Conductive Covalent Organic Frameworks Meet Micro-Electrical Energy Storage: Mechanism, Synthesis and Applications—A Review
Abstract
1. Introduction
2. Conductivity Enhancement Mechanism
2.1. Strategies on Conductivity Enhancing
2.1.1. Ligand-Bond Coordination Strategies
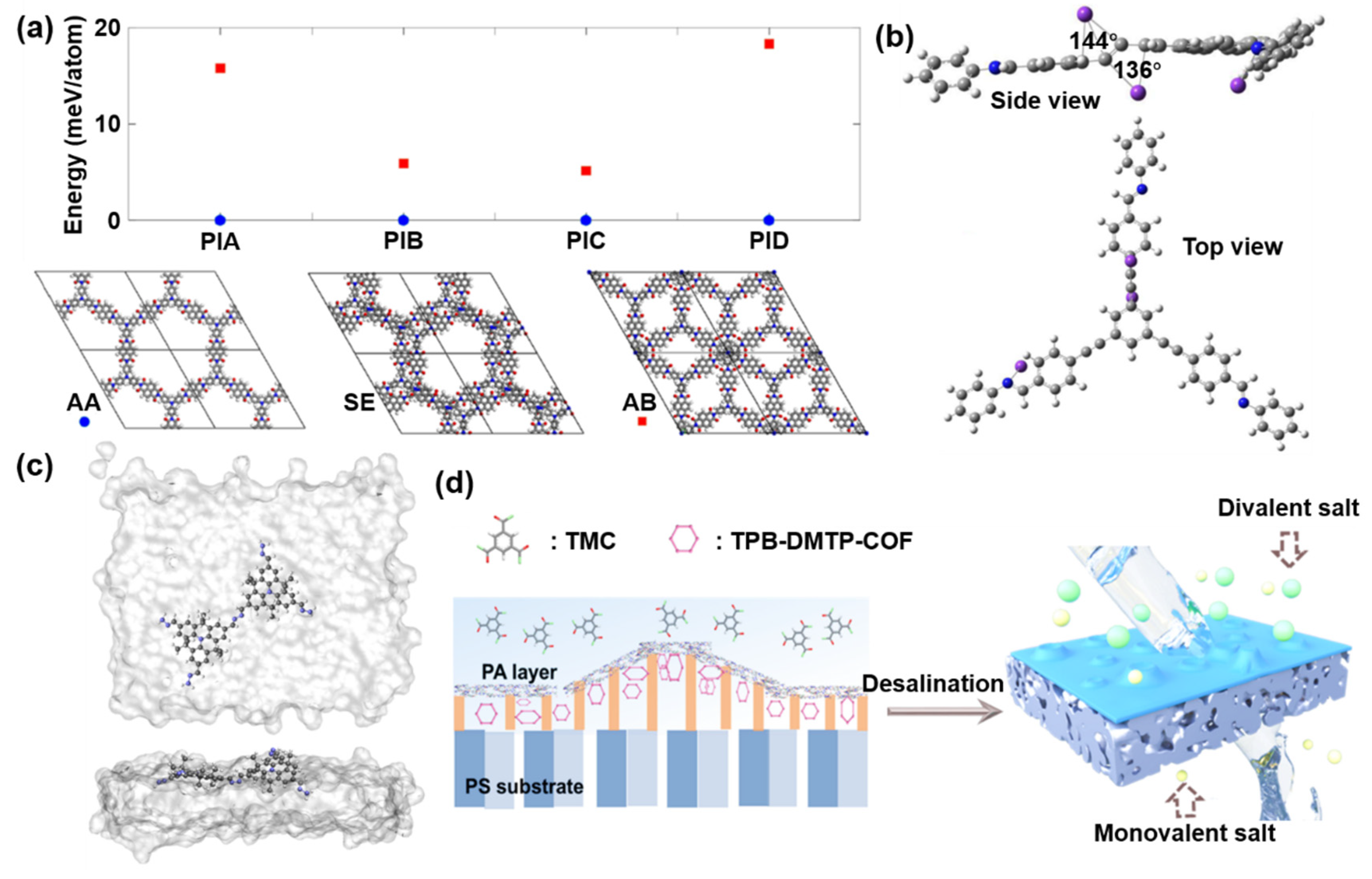
2.1.2. Ligand A—A Stacking Strategy
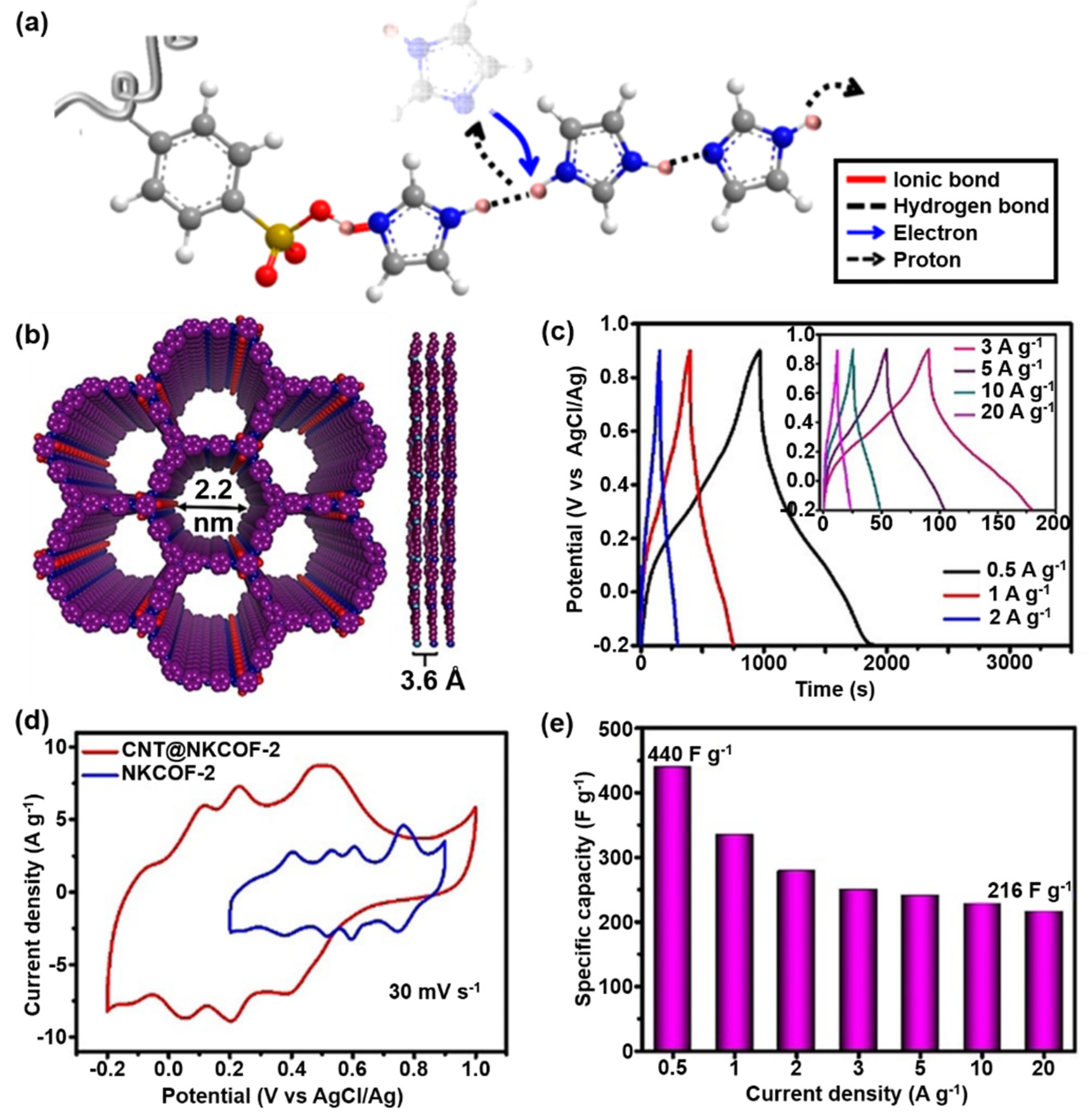
2.1.3. Proton Grotthuss Transfer Strategy
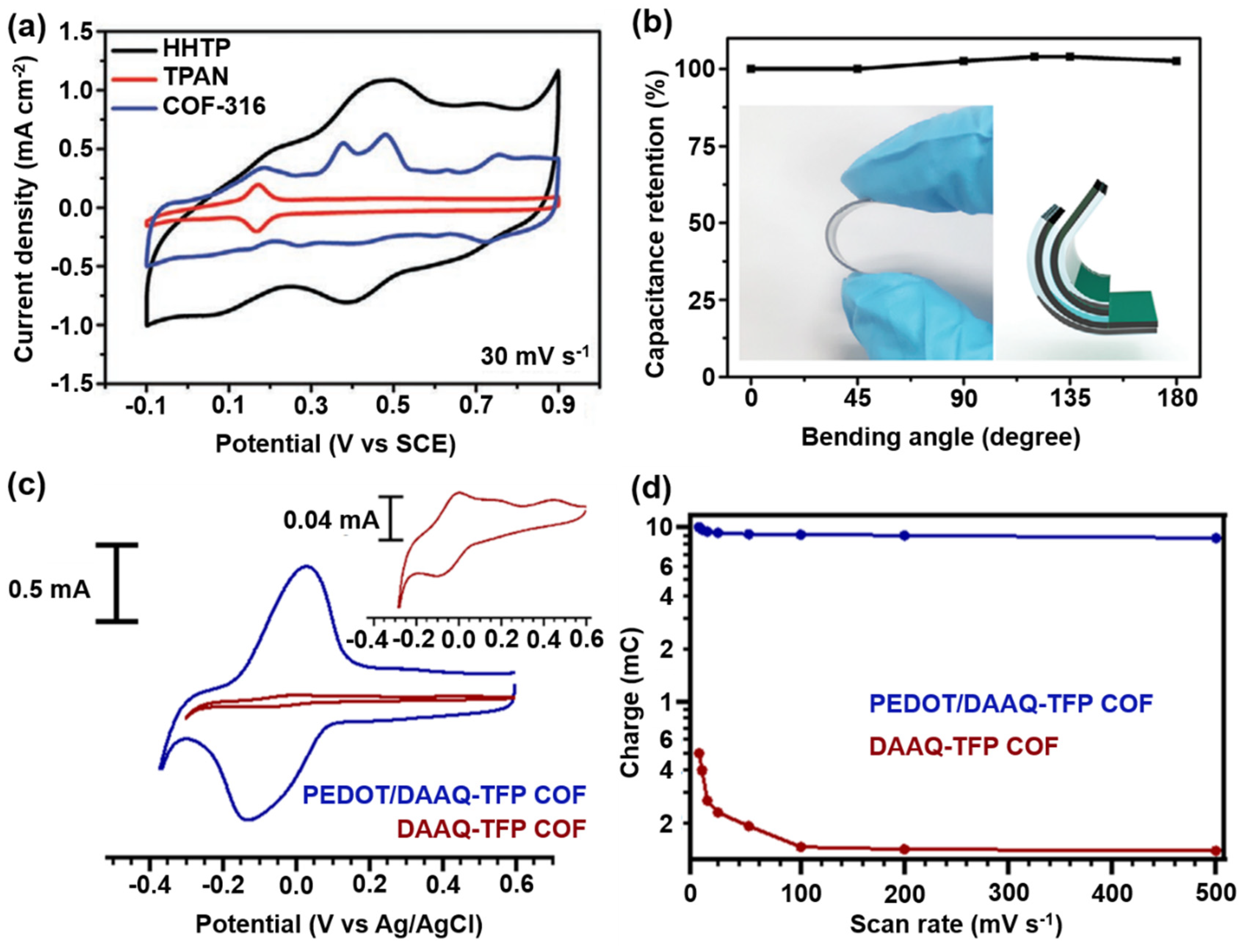
2.1.4. Post-Synthetic Modification Strategy
Polymer Introduction
Surface Doping
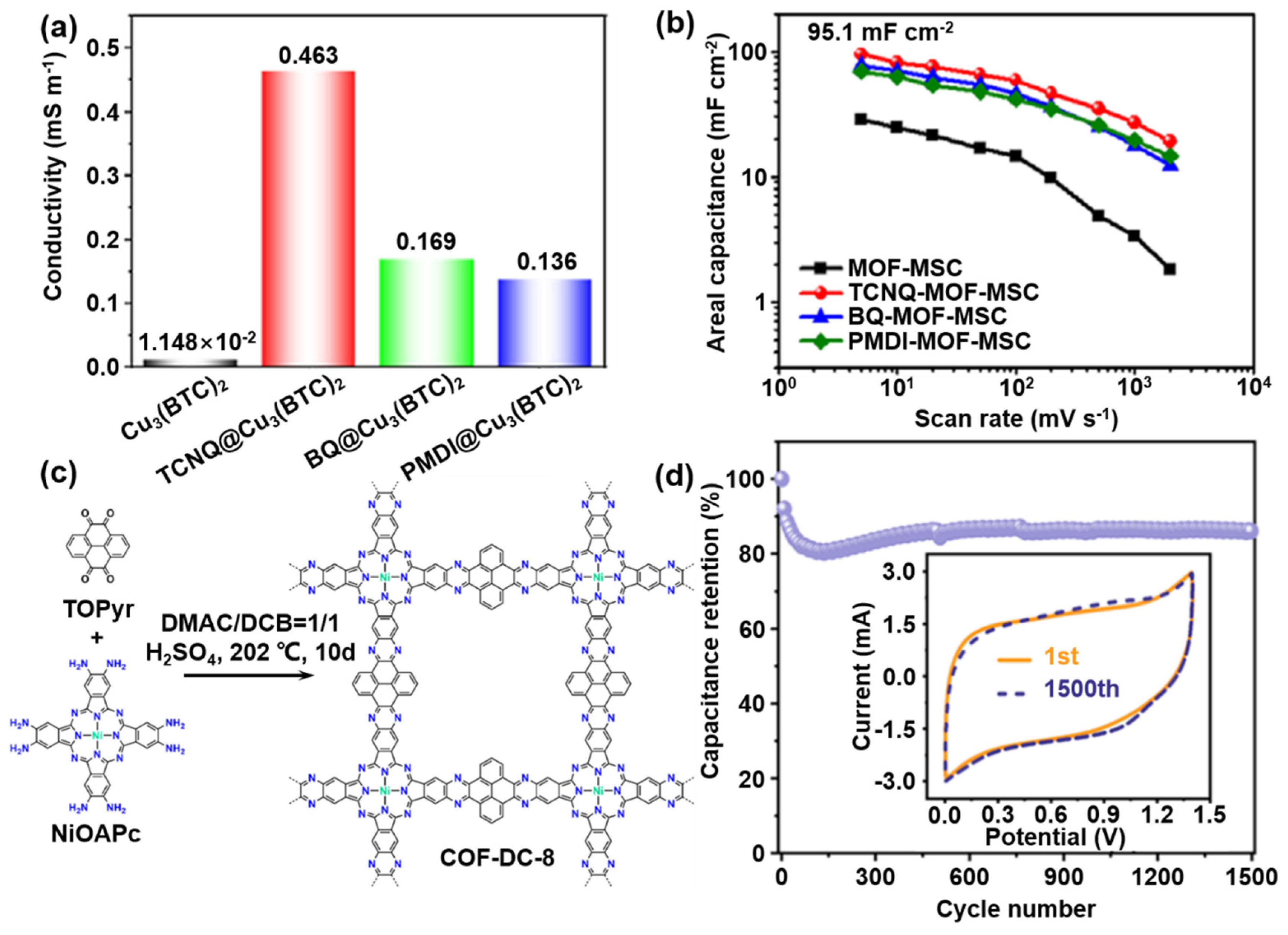
2.2. Theoretical Assistances Explanation
2.2.1. Density Functional Theory
2.2.2. Molecular Dynamics
3. Synthesis Strategies of Organic Frameworks
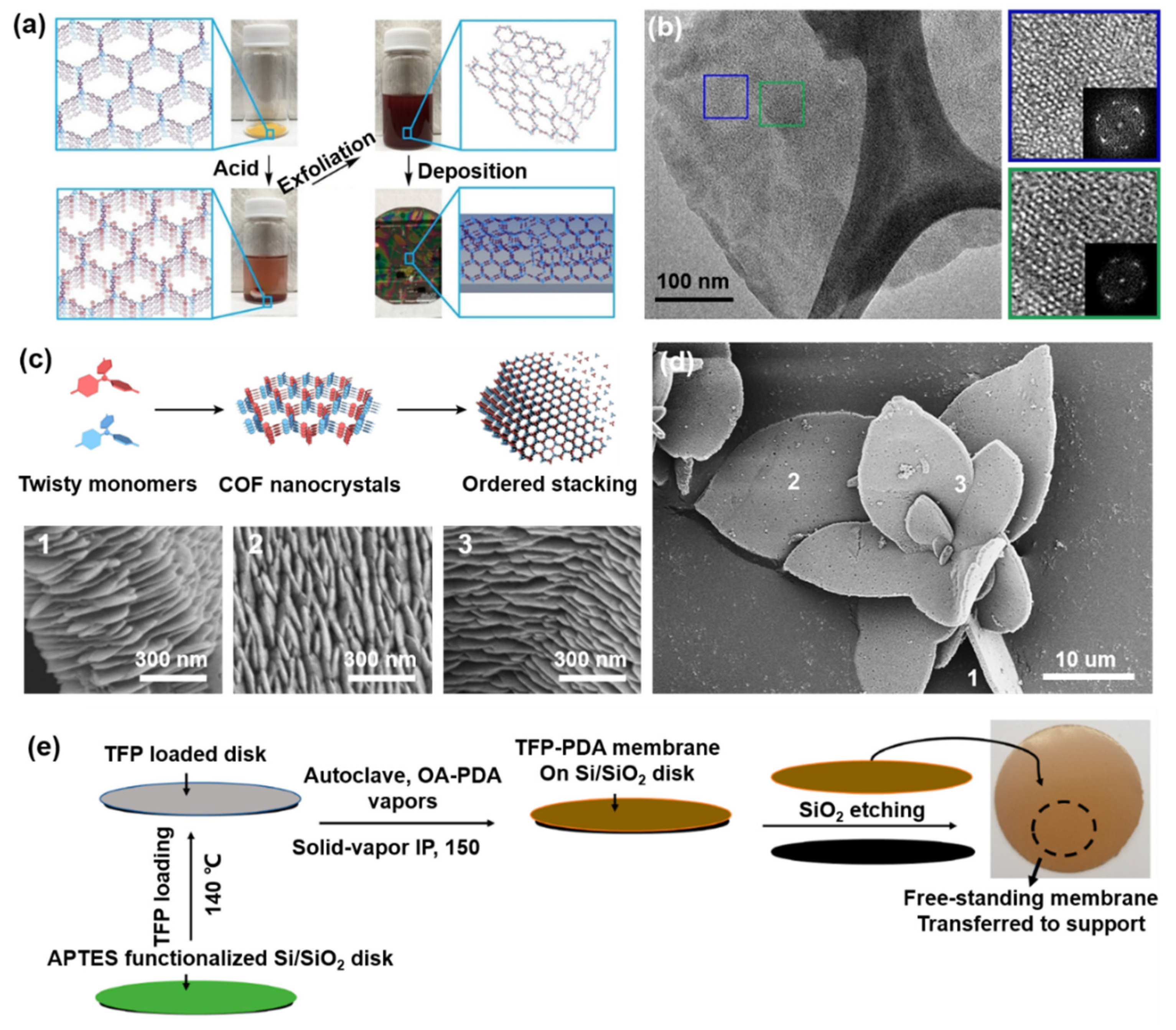
3.1. Top-Down Strategy
3.2. Bottom-Up Strategy
4. Design Criteria for Conductive COF Applied to MEES
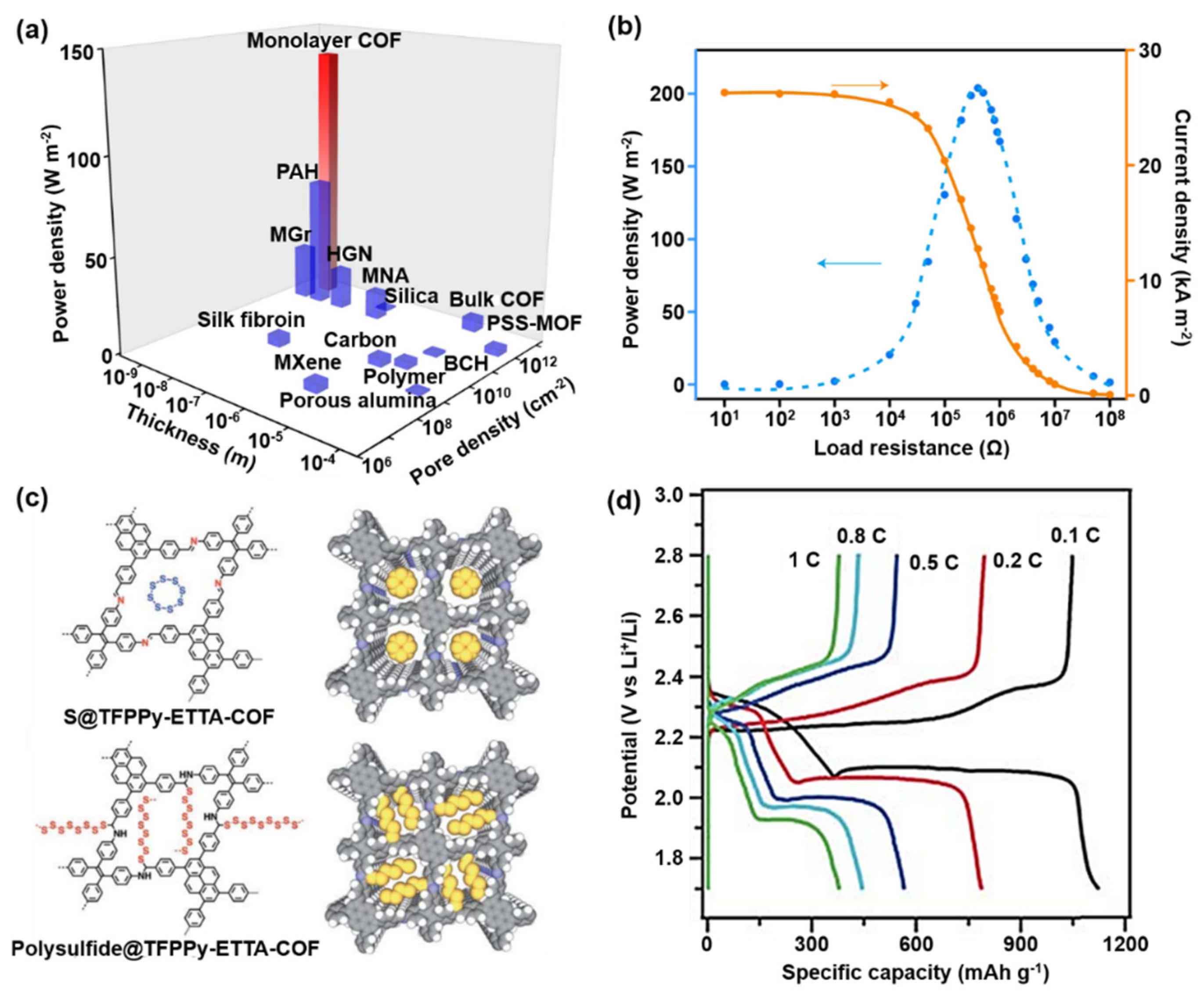
4.1. Good Pore Distribution
4.2. Aligned Channels
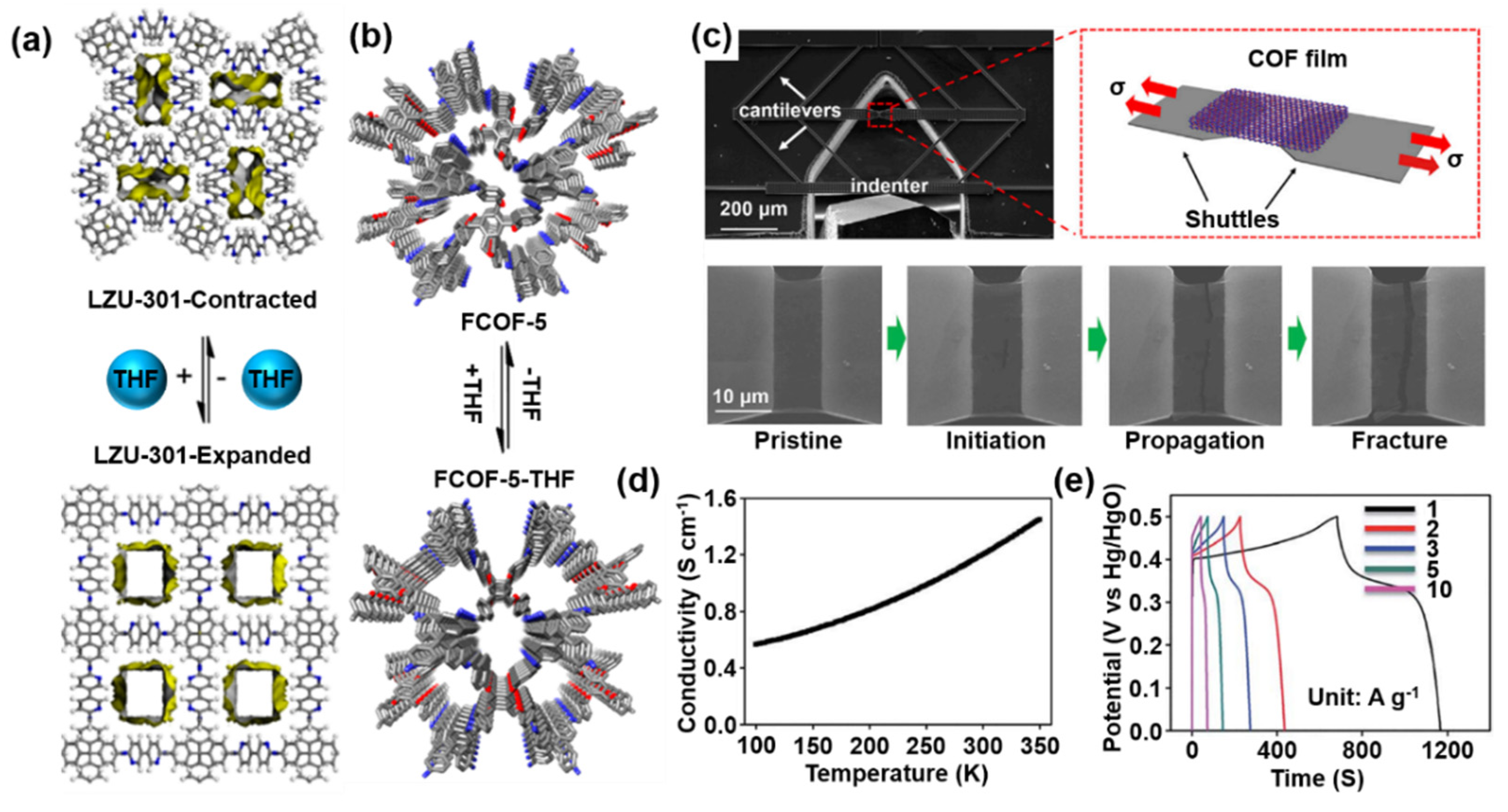
4.3. Dynamic Reversible Crystal Structure
4.4. Excellent Mechanical Properties
4.5. Electrochemical Activity
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miskin, M.Z.; Cortese, A.J.; Dorsey, K.; Esposito, E.P.; Reynolds, M.F.; Liu, Q.; Cao, M.; Muller, D.A.; McEuen, P.L.; Cohen, I. Electronically integrated, mass-manufactured, microscopic robots. Nature 2020, 584, 557–561. [Google Scholar] [CrossRef]
- Wang, R.; Sun, K.; Zhang, Y.; Qian, C.; Bao, W. Dimensional optimization enables high-performance capacitive deionization. J. Mater. Chem. A 2022, 10, 6414–6441. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Sun, K.; Zhang, Y.; Li, J.; Bao, W. Element-Doped Mxenes: Mechanism, Synthesis, and Applications. Small 2022, 18, e2201740. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, K.; Zhang, Y.; Li, B.; Qian, C.; Li, J.; Liu, F.; Bao, W. Nanoscale interface engineering of inorganic Solid-State electrolytes for High-Performance alkali metal batteries. J. Colloid Interface Sci. 2022, 621, 41–66. [Google Scholar] [CrossRef]
- Bao, W.; Wang, R.; Qian, C.; Zhang, Z.; Wu, R.; Zhang, Y.; Liu, F.; Li, J.; Wang, G. Porous Heteroatom-Doped Ti3C2Tx MXene Microspheres Enable Strong Adsorption of Sodium Polysulfides for Long-Life Room-Temperature Sodium-Sulfur Batteries. ACS Nano 2021, 15, 16207–16217. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, R.; Li, B.; Qian, C.; Zhang, Z.; Li, J.; Liu, F. Stable alkali metal anodes enabled by crystallographic optimization—A review. J. Mater. Chem. A 2021, 9, 20957–20984. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Sun, K.; Wang, R.; Qian, C.; Yu, F.; Zhang, L.; Bao, W. Dual-functional iodine photoelectrode enabling high performance photo-assisted rechargeable lithium iodine batteries. J. Mater. Chem. A 2022, 10, 7326–7332. [Google Scholar] [CrossRef]
- Wang, R.; Sun, K.; Liu, H.; Qian, C.; Li, M.; Zhang, Y.; Bao, W. Integrating a Redox-Coupled FeSe2/N-C Photoelectrode into a Potassium Ion Hybrid Capacitors for Photoassisted Charging. J. Mater. Chem. A 2022, 10, 11504–11513. [Google Scholar] [CrossRef]
- Qian, C.; Sun, K.; Bao, W. Recent advance on machine learning of MXenes for energy storage and conversion. Int. J. Energy Res. 2022. [Google Scholar] [CrossRef]
- Shi, X.; Das, P.; Wu, Z.-S. Digital Microscale Electrochemical Energy Storage Devices for a Fully Connected and Intelligent World. ACS Energy Lett. 2021, 7, 267–281. [Google Scholar] [CrossRef]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced Nanocellulose-Based Composites for Flexible Functional Energy Storage Devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Johnson, A.C.; Kim, S.; Kohlmeyer, R.R.; Patra, A.; Grzyb, J.; Padmanabha, A.; Wang, M.; Jiang, Z.; Sun, P.; et al. A Nearly Packaging-Free Design Paradigm for Light, Powerful, and Energy-Dense Primary Microbatteries. Adv. Mater. 2021, 33, e2101760. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Goh, K.; Wang, H.; Wei, L.; Jiang, W.; Zhang, Q.; Dai, L.; Chen, Y. Scalable synthesis of hierarchically structured carbon nanotube–graphene fibres for capacitive energy storage. Nat. Nanotechnol. 2014, 9, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2016, 12, 7–15. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Zhang, N.; Zou, H.; Liu, R.; Tao, C.; Fan, X.; Wang, Z.L. Micro-cable structured textile for simultaneously harvesting solar and mechanical energy. Nat. Energy 2016, 1, 16138. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, H.; Xiao, C.; Zheng, S.; Shi, X.; Qin, J.; Fu, Q.; Bao, X.; Feng, X.; Müllen, K.; et al. Electrochemically Scalable Production of Fluorine-Modified Graphene for Flexible and High-Energy Ionogel-Based Microsupercapacitors. J. Am. Chem. Soc. 2018, 140, 8198–8205. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Zhou, T.; Sun, S. Recent Developments of Planar Micro-Supercapacitors: Fabrication, Properties, and Applications. Adv. Funct. Mater. 2020, 30, 1910000. [Google Scholar] [CrossRef]
- Bao, W.; Wang, R.; Qian, C.; Li, M.; Sun, K.; Yu, F.; Liu, H.; Guo, C.; Li, J. Photoassisted High-Performance Lithium Anode Enabled by Oriented Crystal Planes. ACS Nano 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Zhou, W.; Meng, S.; Qi, C.; Liu, Z.; Kong, T. Biocompatible, High-Performance, Wet-Adhesive, Stretchable All-Hydrogel Supercapacitor Implant Based on PANI@rGO/Mxenes Electrode and Hydrogel Electrolyte. Adv. Energy Mater. 2021, 11, 2101329. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z. Zinc based micro-electrochemical energy storage devices: Present status and future perspective. EcoMat 2020, 2, e12042. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, X.; Ma, M.; Mu, C.; Li, P.; Li, L.; Li, M.; Nie, A.; Xiang, J.; Zhao, Z.; et al. Application of hard ceramic materials B4C in energy storage: Design B4C@C core-shell nanoparticles as electrodes for flexible all-solid-state micro-supercapacitors with ultrahigh cyclability. Nano Energy 2020, 75, 104947. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, D.; Liu, W.; Li, A.; Su, Y.; Jiang, P.; Zou, Y.; Shi, S.; Liu, F. Sputtered titanium nitride films with finely tailored surface activity and porosity for high performance on-chip micro-supercapacitors. J. Power Source 2021, 489, 229406. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef]
- Li, Y.; Karimi, M.; Gong, Y.-N.; Dai, N.; Safarifard, V.; Jiang, H.-L. Integration of metal-organic frameworks and covalent organic frameworks: Design, synthesis, and applications. Matter 2021, 4, 2230–2265. [Google Scholar] [CrossRef]
- Keller, N.; Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 2020, 50, 1813–1845. [Google Scholar] [CrossRef]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef]
- Li, X.; Cai, S.; Sun, B.; Yang, C.; Zhang, J.; Liu, Y. Chemically Robust Covalent Organic Frameworks: Progress and Perspective. Matter 2020, 3, 1507–1540. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Xu, J.; He, Y.; Bi, S.; Wang, M.; Yang, P.; Wu, D.; Wang, J.; Zhang, F. An Olefin-Linked Covalent Organic Framework as a Flexible Thin-Film Electrode for a High-Performance Micro-Supercapacitor. Angew. Chem. Int. Ed. 2019, 58, 12065–12069. [Google Scholar] [CrossRef]
- Bi, S.; Yang, C.; Zhang, W.; Xu, J.; Liu, L.; Wu, D.; Wang, X.; Han, Y.; Liang, Q.; Zhang, F. Two-dimensional semiconducting covalent organic frameworks via condensation at arylmethyl carbon atoms. Nat. Commun. 2019, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Diercks, C.S.; Zhu, C.; Yaghi, O.M. Porous Crystalline Olefin-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 6848–6852. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Scott, T.F. Approaches and challenges in the synthesis of three-dimensional covalent-organic frameworks. Commun. Chem. 2018, 1, 98. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Fang, Q.; Qiu, S. Covalent Organic Frameworks for Catalysis. EnergyChem 2020, 2, 100035. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.-M.; Kirchon, A.; Pang, J.-D.; Yang, X.-Y.; Zhuang, G.-L.; Zhou, H.-C. Unconventional Method for Fabricating Valence Tautomeric Materials: Integrating Redox Center within a Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 6822–6826. [Google Scholar] [CrossRef]
- Wang, D.-G.; Qiu, T.; Guo, W.; Liang, Z.; Tabassum, H.; Xia, D.; Zou, R. Covalent organic framework-based materials for energy applications. Energy Environ. Sci. 2020, 14, 688–728. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Ascherl, L.; Sick, T.; Margraf, J.T.; Lapidus, S.H.; Calik, M.; Hettstedt, C.; Karaghiosoff, K.; Döblinger, M.; Clark, T.; Chapman, K.W.; et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 2016, 8, 310–316. [Google Scholar] [CrossRef]
- Mahmood, J.; Ahmad, I.; Jung, M.; Seo, J.-M.; Yu, S.-Y.; Noh, H.-J.; Kim, Y.H.; Shin, H.-J.; Baek, J.-B. Two-dimensional amine and hydroxy functionalized fused aromatic covalent organic framework. Commun. Chem. 2020, 3, 31. [Google Scholar] [CrossRef]
- Yue, Y.; Li, H.; Chen, H.; Huang, N. Piperazine-Linked Covalent Organic Frameworks with High Electrical Conductivity. J. Am. Chem. Soc. 2022, 144, 2873–2878. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Z.; Wee, V.; Usadi, A.K.; Calabro, D.C.; Baugh, L.S.; Wang, S.; Wang, Y.; Zhao, D. Interlayer Shifting in Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12995–13002. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Chen, Y.; Wang, Y.; Li, G.; Wang, G.; Liu, L.; Zhang, J.; Liu, Y.; Xu, Z.; Tomsia, A.P.; et al. Ultrastrong Graphene Films via Long-Chain π-Bridging. Matter 2019, 1, 389–401. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.; Park, M.J. Proton Hopping and Diffusion Behavior of Sulfonated Block Copolymers Containing Ionic Liquids. Macromolecules 2014, 47, 1099–1108. [Google Scholar] [CrossRef]
- Wu, X.; Hong, J.J.; Shin, W.; Ma, L.; Liu, T.; Bi, X.; Yuan, Y.; Qi, Y.; Surta, T.W.; Huang, W.; et al. Diffusion-free Grotthuss topochemistry for high-rate and long-life proton batteries. Nat. Energy 2019, 4, 123–130. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Zhao, W.; Qin, R.; Ding, S.; Chen, X.; Song, Y.; Yang, L.; Lin, H.; Li, S.; et al. Boosting the Energy Density of Aqueous Batteries via Facile Grotthuss Proton Transport. Angew. Chem. Int. Ed. 2020, 60, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, P.; Hao, L.; Cheng, P.; Chen, Y.; Zhang, Z. Grotthuss Proton-Conductive Covalent Organic Frameworks for Efficient Proton Pseudocapacitors. Angew. Chem. Int. Ed. 2021, 60, 21838–21845. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Aykanat, A.; Mirica, K.A. Proton Conduction in 2D Aza-Fused Covalent Organic Frameworks. Chem. Mater. 2018, 31, 819–825. [Google Scholar] [CrossRef]
- Segura, J.L.; Royuela, S.; Ramos, M.M. Post-synthetic modification of covalent organic frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef] [PubMed]
- Yusran, Y.; Guan, X.; Li, H.; Fang, Q.; Qiu, S. Postsynthetic functionalization of covalent organic frameworks. Natl. Sci. Rev. 2019, 7, 170–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Lin, H.-H.; Ballabio, M.; Zhong, H.; Bonn, M.; Zhou, S.; Heine, T.; Cánovas, E.; Dong, R.; et al. High-Mobility Semiconducting Two-Dimensional Conjugated Covalent Organic Frameworks with p-Type Doping. J. Am. Chem. Soc. 2020, 142, 21622–21627. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, W.; Chen, T.; Bai, Y.; Xu, H.; Jiang, M.; Liu, S.; Huang, W.; Zhao, Q. All-in-One Hollow Flower-Like Covalent Organic Frameworks for Flexible Transparent Devices. Adv. Funct. Mater. 2021, 31, 2010306. [Google Scholar] [CrossRef]
- Mulzer, C.R.; Shen, L.; Bisbey, R.P.; McKone, J.R.; Zhang, N.; Abruña, H.D.; Dichtel, W.R. Superior Charge Storage and Power Density of a Conducting Polymer-Modified Covalent Organic Framework. ACS Central Sci. 2016, 2, 667–673. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.J.; Huang, X.; Dong, L.Z.; Lu, M.; Guo, C.; Yuan, D.; Chen, Y.; Xu, G.; Li, S.L.; et al. Porphyrin-Based COF 2D Materials: Variable Modification of Sensing Performances by Post-Metallization. Angew. Chem. Int. Ed. 2022, 61, e202115308. [Google Scholar]
- He, Y.; Yang, S.; Fu, Y.; Wang, F.; Ma, J.; Wang, G.; Chen, G.; Wang, M.; Dong, R.; Zhang, P.; et al. Electronic Doping of Metal-Organic Frameworks for High-Performance Flexible Micro-Supercapacitors. Small Struct. 2021, 2, 2000095. [Google Scholar] [CrossRef]
- Wang, L.; Dong, B.; Ge, R.; Jiang, F.; Xu, J. Fluorene-Based Two-Dimensional Covalent Organic Framework with Thermo-electric Properties through Doping. ACS Appl. Mater. Interfaces 2017, 9, 7108–7114. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mirica, K.A. Two-Dimensional Chemiresistive Covalent Organic Framework with High Intrinsic Conductivity. J. Am. Chem. Soc. 2019, 141, 11929–11937. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, S.K.S.; Oliveira, F.L.; Santos, T.C.; Hisse, D.; Merlini, C.; Ronconi, C.M.; Esteves, P.M. A Carbocationic Triarylmethane-Based Porous Covalent Organic Network. Chem. Eur. J. 2020, 27, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, M.; Zhao, Y.; Wang, D.; Li, Y.; Sui, Z.; Xiao, J.; Chen, Q. Functionalized triazine-based covalent organic frameworks containing quinoline via aza-Diels-Alder reaction for enhanced lithium-sulfur batteries performance. J. Colloid Interface Sci. 2021, 608, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Y.; Wang, Z.Q.; Cui, F.Z.; Li, J.Y.; Han, X.H.; Qi, Q.Y.; Ma, D.L.; Jiang, G.F.; Zeng, X.X.; Zhao, X. A Covalent Organic Framework with Extended pi-Conjugated Building Units as a Highly Efficient Recipient for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 34990–34998. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiang, K.; Zheng, Q.; Li, X.; Mao, H.; Zhong, W.; Chen, C.; Sun, B.; Zheng, H.; Zhuang, X.; et al. Chemically Stable Polyarylether-Based Metallophthalocyanine Frameworks with High Carrier Mobilities for Capacitive Energy Storage. J. Am. Chem. Soc. 2021, 143, 17701–17707. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, D.; Zhang, Z.; Matsushita, M.M.; Awaga, K. Electron Highways into Nanochannels of Covalent Organic Frameworks for High Electrical Conductivity and Energy Storage. ACS Appl. Mater. Interfaces 2019, 11, 7661–7665. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.; Piao, Q.; Zhong, J.; Wang, Y.; Li, H.; Chen, Y.; Wang, B. Flexible Solid-State Supercapacitor Based on a Metal–Organic Framework Interwoven by Electrochemically-Deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Srepusharawoot, P.; Scheicher, R.H.; Araújo, C.M.; Blomqvist, A.; Pinsook, U.; Ahuja, R. Ab Initio Study of Molecular Hydrogen Adsorption in Covalent Organic Framework-1. J. Phys. Chem. C 2009, 113, 8498–8504. [Google Scholar] [CrossRef]
- Koo, B.T.; Dichtel, W.R.; Clancy, P. A classification scheme for the stacking of two-dimensional boronate ester-linked covalent organic frameworks. J. Mater. Chem. 2012, 22, 17460–17469. [Google Scholar] [CrossRef]
- Keller, N.; Calik, M.; Sharapa, D.; Soni, H.R.; Zehetmaier, P.M.; Rager, S.; Auras, F.; Jakowetz, A.C.; Goerling, A.; Clark, T.; et al. Enforcing Extended Porphyrin J-Aggregate Stacking in Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 16544–16552. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.-N.; Zhu, C.-Y.; Zhang, M.; Geng, Y.; Li, Y.-G.; Su, Z.-M. A two-dimensional conductive Mo-based covalent organic framework as an efficient electrocatalyst for nitrogen fixation. J. Mater. Chem. A 2020, 8, 23599–23606. [Google Scholar] [CrossRef]
- Lv, B.Q.; Qian, T.; Ding, H. Experimental perspective on three-dimensional topological semimetals. Rev. Mod. Phys. 2021, 93, 025002. [Google Scholar] [CrossRef]
- Peng, H.; Huang, S.; Tranca, D.; Richard, F.; Baaziz, W.; Zhuang, X.; Samori, P.; Ciesielski, A. Quantum Capacitance through Molecular Infiltration of 7,7,8,8-Tetracyanoquinodimethane in Metal-Organic Framework/Covalent Organic Framework Hybrids. ACS Nano 2021, 15, 18580–18589. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically Conductive Porous Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Henfling, S.; Zhang, N.N.; Krautscheid, H. Semiconductive coordination polymers with continuous pi-pi interactions and defined crystal structures. Chem. Commun. 2021, 57, 10407–10410. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Deng, W.-H.; Wang, G.-E.; Xu, G. Conductive MOFs. EnergyChem 2020, 2, 100029. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Xie, Z.; Li, Y.; Liu, Y.; Sun, J.; Ma, Y.; Terasaki, O.; Chen, L. Conjugated Copper–Catecholate Framework Electrodes for Efficient Energy Storage. Angew. Chem. Int. Ed. 2019, 59, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Qin, Y.; Cheng, Y.; Fan, W.; Lang, X.; Zheng, L.; Cao, Q. Modulating the Stacking Model of Covalent Organic Framework Isomers with Different Generation Efficiencies of Reactive Oxygen Species. ACS Appl. Mater. Interfaces 2021, 13, 29471–29481. [Google Scholar] [CrossRef] [PubMed]
- van der Jagt, R.; Vasileiadis, A.; Veldhuizen, H.; Shao, P.; Feng, X.; Ganapathy, S.; Habisreutinger, N.C.; van der Veen, M.A.; Wang, C.; Wagemaker, M.; et al. Synthesis and Structure–Property Relationships of Polyimide Covalent Organic Frameworks for Carbon Dioxide Capture and (Aqueous) Sodium-Ion Batteries. Chem. Mater. 2021, 33, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-B.; Tian, P.-J.; Yao, J.; Lu, Y.; Qi, Q.-Y.; Zhao, X. Toward azo-linked covalent organic frameworks by developing linkage chemistry via linker exchange. Nat. Commun. 2022, 13, 2180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z. High performance anode material for sodium-ion batteries derived from covalent-organic frameworks. Electrochim. Acta 2019, 301, 23–28. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Y.; Yang, Y.; Zhang, C.; An, Q.; Guo, H. Molecular engineering regulation redox-dual-active-center covalent organic frameworks-based anode for high-performance Li storage. EcoMat 2022, e212221. [Google Scholar] [CrossRef]
- Luo, X.X.; Li, W.H.; Liang, H.J.; Zhang, H.X.; Du, K.D.; Wang, X.T.; Liu, X.F.; Zhang, J.P.; Wu, X.L. Covalent Organic Framework with Highly Accessible Carbonyls and pi-Cation Effect for Advanced Potassium-Ion Batteries. Angew. Chem.-Int. Ed. 2022, 61, e202117661. [Google Scholar]
- Xia, Z.; Jia, X.; Ge, X.; Ren, C.; Yang, Q.; Hu, J.; Chen, Z.; Han, J.; Xie, G.; Chen, S.; et al. Tailoring Electronic Structure and Size of Ultrastable Metalated Metal-Organic Frameworks with Enhanced Electroconductivity for High-Performance Super-capacitors. Angew. Chem. Int. Ed. 2021, 60, 10228–10238. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, E.R.; Schkeryantz, L.; Moscarello, E.M.; Fernandez, J.P.; Paszek, J.; Wu, Y.; Hadad, C.M.; McGrier, P.L. Al-kynyl-Based Covalent Organic Frameworks as High-Performance Anode Materials for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 41628–41636. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; An, N.; Meng, C.; Xie, K.-F.; Wang, X.; Dong, X.-Y.; Sun, D.; Yang, Y.; Hu, Z.-A. High-density active site COFs with a flower-like morphology for energy storage applications. J. Mater. Chem. A 2022, 10, 11030–11038. [Google Scholar] [CrossRef]
- Ortega-Guerrero, A.; Sahabudeen, H.; Croy, A.; Dianat, A.; Dong, R.; Feng, X.; Cuniberti, G. Multiscale Modeling Strategy of 2D Covalent Organic Frameworks Confined at an Air–Water Interface. ACS Appl. Mater. Interfaces 2021, 13, 26411–26420. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mai, Z.; Wang, Z.; Zhang, X.; Zhu, J.; Shen, J.; Wang, J.; Wang, Y.; Zhang, Y. Covalent Organic Frame-work-Mediated Thin-Film Composite Polyamide Membranes toward Precise Ion Sieving. ACS Appl. Mater. Interfaces 2022, 14, 3427–3436. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.-H.; Ma, M.-Q.; Wang, Z.; Xu, Z.-K. Ultrathin metal/covalent–organic framework membranes towards ultimate separation. Chem. Soc. Rev. 2019, 48, 3811–3841. [Google Scholar] [CrossRef]
- Guan, X.; Li, H.; Ma, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 2019, 11, 587–594. [Google Scholar] [CrossRef]
- Ji, X.; Kong, N.; Wang, J.; Li, W.; Xiao, Y.; Gan, S.T.; Zhang, Y.; Li, Y.; Song, X.; Xiong, Q.; et al. A Novel Top-Down Synthesis of Ultrathin 2D Boron Nanosheets for Multimodal Imaging-Guided Cancer Therapy. Adv. Mater. 2018, 30, e1803031. [Google Scholar] [CrossRef]
- Zhou, H.G.; Xia, R.Q.; Zheng, J.; Yuan, D.; Ning, G.H.; Li, D. Acid-triggered interlayer sliding of two-dimensional cop-per(i)-organic frameworks: More metal sites for catalysis. Chem. Sci. 2021, 12, 6280–6286. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Tsuru, T. Two-Dimensional Covalent Organic Framework (COF) Membranes Fabricated via the Assembly of Exfoliated COF Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 8433–8436. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.W.; Sun, C.; Castano, I.; Flanders, N.C.; Evans, A.M.; Vitaku, E.; McLeod, D.C.; Lambeth, R.H.; Chen, L.X.; Gianneschi, N.C.; et al. Acid Exfoliation of Imine-linked Covalent Organic Frameworks Enables Solution Processing into Crystalline Thin Films. Angew. Chem. Int. Ed. 2020, 59, 5165–5171. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Zhang, H.; Zhang, Z.; Zhang, C.; Huang, X.; Kozawa, D.; Liu, P.; Li, B.-G.; Wang, W.-J. Toward Covalent Organic Framework Metastructures. J. Am. Chem. Soc. 2021, 143, 5003–5010. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.M.; Xiao, Y.; Peng, L.; Duo, Y.; Wang, L.; Zhang, L.; Wang, B.; Zhang, H. Recent Advancement for the Synthesis of MXene Derivatives and Their Sensing Protocol. Adv. Mater. Technol. 2021, 6, 2001197. [Google Scholar] [CrossRef]
- Khan, N.A.; Zhang, R.; Wu, H.; Shen, J.; Yuan, J.; Fan, C.; Cao, L.; Olson, M.A.; Jiang, Z. Solid–Vapor Interface Engineered Covalent Organic Framework Membranes for Molecular Separation. J. Am. Chem. Soc. 2020, 142, 13450–13458. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhao, H.; Li, B.; Ma, H. A two-dimensional cationic covalent organic framework membrane for selective molecular sieving. J. Mater. Chem. A 2018, 6, 13331–13339. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Fang, Q.; Liu, F. Covalent triazine frameworks membrane with highly ordered skeleton nanopores for robust and precise molecule/ion separation. J. Membr. Sci. 2020, 595, 117525. [Google Scholar] [CrossRef]
- Halder, A.; Kandambeth, S.; Biswal, B.P.; Kaur, G.; Roy, N.C.; Addicoat, M.; Salunke, J.K.; Banerjee, S.; Vanka, K.; Heine, T.; et al. Decoding the Morphological Diversity in Two Dimensional Crystalline Porous Polymers by Core Planarity Modulation. Angew. Chem. Int. Ed. 2016, 55, 7806–7810. [Google Scholar] [CrossRef]
- Nguyen, V.; Grunwald, M. Microscopic Origins of Poor Crystallinity in the Synthesis of Covalent Organic Framework COF-5. J. Am. Chem. Soc. 2018, 140, 3306–3311. [Google Scholar] [CrossRef]
- Koo, B.T.; Heden, R.F.; Clancy, P. Nucleation and growth of 2D covalent organic frameworks: Polymerization and crystallization of COF monomers. Phys. Chem. Chem. Phys. 2017, 19, 9745–9754. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Karak, S.; Addicoat, M.; Bera, S.; Chakraborty, A.; Kunjattu, S.H.; Pachfule, P.; Heine, T.; Banerjee, R. Ultrastable Imine-Based Covalent Organic Frameworks for Sulfuric Acid Recovery: An Effect of Interlayer Hydrogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 5797–5802. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu H, S.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes. Adv. Mater. 2016, 29, 1603945. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-Flux Membranes Based on the Covalent Organic Framework COF-LZU1 for Selective Dye Separation by Nanofiltration. Angew. Chem. Int. Ed. 2018, 57, 4083–4087. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, C.; Yao, M.-S.; Kitagawa, S. Hybridization of Emerging Crystalline Porous Materials: Synthesis Dimensionality and Electrochemical Energy Storage Application. Adv. Energy Mater. 2022, 12, 2100321. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, W.; Wang, X. 3D Hierarchical Carbon-Rich Micro-/Nanomaterials for Energy Storage and Catalysis. Electrochem. Energy Rev. 2021, 4, 269–335. [Google Scholar] [CrossRef]
- Hu, Y.; Wayment, L.J.; Haslam, C.; Yang, X.; Lee, S.-H.; Jin, Y.; Zhang, W. Covalent organic framework based lithium-ion battery: Fundamental, design and characterization. EnergyChem 2021, 3, 100048. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Gao, Z.; Li, H.; Liu, S.; Cai, S.; Yang, X.; Guo, H.; Sun, X. Dual Active Site of the Azo and Carbon-yl-Modified Covalent Organic Framework for High-Performance Li Storage. ACS Energy Lett. 2020, 5, 1022–1031. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Hao, Z.; Zhao, Q.; Tang, J.; Zhang, Q.; Liu, J.; Jin, Y.; Wang, H. Functional separators towards the suppression of lithium dendrites for rechargeable high-energy batteries. Mater. Horizons 2020, 8, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liu, L.; Lu, Y.; Wang, C.; Li, Y.; Li, L.; Yan, Z.; Chen, J. Nitrogen-rich covalent organic frameworks with multiple carbonyls for high-performance sodium batteries. Nat. Commun. 2020, 11, 178. [Google Scholar] [CrossRef]
- Huang, J.; Golomb, M.J.; Kavanagh, S.R.; Tolborg, K.; Ganose, A.M.; Walsh, A. Band gap opening from displacive instabilities in layered covalent-organic frameworks. J. Mater. Chem. A 2022, 10, 13500–13507. [Google Scholar] [CrossRef]
- Zhai, L.; Wei, W.; Ma, B.; Ye, W.; Wang, J.; Chen, W.; Yang, X.; Cui, S.; Wu, Z.; Soutis, C.; et al. Cationic Covalent Organic Frameworks for Fabricating an Efficient Triboelectric Nanogenerator. ACS Mater. Lett. 2020, 2, 1691–1697. [Google Scholar] [CrossRef]
- Kong, L.; Liu, M.; Huang, H.; Xu, Y.; Bu, X.-H. Metal/Covalent-Organic Framework Based Cathodes for Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2100172. [Google Scholar] [CrossRef]
- Liu, M.; Kong, L.; Wang, X.; He, J.; Bu, X. Engineering Bimetal Synergistic Electrocatalysts Based on Metal–Organic Frameworks for Efficient Oxygen Evolution. Small 2019, 15, e1903410. [Google Scholar] [CrossRef]
- Yang, J.; Bin Tu, B.; Zhang, G.; Liu, P.; Hu, K.; Wang, J.; Yan, Z.; Huang, Z.; Fang, M.; Hou, J.; et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 2022, 17, 622–628. [Google Scholar] [CrossRef]
- Xu, F.; Yang, S.; Chen, X.; Liu, Q.; Li, H.; Wang, H.; Wei, B.; Jiang, D. Energy-storage covalent organic frameworks: Improving performance via engineering polysulfide chains on walls. Chem. Sci. 2019, 10, 6001–6006. [Google Scholar] [CrossRef]
- Meng, Y.; Luo, Y.; Shi, J.; Ding, H.; Lang, X.; Chen, W.; Zheng, A.; Sun, J.; Wang, C. 2D and 3D Porphyrinic Covalent Organic Frameworks: The Influence of Dimensionality on Functionality. Angew. Chem. Int. Ed. 2019, 59, 3624–3629. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Li, Z.-J.; Wei, L.; Ding, S.-Y.; Zhang, Y.-B.; Wang, W. A Dynamic Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 4995–4998. [Google Scholar] [CrossRef]
- Fang, Q.; Sui, C.; Wang, C.; Zhai, T.; Zhang, J.; Liang, J.; Guo, H.; Sandoz-Rosado, E.; Lou, J. Strong and flaw-insensitive two-dimensional covalent organic frameworks. Matter 2021, 4, 1017–1028. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Gui, B.; Lin, G.; Fu, Q.; Yin, S.; Liu, X.; Sun, J.; Wang, C. A Crystalline Three-Dimensional Covalent Organic Framework with Flexible Building Blocks. J. Am. Chem. Soc. 2021, 143, 2123–2129. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.-D.; Liu, Y.; Li, Y.; Cheng, C.; Zhu, H.; Yan, X.; Li, Z.; Gu, Z.-G. A two-dimensional semiconducting covalent organic framework with nickel(ii) coordination for high capacitive performance. J. Mater. Chem. A 2019, 7, 19676–19681. [Google Scholar] [CrossRef]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D Covalent Organic Framework Thin Films on Single-Layer Graphene. Science 2011, 332, 228–231. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2012, 42, 548–568. [Google Scholar] [CrossRef]
- Spitler, E.L.; Koo, B.T.; Novotney, J.L.; Colson, J.W.; Uribe-Romo, F.J.; Gutierrez, G.D.; Clancy, P.; Dichtel, W.R. A 2D Covalent Organic Framework with 4.7-nm Pores and Insight into Its Interlayer Stacking. J. Am. Chem. Soc. 2011, 133, 19416–19421. [Google Scholar] [CrossRef]
- Yan, W.; Gao, X.; Yang, J.; Xiong, X.; Xia, S.; Huang, W.; Chen, Y.; Fu, L.; Zhu, Y.; Wu, Y. Boosting Polysulfide Catalytic Conversion and Facilitating Li + Transportation by Ion-Selective COFs Composite Nanowire for Li-S Batteries. Small 2022, 18, 2106679. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.; Li, X.; Cui, M.; Gao, Y.; Xu, H.; Xu, X. In situ form core-shell carbon nanotube-imide COF composite for high performance negative electrode of pseudocapacitor. Electrochim. Acta 2022, 421. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, H.; Wang, J.; Hong, J.; Sun, W.; Wu, Y.; Wang, Y. Boosting the Capacity of Aqueous Li-Ion Capacitors via Pinpoint Surgery in Nanocoral-Like Covalent Organic Frameworks. Small Methods 2022. [Google Scholar] [CrossRef]
- Haldar, S.; Rase, D.; Shekhar, P.; Jain, C.; Vinod, C.P.; Zhang, E.; Shupletsov, L.; Kaskel, S.; Vaidhyanathan, R. Incorporating Conducting Polypyrrole into a Polyimide COF for Carbon-Free Ultra-High Energy Supercapacitor. Adv. Energy Mater. 2022, 12. [Google Scholar] [CrossRef]
- Kang, K.; Wu, Z.; Zhao, M.; Li, Z.; Ma, Y.; Zhang, J.; Wang, Y.; Sajjad, M.; Tao, R.; Qiu, L. A nanostructured covalent organic framework with readily accessible triphenylstibine moieties for high-performance supercapacitors. Chem. Commun. 2022, 58, 3649–3652. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yu, F.; Jiang, Y.; Su, J.; Ke, S.-W.; Tie, Z.; Zuo, J.-L.; Jin, Z. Self-Assembly Construction of Carbon Nanotube Network-Threaded Tetrathiafulvalene-Bridging Covalent Organic Framework Composite Anodes for High-Performance Hybrid Lithium-Ion Capacitors. Small Struct. 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; An, Y.; Wei, C.; Tan, L.; Xi, B.; Xiong, S.; Feng, J. Ultrastable and High-Rate 2D Siloxene Anode Enabled by Covalent Organic Framework Engineering for Advanced Lithium-lon Batteries. Small Methods 2022, 6, 2200306. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Li, Y.; Zhang, G.; Xing, G.; Chen, L. Arylamine-Linked 2D Covalent Organic Frameworks for Efficient Pseudocapacitive Energy Storage. Angew. Chem. Int. Ed. 2021, 60, 20754–20759. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kim, J.; Kang, B.; Moon, J.; Kim, S.; Kim, W.Y.; Byon, H.R. Thiazole-Linked Covalent Organic Framework Promoting Fast Two-Electron Transfer for Lithium-Organic Batteries. Adv. Energy Mater. 2021, 11, 2003735. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Li, D.; Tang, L.; Xue, M.; Zhuang, Z.; Yan, Y.; Valtchev, V.; Qiu, S.; et al. Exfoliated Mesoporous 2D Covalent Organic Frameworks for High-Rate Electrochemical Double-Layer Capacitors. Adv. Mater. 2020, 32, 1907289. [Google Scholar] [CrossRef]
- An, N.; Guo, Z.; Xin, J.; He, Y.; Xie, K.; Sun, D.; Dong, X.; Hu, Z. Hierarchical porous covalent organic framework/graphene aerogel electrode for high-performance supercapacitors. J. Mater. Chem. A 2021, 9, 16824–16833. [Google Scholar] [CrossRef]
- Amin, K.; Zhang, J.; Zhou, H.-Y.; Lu, R.; Zhang, M.; Ashraf, N.; YueLi, C.; Mao, L.; Faul, C.F.J.; Wei, Z. Surface controlled pseudo-capacitive reactions enabling ultra-fast charging and long-life organic lithium ion batteries. Sustain. Energy Fuels 2020, 4, 4179–4185. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, S.; Strømme, M.; Xu, C. Redox active covalent organic framework-based conductive nanofibers for flexible energy storage device. Carbon 2021, 171, 248–256. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Lu, Y.; Hua, X.; Liu, J.; Yang, Z.; Wei, H.; Mai, Y. All-organic covalent organic framework/polyaniline composites as stable electrode for high-performance supercapacitors. Mater. Lett. 2018, 236, 354–357. [Google Scholar] [CrossRef]
| COFs | BET (m2 g−1) | Pores Size (nm) | Specific Capacitance | Power Density | Energy Density | Capacity Retention | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Tf–TAPA | 159.55 | 1.47 | 583 mAh g−1 at 2 A g−1 | 96% (1500) at 8 A g−1 | LIBs | [132] | ||
| AAm–TPB | 403 | 2.99 | 271 F g−1 at 1 A g−1 | 19.16 Wh kg−1 at 350 W kg−1 | 92% (10000) at 5 A g−1 | ASCs | [133] | |
| AZO–1 | 649 | 2.8 | 2800 W kg−1 | 140 mAh g−1 at 0.5 C | (5000) at 10 C | LIBs | [134] | |
| COF–316@PPy | 452 | 1.56 | 783.6 µF cm−2 at 3 µA cm−2 | 100% (3400) at 20 uA cm−2 | ASCs | [52] | ||
| g–C34N6–COF | 1003 | 15.2 mF cm−2 at 2 mV s−1 | 7.3 mWh cm−3 at 50 mW cm−3 | 93.1% (5000) | ASCs | [30] | ||
| e-COFs | 1170 | 3.4 | 5.46 mF cm−2 at 1000 mV s−1 | 1002 mW cm−3 at 0.23 mWh cm−3 | 100% (10,000) | EDLCs | [135] | |
| DAAQ–COFs/GA | 425.3 | 1.77 | 378 F g−1 at 1 A g−1 | 30.5 Wh kg−1 at 700 W kg−1 | 88.9% (20,000) at 5 A g−1 | ASCs | [136] | |
| AQ–COF@CNTs | 905 | 144 mAh g−1 at 50 mA g−1 | 100% (3000) at 250 mA g−1 | LIBs | [137] | |||
| c-CNT@COF–3 | 576.7 | 1.5 | 418.7 F g−1 at 0.2 A g−1 | 30.7 mWh cm−2 and 591.9 mW cm−2 | 94% (10,000) at 10 mA cm−2 | ASCs | [138] | |
| TpPa–COF@PANI | 574 | 1.3 | 95 F g−1 at 0.2 A g−1 | 83% (30000) at 5 A g−1 | ASCs | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, C.; Wang, R.; Yu, F.; Liu, H.; Guo, C.; Sun, K.; Li, J.; Bao, W. Conductive Covalent Organic Frameworks Meet Micro-Electrical Energy Storage: Mechanism, Synthesis and Applications—A Review. Crystals 2022, 12, 1405. https://doi.org/10.3390/cryst12101405
Qian C, Wang R, Yu F, Liu H, Guo C, Sun K, Li J, Bao W. Conductive Covalent Organic Frameworks Meet Micro-Electrical Energy Storage: Mechanism, Synthesis and Applications—A Review. Crystals. 2022; 12(10):1405. https://doi.org/10.3390/cryst12101405
Chicago/Turabian StyleQian, Chengfei, Ronghao Wang, Feng Yu, He Liu, Cong Guo, Kaiwen Sun, Jingfa Li, and Weizhai Bao. 2022. "Conductive Covalent Organic Frameworks Meet Micro-Electrical Energy Storage: Mechanism, Synthesis and Applications—A Review" Crystals 12, no. 10: 1405. https://doi.org/10.3390/cryst12101405
APA StyleQian, C., Wang, R., Yu, F., Liu, H., Guo, C., Sun, K., Li, J., & Bao, W. (2022). Conductive Covalent Organic Frameworks Meet Micro-Electrical Energy Storage: Mechanism, Synthesis and Applications—A Review. Crystals, 12(10), 1405. https://doi.org/10.3390/cryst12101405










