater. Res. 2009, 100, 1739–1746. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Tessier, P.; Strom-Olsen, J.; Schulz, R. Nanocrystalline hydrogen absorbing alloys. In Proceedings of the Materials Science Forum, 10 July 1996; p. 2. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M.; Schafler, E.; Calizzi, M.; Pasquini, L. Dehydrogenation-hydrogenation characteristics of nanocrystalline Mg2Ni powders compacted by high-pressure torsion. J. Alloys Compd. 2017, 702, 84–91. [Google Scholar] [CrossRef]
- Skripnyuk, V.; Rabkin, E.; Bendersky, L.; Magrez, A.; Carreño-Morelli, E.; Estrin, Y.J. Hydrogen storage properties of as-synthesized and severely deformed magnesium–multiwall carbon nanotubes composite. Int. J. Hydrogen Energy 2010, 35, 5471–5478. [Google Scholar] [CrossRef]
- Khani, S.; Salehi, M.; Samim, H.; Aboutalebi, M.; Palkowski, H. The effect of severe plastic deformation on the microstructure and mechanical properties of as-cast AZ31. Iran. J. Mater. Sci. Eng. 2016, 13, 29–38. [Google Scholar]
- Malik, A.; Wang, Y.; Nazeer, F.; Khan, M.A.; Ali, T.; Ain, Q.T. Effect of pre-straining on twinning, texture and mechanical behavior of magnesium alloys A-review. J. Mater. Res. Technol. 2020, 9, 14478–14499. [Google Scholar] [CrossRef]
- Skripnyuk, V.; Rabkin, E.; Estrin, Y.; Lapovok, R. Improving hydrogen storage properties of magnesium based alloys by equal channel angular pressing. Int. J. Hydrogen Energy 2009, 34, 6320–6324. [Google Scholar] [CrossRef]
- Chiu, C.; Huang, S.-J.; Chou, T.-Y.; Rabkin, E. Improving hydrogen storage performance of AZ31 Mg alloy by equal channel angular pressing and additives. J. Alloys Compd. 2018, 743, 437–447. [Google Scholar] [CrossRef]
- Jorge, A.M., Jr.; Prokofiev, E.; de Lima, G.F.; Rauch, E.; Veron, M.; Botta, W.J.; Kawasaki, M.; Langdon, T.G. An investigation of hydrogen storage in a magnesium-based alloy processed by equal-channel angular pressing. Int. J. Hydrogen Energy 2013, 38, 8306–8312. [Google Scholar] [CrossRef]
- Edalati, K.; Yamamoto, A.; Horita, Z.; Ishihara, T. High-pressure torsion of pure magnesium: Evolution of mechanical properties, microstructures and hydrogen storage capacity with equivalent strain. Scr. Mater. 2011, 64, 880–883. [Google Scholar] [CrossRef]
- Hongo, T.; Edalati, K.; Arita, M.; Matsuda, J.; Akiba, E.; Horita, Z. Significance of grain boundaries and stacking faults on hydrogen storage properties of Mg2Ni intermetallics processed by high-pressure torsion. Acta Mater. 2015, 92, 46–54. [Google Scholar] [CrossRef]
- Jorge, A.M., Jr.; Prokofiev, E.; Triques, M.R.M.; Roche, V.; Botta, W.J.; Kiminami, C.S.; Raab, G.I.; Valiev, R.Z.; Langdon, T.G. Effect of cold rolling on the structure and hydrogen properties of AZ91 and AM60D magnesium alloys processed by ECAP. Int. J. Hydrogen Energy 2017, 42, 21822–21831. [Google Scholar] [CrossRef]
- Skripnyuk, V.; Buchman, E.; Rabkin, E.; Estrin, Y.; Popov, M.; Jorgensen, S. The effect of equal channel angular pressing on hydrogen storage properties of a eutectic Mg–Ni alloy. J. Alloys Compd. 2007, 436, 99–106. [Google Scholar] [CrossRef]
- Skripnyuk, V.; Rabkin, E.; Estrin, Y.; Lapovok, R. The effect of ball milling and equal channel angular pressing on the hydrogen absorption/desorption properties of Mg–4.95 wt.% Zn–0.71 wt.% Zr (ZK60) alloy. Acta Mater. 2004, 52, 405–414. [Google Scholar] [CrossRef]
- Asselli, A.; Leiva, D.; Huot, J.; Kawasaki, M.; Langdon, T.; Botta, W. Effects of equal-channel angular pressing and accumulative roll-bonding on hydrogen storage properties of a commercial ZK60 magnesium alloy. Int. J. Hydrogen Energy 2015, 40, 16971–16976. [Google Scholar] [CrossRef]
- Botta, W.; Jorge, A., Jr.; Veron, M.; Rauch, E.; Ferrie, E.; Yavari, A.; Huot, J.; Leiva, D. H-sorption properties and structural evolution of Mg processed by severe plastic deformation. J. Alloys Compd. 2013, 580, S187–S191. [Google Scholar] [CrossRef]
- Jorge, A.M., Jr.; de Lima, G.F.; Triques, M.R.M.; Botta, W.J.; Kiminami, C.S.; Nogueira, R.P.; Yavari, A.R.; Langdon, T.G. Correlation between hydrogen storage properties and textures induced in magnesium through ECAP and cold rolling. Int. J. Hydrogen Energy 2014, 39, 3810–3821. [Google Scholar] [CrossRef]
- Huot, J.; Skryabina, N.Y.; Fruchart, D. Application of severe plastic deformation techniques to magnesium for enhanced hydrogen sorption properties. Metals 2012, 2, 329–343. [Google Scholar] [CrossRef]
- Abdi, M.; Ebrahimi, R. Twin parallel channel angular extrusion as a development of ECAE in parallel channels. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.; Ebrahimi, R. Microstructure Evolution of AZ91 Alloy Processed by Twin Parallel Channel Angular Extrusion Technique. J. Mater. Eng. Perform. 2022, 31, 5358–5373. [Google Scholar] [CrossRef]
- Asselli, A.A.C.; Hébert, N.B.; Huot, J. The role of morphology and severe plastic deformation on the hydrogen storage properties of magnesium. Int. J. Hydrogen Energy 2014, 39, 12778–12783. [Google Scholar] [CrossRef]
- Dini, H.; Svoboda, A.; Andersson, N.-E.; Ghassemali, E.; Lindgren, L.-E.; Jarfors, A.E. Optimization and validation of a dislocation density based constitutive model for as-cast Mg-9% Al-1% Zn. Mater. Sci. Eng. A 2018, 710, 17–26. [Google Scholar] [CrossRef]
- Khani, S.; Aboutalebi, M.; Salehi, M.; Samim, H.; Palkowski, H. Microstructural development during equal channel angular pressing of as-cast AZ91 alloy. Mater. Sci. Eng. A 2016, 678, 44–56. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, M.; Lin, J.; Roven, H.J. Microstructure evolution of AZ series magnesium alloys during cyclic extrusion compression. Mater. Sci. Eng. A 2010, 527, 2265–2273. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, Q.; Sun, J.; Liu, H.; Xu, Q.; Wu, Y.; Song, D.; Jiang, J.; Ma, A. High mechanical properties of AZ91 Mg alloy processed by equal channel angular pressing and rolling. Metals 2019, 9, 386. [Google Scholar] [CrossRef]
- Peng, W.; Lan, Z.; Wei, W.; Xu, L.; Guo, J. Investigation on preparation and hydrogen storage performance of Mg17Al12 alloy. Int. J. Hydrogen Energy 2016, 41, 1759–1765. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, C.; Shen, S.; Zou, J.; Mao, S.S.; Yao, X. Combination of nanosizing and interfacial effect: Future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew. Sustain. Energy Rev. 2015, 44, 289–303. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.; Melo, G.; Ishikawa, T.; Huot, J.; Kaufman, M.; Figueroa, S.; Mendoza-Zélis, L.A.; Damonte, L.C.; Botta, W. Low temperature rolling of AZ91 alloy for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 29394–29405. [Google Scholar] [CrossRef]
- Amira, S.; Huot, J. Effect of cold rolling on hydrogen sorption properties of die-cast and as-cast magnesium alloys. J. Alloys Compd. 2012, 520, 287–294. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, X.; Liu, T.; Liu, W.; Wang, B. First-principles calculation of twin boundary energy and strength/embrittlement in hexagonal close-packed titanium. Mater. Des. 2022, 213, 110331. [Google Scholar] [CrossRef]
- Töpler, J.; Buchner, H.; Säufferer, H.; Knorr, K.; Prandl, W. Measurements of the diffusion of hydrogen atoms in magnesium and Mg2Ni by neutron scattering. J. Less Common Met. 1982, 88, 397–404. [Google Scholar] [CrossRef]
- Andreasen, A. Hydrogenation properties of Mg–Al alloys. Int. J. Hydrogen Energy 2008, 33, 7489–7497. [Google Scholar] [CrossRef]
- Karty, A.; Grunzweig-Genossar, J.; Rudman, P. Hydriding and dehydriding kinetics of Mg in a Mg/Mg2Cu eutectic alloy: Pressure sweep method. J. Appl. Phys. 1979, 50, 7200–7209. [Google Scholar] [CrossRef]
- Galey, B.; Auroux, A.; Sabo-Etienne, S.; Dhaher, S.; Grellier, M.; Postole, G. Improved hydrogen storage properties of Mg/MgH2 thanks to the addition of nickel hydride complex precursors. Int. J. Hydrogen Energy 2019, 44, 28848–28862. [Google Scholar] [CrossRef]
- Johansson, M.; Ostenfeld, C.W.; Chorkendorff, I. Adsorption of hydrogen on clean and modified magnesium films. Phys. Rev. B 2006, 74, 193408. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Nobuki, T.; Kato, S.; Abe, M.; Kuji, T. Hydrogen absorption properties of the γ-Mg17Al12 phase and its Al-richer domain. J. Alloys Compd. 2007, 446, 157–161. [Google Scholar] [CrossRef]


| Mg | Al | Zn | Mn | Fe | Si | Cu | Ca |
|---|---|---|---|---|---|---|---|
| Base | 8.9 | 0.85 | 0.338 | 0.01 | 0.08 | 0.004 | 0.04 |
| Sample | Pass 1 | Pass 2 | Pass 3 | Pass 4 |
|---|---|---|---|---|
| S1 | 340 °C | - | - | - |
| S2 | 340 °C | 300 °C | - | - |
| S3 | 340 °C | 250 °C | - | - |
| S4 | 340 °C | 200 °C | - | - |
| S5 | 340 °C | 300 °C | 250 °C | - |
| S6 | 340 °C | 300 °C | 250 °C | 200 °C |
| Sample | Mean Grain Size (µm) | Dislocation Density (×1014/m2) | Volume Fraction of β-Phase Precipitate (%) |
|---|---|---|---|
| As cast | 550 | 0.1 [32] | - |
| S1 | 13.54 | 2.73 | 3 |
| S2 | 4.29 | 1.33 | 8.7 |
| S3 | 2.39 | 0.36 | 13.3 |
| S4 | 5.67 | 4.1 | 15.3 |
| S5 | 2.19 | 0.61 | 15.4 |
| S6 | 3.66 | 3.28 | 15.6 |
| Sample | |||
|---|---|---|---|
| As cast | 0.73 | 0.48 | 0.39 |
| S1 | 0.52 | 0.55 | 0.40 |
| S2 | 0.72 | 0.4 | 0.66 |
| S3 | 0.57 | 0.58 | 0.46 |
| S4 | 0.56 | 0.57 | 0.47 |
| S5 | 0.58 | 0.67 | 0.59 |
| S6 | 0.6 | 0.28 | 0.59 |
| Sample | k250 [10−2 s−1] | k300 [10−2 s−1] | k350 [10−2 s−1] | Apparent Activation Energy (kJ/mol) |
|---|---|---|---|---|
| As cast | 0.08 | 0.69 | 1.7 | 82.9 |
| S1 | 0.8 | 1.7 | 5.4 | 51.2 |
| S2 | 0.2 | 4.3 | 1.2 | 48.2 |
| S3 | 1.2 | 1.4 | 4.2 | 34.2 |
| S4 | 0.6 | 1.7 | 4.3 | 54.9 |
| S5 | 1.1 | 1.4 | 2.9 | 27 |
| S6 | 0.6 | 15.9 | 1.6 | 29.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, M.; Ebrahimi, R.; Bagherpour, E. Improvement of Hydrogenation and Dehydrogenation Kinetics of As-Cast AZ91 Magnesium Alloy via Twin Parallel Channel Angular Extrusion Processing. Crystals 2022, 12, 1428. https://doi.org/10.3390/cryst12101428
Abdi M, Ebrahimi R, Bagherpour E. Improvement of Hydrogenation and Dehydrogenation Kinetics of As-Cast AZ91 Magnesium Alloy via Twin Parallel Channel Angular Extrusion Processing. Crystals. 2022; 12(10):1428. https://doi.org/10.3390/cryst12101428
Chicago/Turabian StyleAbdi, Mohammad, Ramin Ebrahimi, and Ebad Bagherpour. 2022. "Improvement of Hydrogenation and Dehydrogenation Kinetics of As-Cast AZ91 Magnesium Alloy via Twin Parallel Channel Angular Extrusion Processing" Crystals 12, no. 10: 1428. https://doi.org/10.3390/cryst12101428
APA StyleAbdi, M., Ebrahimi, R., & Bagherpour, E. (2022). Improvement of Hydrogenation and Dehydrogenation Kinetics of As-Cast AZ91 Magnesium Alloy via Twin Parallel Channel Angular Extrusion Processing. Crystals, 12(10), 1428. https://doi.org/10.3390/cryst12101428








