Bis-Citrullinato Copper(II) Complex: Synthesis, Crystal Structure, and Non-Covalent Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of [Cu(Citr)2]n
2.2. Characterization Methods
2.3. Computational Details
3. Results
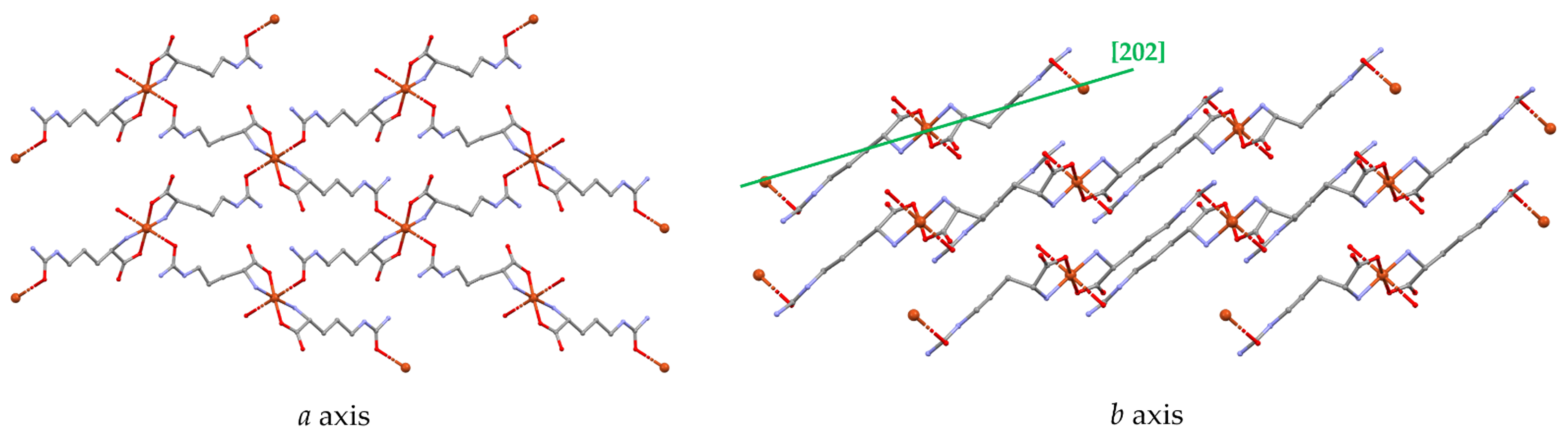
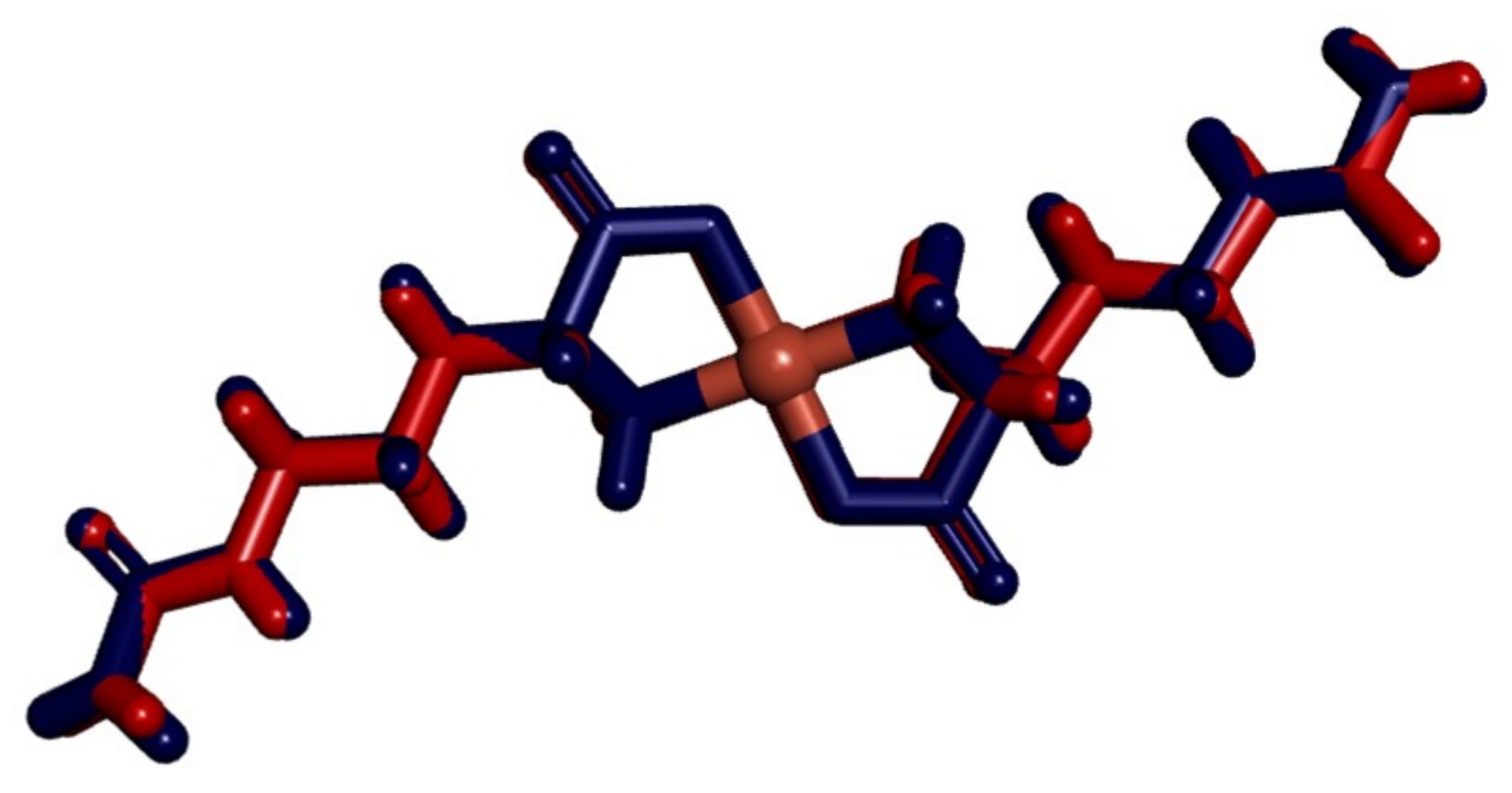
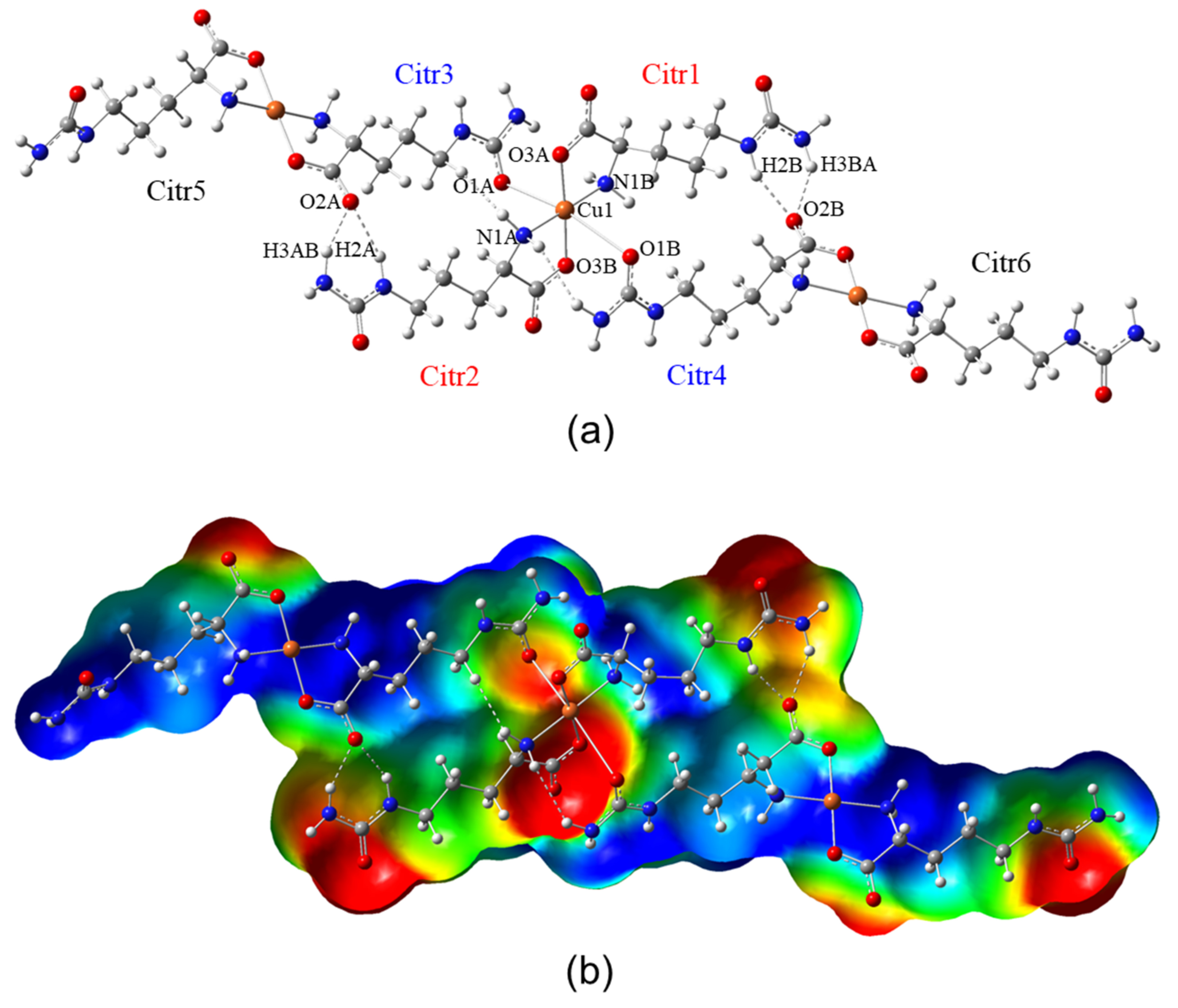
3.1. Structural Description
3.2. Characterization of the Complex
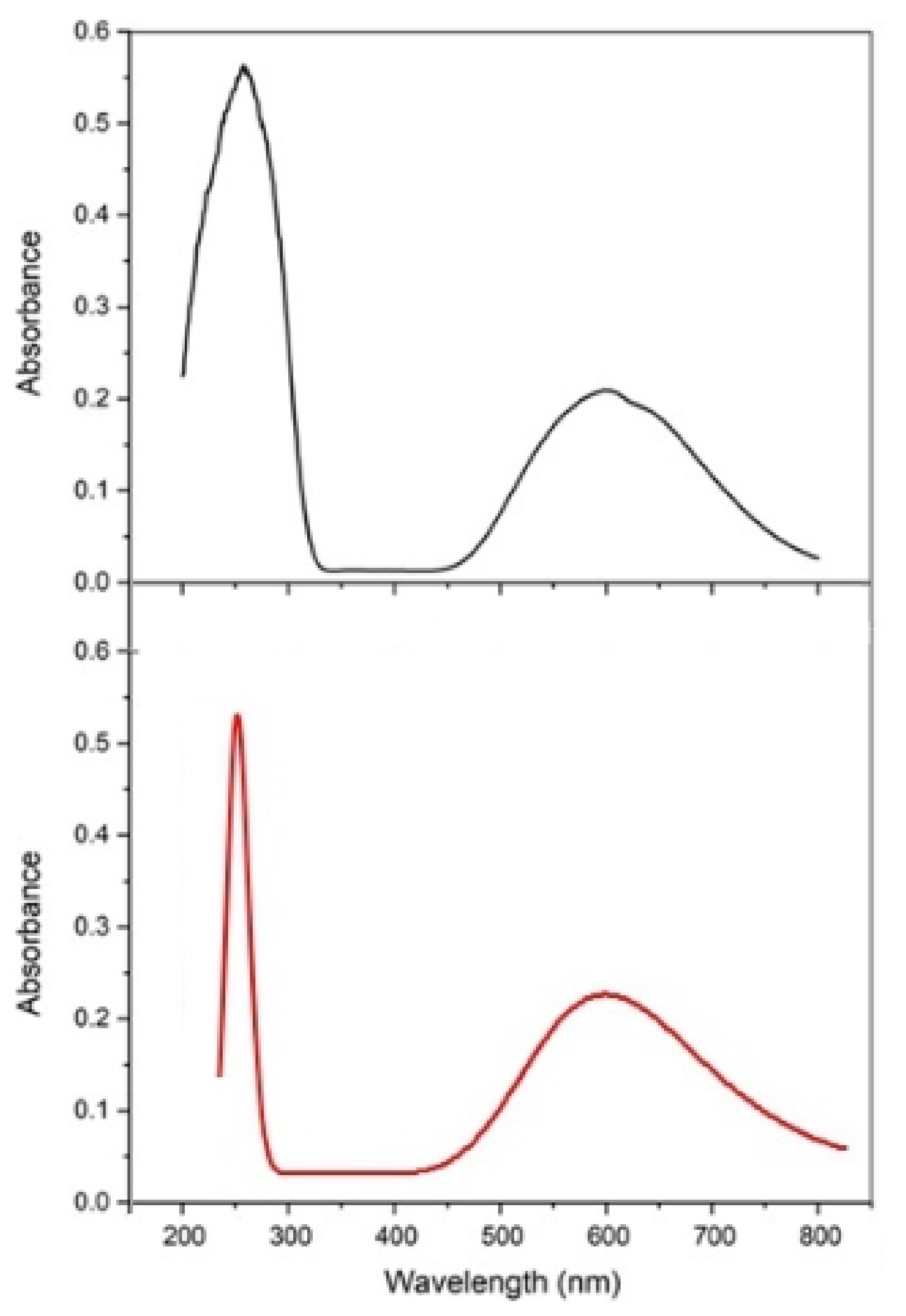
3.2.1. UV-Visible Spectroscopy
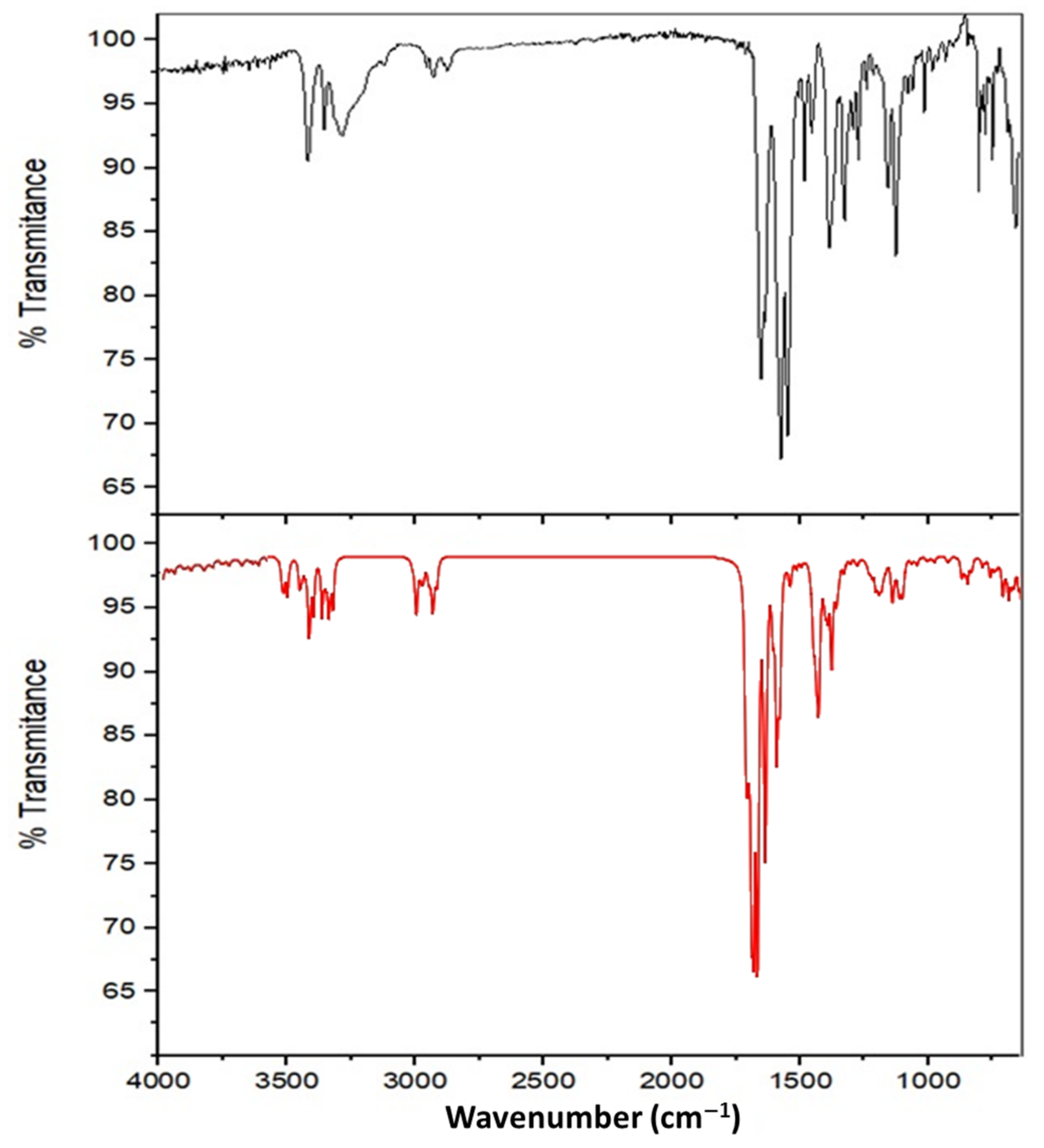
3.2.2. Infrared Spectroscopy
3.2.3. Raman Spectroscopy
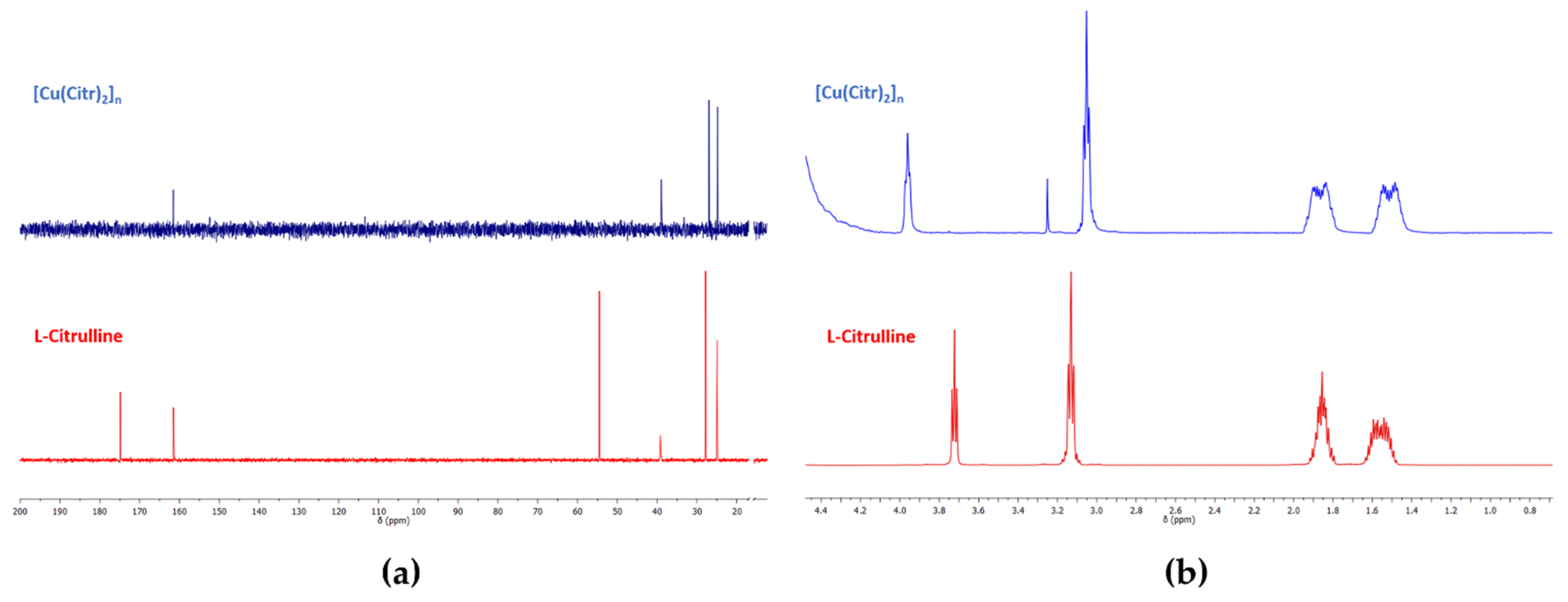
3.2.4. NMR Studies
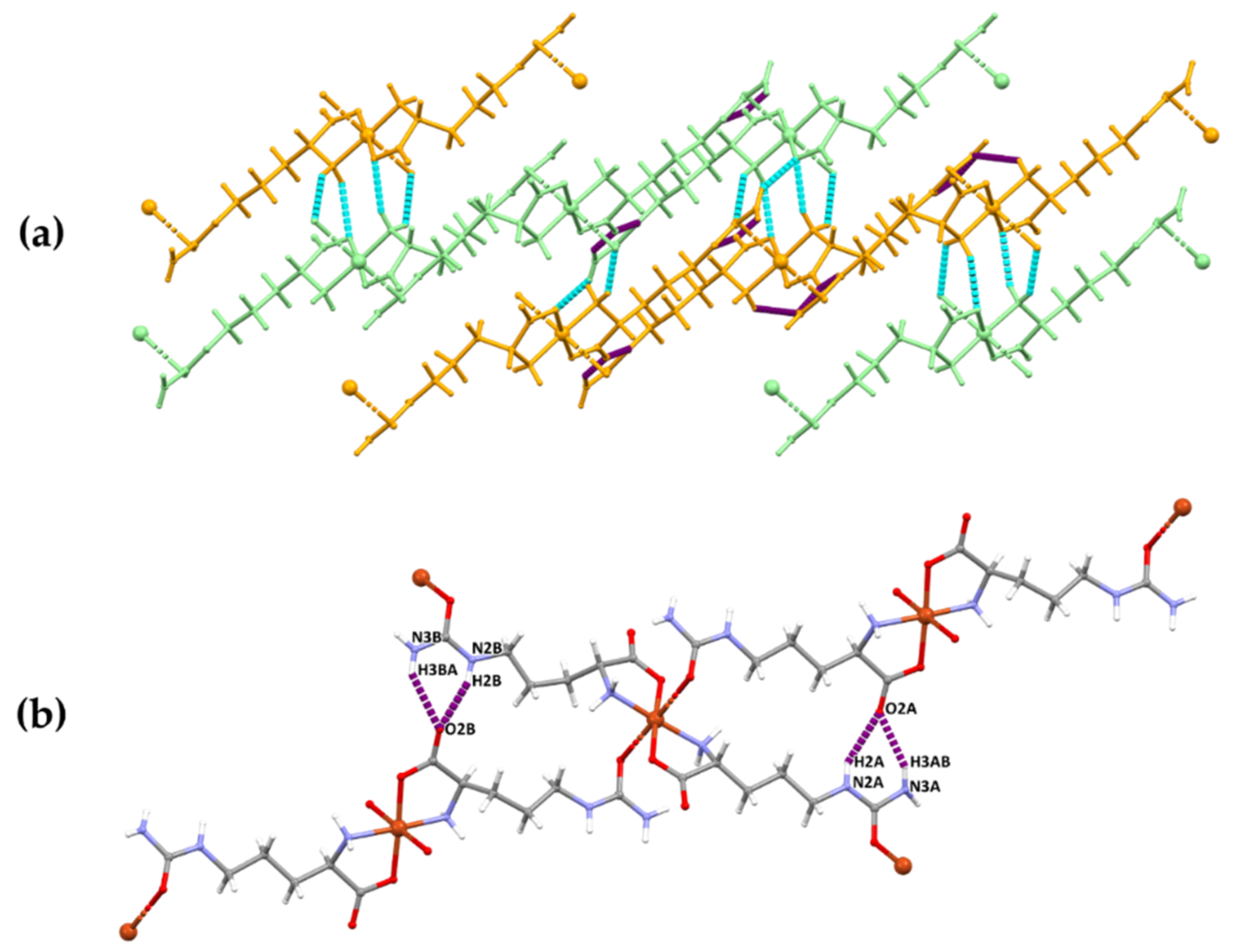
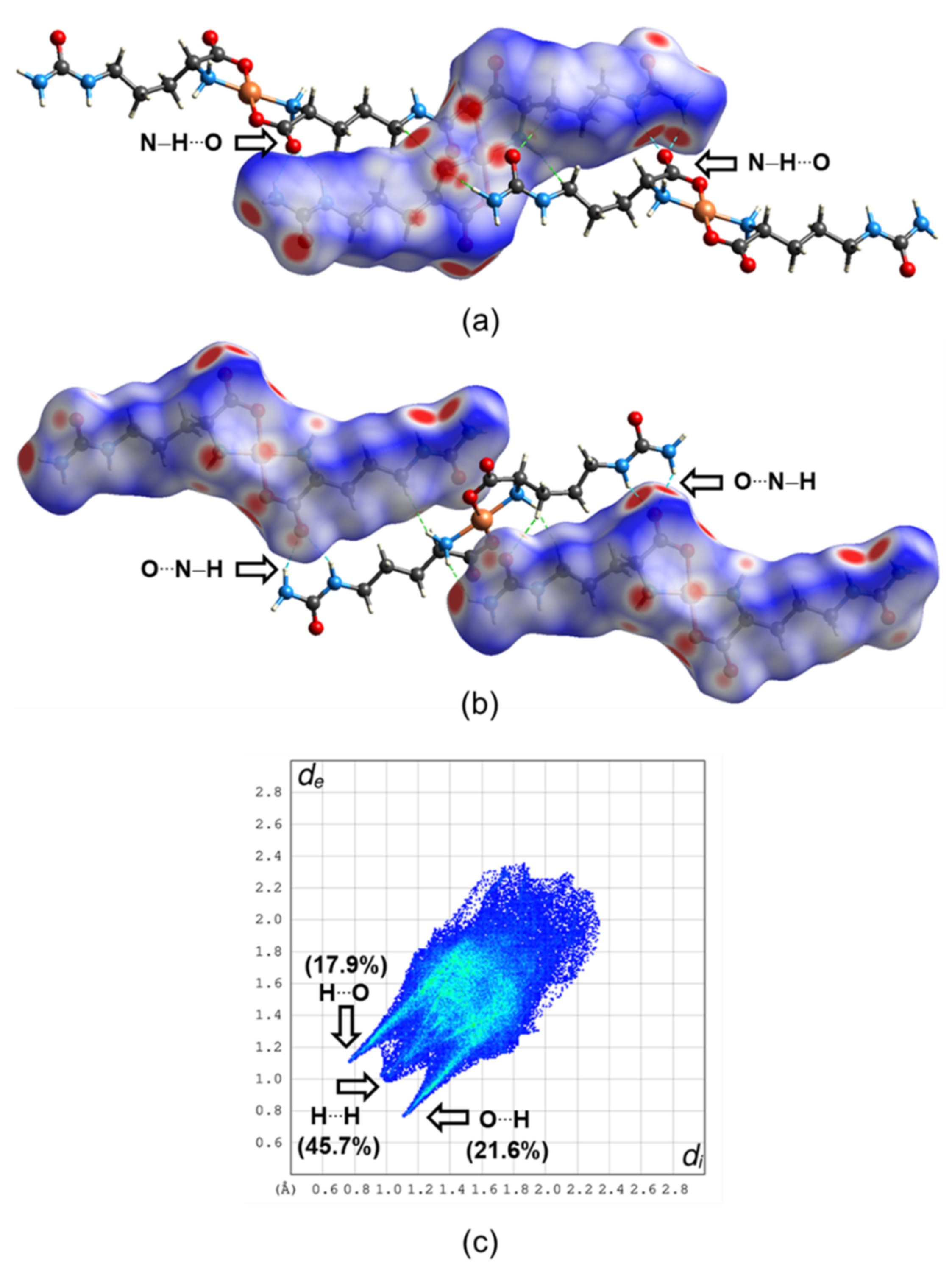
3.3. Molecular Structure and Non-Covalent Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wada, M. On the Occurrence of a New Amino Acid in Watermelon, Citrullus Vulgaris, Schrad. Bull. Agric. Chem. Soc. Jpn. 1930, 6, 32–34. [Google Scholar] [CrossRef]
- Fragkos, K.C.; Forbes, A. Was Citrulline First a Laxative Substance? The Truth about Modern Citrulline and Its Isolation. Nihon Ishigaku Zasshi 2011, 57, 275–292. [Google Scholar] [PubMed]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of Citrulline in Watermelon Rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Kaore, S.N.; Kaore, N.M. Citrulline: Pharmacological Perspectives and Role as a Biomarker in Diseases and Toxicities. In Biomarkers in Toxicology; Gupta, R.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 883–905. ISBN 978-0-12-404630-6. [Google Scholar]
- Curis, E.; Crenn, P.; Cynober, L. Citrulline and the Gut. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 620–626. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost All about Citrulline in Mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in Health and Disease. Review on Human Studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef]
- Endo, F.; Matsuura, T.; Yanagita, K.; Matsuda, I. Clinical Manifestations of Inborn Errors of the Urea Cycle and Related Metabolic Disorders during Childhood. J. Nutr. 2004, 134, 1605S–1609S. [Google Scholar] [CrossRef]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral L-Arginine and L-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon Consumption Increases Plasma Arginine Concentrations in Adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef]
- Gramaglia, I.; Velez, J.; Chang, Y.-S.; Caparros-Wanderley, W.; Combes, V.; Grau, G.; Stins, M.F.; van der Heyde, H.C. Citrulline Protects Mice from Experimental Cerebral Malaria by Ameliorating Hypoargininemia, Urea Cycle Changes and Vascular Leak. PLoS ONE 2019, 14, e0213428. [Google Scholar] [CrossRef]
- Kaore, S.N.; Amane, H.S.; Kaore, N.M. Citrulline: Pharmacological Perspectives and Its Role as an Emerging Biomarker in Future. Fundam. Clin. Pharmacol. 2013, 27, 35–50. [Google Scholar] [CrossRef]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.-C.; Cynober, L.; Sfar, S. Citrulline: From Metabolism to Therapeutic Use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef]
- Rashid, J.; Kumar, S.S.; Job, K.M.; Liu, X.; Fike, C.D.; Sherwin, C.M.T. Therapeutic Potential of Citrulline as an Arginine Supplement: A Clinical Pharmacology Review. Paediatr. Drugs 2020, 22, 279–293. [Google Scholar] [CrossRef]
- Alghamdi, M.; Alasmari, D.; Assiri, A.; Mattar, E.; Aljaddawi, A.A.; Alattas, S.G.; Redwan, E.M. An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders. J. Immunol. Res. 2019, 2019, 7592851. [Google Scholar] [CrossRef]
- Maric, S.; Restin, T.; Muff, J.L.; Camargo, S.M.; Guglielmetti, L.C.; Holland-cunz, S.G.; Crenn, P.; Vuille-dit-bille, R.N. Citrulline, Biomarker of Enterocyte Functional Mass and Dietary Supplement. Metabolism, Transport, and Current Evidence for Clinical Use. Nutrients 2021, 13, 2794. [Google Scholar] [CrossRef]
- Crans, D.C.; Kostenkova, K. Open Questions on the Biological Roles of First-Row Transition Metals. Commun. Chem. 2020, 3, 104. [Google Scholar] [CrossRef]
- Fox, S.W. The Preparation of Citrulline by Hydrolysis of Arginine. J. Biol. Chem. 1938, 123, 687–690. [Google Scholar] [CrossRef]
- Kurtz, A.C. A Simple Synthesis of Dl-Citrulline. J. Biol. Chem. 1938, 122, 477–484. [Google Scholar] [CrossRef]
- Trikha, K.C.; Nair, B.C.; Singh, R.P. Complexation of Bivalent Metal Ions with Amino Acids. I. L-Citrulline Complexes. Indian J. Chem. 1968, 6, 532. [Google Scholar]
- Clarke, E.R.; Martell, A.E. Metal Chelates of Arginine and Related Ligands. J. Inorg. Nucl. Chem. 1970, 32, 911–926. [Google Scholar] [CrossRef]
- Yamauchi, O.; Sakurai, T.; Nakahara, A. Histidine-Containing Ternary Amino Acid-Copper(II) Complexes. Syntheses and Properties. J. Am. Chem. Soc. 1979, 101, 4164–4172. [Google Scholar] [CrossRef]
- Ganadu, M.L.; Leoni, V.; Crisponi, G.; Nurchi, V. An Investigation on the Interaction between Palladium(II) and L-Citrulline by 1H and 13C NMR Spectroscopy and Potentiometry. Polyhedron 1991, 10, 333–336. [Google Scholar] [CrossRef]
- Mascaliovas, B.Z.; Bergamini, F.R.G.; Cuin, A.; Corbi, P.P. Synthesis and Crystal Structure of a Palladium(II) Complex with the Amino Acid L-Citrulline. Powder Diffr. 2015, 30, 357–361. [Google Scholar] [CrossRef]
- Singh, M.; Shankar, V.; Singh, D.; Krishna, V. Chelation and Stabilization Properties of Citrulline and Uracil with Hg(II) as a Heavy Metal Ion in Solution. Chem. Sci. Trans. 2017, 6, 646–652. [Google Scholar] [CrossRef]
- Singh, M.; Sinha, S.; Krishna, V. Computed Distribution of Quaternary Complexes of Cu(II), Zn(II) Co(II) and Ni(II) with Citrulline and Tryphtophan as Primary Ligand and Thymine as Secondary Ligand. Proc. Natl. Acad. Sci. India Sect. A—Phys. Sci. 2021, 91, 1–7. [Google Scholar] [CrossRef]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Noriega, L.; Sánchez-Gaytán, B.L.; Castro, M.E.; Meléndez-Bustamante, F.; González-Vergara, E. Cyclo-Tetravanadate Bridged Copper Complexes as Potential Double Bullet pro-Metallodrugs for Cancer Treatment. J. Inorg. Biochem. 2020, 208, 111081. [Google Scholar] [CrossRef]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Sánchez-Gaytán, B.L.; Cerro-López, M.; Mendoza, A.; Castro, M.E.; Meléndez-Bustamante, F.J.; González-Vergara, E. Synthesis and Experimental-Computational Characterization of a Copper/Vanadium Compound with Potential Anticancer Activity. Crystals 2020, 10, 492. [Google Scholar] [CrossRef]
- Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Mendoza, A.; Meléndez-Bustamante, F.J.; Castro, M.E.; González-Vergara, E. Ternary Copper Complex of L-Glutamine and Phenanthroline as Counterions of Cyclo-Tetravanadate Anion: Experimental-Theoretical Characterization and Potential Antineoplastic Activity. Metals 2021, 11, 1541. [Google Scholar] [CrossRef]
- Su, C.-C.; Tai, T.-Y.; Wu, S.-P.; Wang, S.-L.; Liao, F.-L. Spectroscopic and Electronic Properties of Mixed Ligand Aminoacidatocopper(II) Complexes: Molecular Structure of [Cu(4,7-Dimethyl-1,10-Phenanthroline)(l-Phenylalaninato)](ClO4). Polyhedron 1999, 18, 2361–2368. [Google Scholar] [CrossRef]
- Pérez-Benítez, A.; Méndez-Rojas, M.Á.; Bernès, S.; González-Vergara, E. Hybrid (Electrochemical-Chemical) Single Crystal Synthesis of Copper Aspirinate Starting from an Aspirin Tablet: An Undergraduate Bioinorganic Experiment. Chem. Educ. J. 2008, 11, 11–18. [Google Scholar]
- Schotten, C.; Nicholls, T.P.; Bourne, R.A.; Kapur, N.; Nguyen, B.N.; Willans, C.E. Making Electrochemistry Easily Accessible to the Synthetic Chemist. Green Chem. 2020, 22, 3358–3375. [Google Scholar] [CrossRef]
- Bruker. APEX3, SAINT, and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The Calculations of Excited-State Properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Exchange Functionals with Improved Long-Range Behavior and Adiabatic Connection Methods without Adjustable Parameters: The MPW and MPW1PW Models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G * Basis Set for Atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 16, Revision, B.01; Gaussian Inc.: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Dennington, R.D.; Keith, T.A.; Millam, J.M. Gauss View; Version 6.0.16; Semichem, Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Keith, T.A. TK Gristmill Software; Version 19.02.13; AIMAll; TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Halcrow, M.A. Interpreting and Controlling the Structures of Six-Coordinate Copper(II) Centres—When Is a Compression Really a Compression? Dalt. Trans. 2003, 23, 4375–4384. [Google Scholar] [CrossRef]
- Hemissi, H.; Nasri, M.; Abid, S.; Al-Deyab, S.S.; Dhahri, E.; Hlil, E.K.; Rzaigui, M. Crystal Structure, Spectroscopic, Magnetic and Electronic Structure Studies of a Novel Cu(II) Amino Acid Complex [Cu(l-Arg)2(H2O)]2(P4O12)·8H2O. J. Solid State Chem. 2012, 196, 489–497. [Google Scholar] [CrossRef]
- Stevanović, N.; Zlatar, M.; Novaković, I.; Pevec, A.; Radanović, D.; Matić, I.Z.; Đorđić Crnogorac, M.; Stanojković, T.; Vujčić, M.; Gruden, M.; et al. Cu(II), Mn(II) and Zn(II) Complexes of Hydrazones with a Quaternary Ammonium Moiety: Synthesis, Experimental and Theoretical Characterization and Cytotoxic Activity. Dalt. Trans. 2022, 51, 185–196. [Google Scholar] [CrossRef]
- Suktanarak, P.; Watchasit, S.; Chitchak, K.; Plainpan, N.; Chainok, K.; Vanalabhpatana, P.; Pienpinijtham, P.; Suksai, C.; Tuntulani, T.; Ruangpornvisuti, V.; et al. Tuning the Reactivity of Copper Complexes Supported by Tridentate Ligands Leading to Two-Electron Reduction of Dioxygen. Dalt. Trans. 2018, 47, 16337–16349. [Google Scholar] [CrossRef]
- Halcrow, M.A. Jahn-Teller Distortions in Transition Metal Compounds, and Their Importance in Functional Molecular and Inorganic Materials. Chem. Soc. Rev. 2013, 42, 1784–1795. [Google Scholar] [CrossRef]
- Jezowska-Trzebiatowska, B.; Gerega, K.; Vogt, A. Cobalt(II) Complexes Reversibly Uptaking Nitric Oxide in Aqueous Solution. Inorg. Chim. Acta 1978, 31, 183–185. [Google Scholar] [CrossRef]
- Mofidabadi, A.H.J.; Dehghani, A.; Ramezanzadeh, B. Investigating the Effectiveness of Watermelon Extract-Zinc Ions for Steel Alloy Corrosion Mitigation in Sodium Chloride Solution. J. Mol. Liq. 2022, 346, 117086. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Smith, D.W. Ligand Field Splittings in Copper (II) Compounds. In Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1972; Volume 12, pp. 49–112. [Google Scholar]
- Hathaway, B.J. A New Look at the Stereochemistry and Electronic Properties of Complexes of the Copper(II) Ion. In Complex Chemistry. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1984; pp. 55–118. ISBN 978-3-540-13411-4. [Google Scholar]
- Stanila, A.; Marcu, A.; Rusu, D.; Rusu, M.; David, L. Spectroscopic Studies of Some Copper(II) Complexes with Amino Acids. J. Mol. Struct. 2007, 834, 364–368. [Google Scholar] [CrossRef]
- Reddy, S.L.; Endo, T.; Reddy, G.S. Electronic (Absorption) Spectra of 3d Transition Metal Complexes. In Advanced Aspects of Spectroscopy; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2012; pp. 3–48. [Google Scholar]
- Wojciechowska, A.; Szuster-Ciesielska, A.; Sztandera, M.; Bregier-Jarzębowska, R.; Jarząb, A.; Rojek, T.; Komarnicka, U.K.; Bojarska-Junak, A.; Jezierska, J. L-Argininato Copper(II) Complexes in Solution Exert Significant Selective Anticancer and Antimicrobial Activities. Appl. Organomet. Chem. 2020, 34, e5698. [Google Scholar] [CrossRef]
- Sciortino, G.; Maréchal, J.-D.; Fábián, I.; Lihi, N.; Garribba, E. Quantitative Prediction of Electronic Absorption Spectra of Copper(II)-Bioligand Systems: Validation and Applications. J. Inorg. Biochem. 2020, 204, 110953. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.; Viera, I.; Torre, M.H.; Kremer, E.; Etcheverry, S.B.; Baran, E.J. Infrared Spectra of the Copper (II) Complexes of Amino Acids with Hydrophobic Residues. Acta Farm. Bonaer. 1998, 17, 213–218. [Google Scholar]
- Baran, E.J.; Wagner, C.C.; Torre, M.H.; Kremer, E.; Kögerler, P. Vibrational Spectra of the Cu(II) Complexes of Aspartic and Glutamic Acids. Acta Farm. Bonaer. 2000, 19, 231–234. [Google Scholar]
- Jenkins, A.L.; Larsen, R.A.; Williams, T.B. Characterization of Amino Acids Using Raman Spectroscopy. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2005, 61, 1585–1594. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2009; ISBN 9780471743392. [Google Scholar]
- Sreevalsa, V.G.; Jayalekshmi, S. Investigations on the Growth and Characterization of L-Citrulline Oxalate Monohydrate Single Crystal. J. Cryst. Growth 2011, 324, 172–176. [Google Scholar] [CrossRef]
- Freire, P.T.C.; Barboza, F.M.; Lima, J.A.; Melo, F.E.A.; Filho, J.M. Raman Spectroscopy of Amino Acid Crystals. In Raman Spectroscopy and Applications; Khan, M., Ed.; IntechOpen: London, UK, 2017; pp. 201–223. [Google Scholar]
- Fernández, L.E.; Delgado, G.E.; Maturano, L.V.; Tótaro, R.M.; Varetti, E.L. Experimental and Theoretical Vibrational Study of N-Carbamoyl-L-Proline. J. Mol. Struct. 2018, 1168, 84–91. [Google Scholar] [CrossRef]
- Vusak, D.; Pejic, J.; Jurkovic, M.; Szalontai, G.; Sabolovic, J. Coordination Polymers of Paramagnetic Bis(Leucinato)Copper(Ii) Diastereomers: Experimental and Computational Study of the Stereoisomerism and Conformations. CrystEngComm 2020, 22, 5587–5600. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Y.; Oldfield, E. Nuclear Magnetic Resonance Shifts in Paramagnetic Metalloporphyrins and Metalloproteins. J. Am. Chem. Soc. 2002, 124, 13911–13920. [Google Scholar] [CrossRef]
- Patil, B.H.; Peraje, P.; Naik, D.; Rajaramakrishna, R.; Dittmer, J.; Kumar, S.; Swamy, K. Experimental 1H and 13C Solid-State NMR Signal Assignment of Paramagnetic Copper (II) 2-Pyrazine-Carboxylate Complex Using Density Functional Theory Calculations. J. Phys. Conf. Ser. 2021, 1819, 012032. [Google Scholar] [CrossRef]
- Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Méndez-Rojas, M.Á.; Melendez, F.J.; Castro, M.E.; González-Vergara, E. Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment. Molecules 2020, 25, 4679. [Google Scholar] [CrossRef]
- Caruso, A.; Rossi, M.; Gahn, C.; Caruso, F. A Structural and Computational Study of Citrulline in Biochemical Reactions. Struct. Chem. 2017, 28, 1581–1589. [Google Scholar] [CrossRef]
- Jia, G.-G.; Liu, Y.; Zheng, Y.-G.; Zhang, L.; Liu, L.; Ling, X.; Gou, L.-S.; Yin, C.; Zhuang, X.-M. A Novel Preparation of L-Citrulline and L-Homocitrulline. J. Chem. Soc. Pak. 2012, 34, 451–454. [Google Scholar]
- Olivard, J.; Metzler, D.E.; Snell, E.E. Catalytic Racemization of Amino Acids by Pyridoxal and Metal Salts. J. Biol. Chem. 1952, 199, 669–674. [Google Scholar] [CrossRef]
- Abbott, E.H.; Martell, A.E. Mechanism of Formation, Structure, Stereochemistry, and Racemization of Bis[Pyridoxylidene (Amino Acidato)]Aluminum(III) Complexes. J. Am. Chem. Soc. 1970, 92, 5845–5851. [Google Scholar] [CrossRef]
- Fu, R.; So, S.M.; Lough, A.J.; Chin, J. Hydrogen Bond Assisted L to D Conversion of α-Amino Acids. Angew. Chemie Int. Ed. 2020, 59, 4335–4339. [Google Scholar] [CrossRef]
- Kolarovič, A.; Jakubec, P. State of the Art in Crystallization-Induced Diastereomer Transformations. Adv. Synth. Catal. 2021, 363, 4110–4158. [Google Scholar] [CrossRef]
- Augeri, D.J.; Robl, J.A.; Betebenner, D.A.; Magnin, D.R.; Khanna, A.; Robertson, J.G.; Wang, A.; Simpkins, L.M.; Taunk, P.; Huang, Q.; et al. Discovery and Preclinical Profile of Saxagliptin (BMS-477118): A Highly Potent, Long-Acting, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 5025–5037. [Google Scholar] [CrossRef]
- Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier De Gouville, A.C.; Coste, H.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. The Discovery of Tadalafil: A Novel and Highly Selective PDE5 Inhibitor. 1: 5,6,11,11a-Tetrahydro-1H-Imidazo[1′,5′:1,6]Pyrido[3,4-b]Indole-1,3(2H)-Dione Analogues. J. Med. Chem. 2003, 46, 4525–4532. [Google Scholar] [CrossRef]
- Herbert, J.M.; Frehel, D.; Vallee, E.; Kieffer, G.; Gouy, D.; Berger, Y.; Necciari, J.; Defreyn, G.; Maffrand, J.P. Clopidogrel, A Novel Antiplatelet and Antithrombotic Agent. Cardiovasc. Drug Rev. 1993, 11, 180–198. [Google Scholar] [CrossRef]
- Kuehl, F.A.; Wolf, F.J.; Trenner, N.R.; Peck, R.L.; Howe, E.; Hunnewell, B.D.; Downing, G.; Newstead, E.; Folkers, K.; Buhs, R.P.; et al. D-4-Amino-3-Isoxazolidone, A New Antibiotic. J. Am. Chem. Soc. 1955, 77, 2344–2345. [Google Scholar] [CrossRef]
- Kwong, A.D.; Kauffman, R.S.; Hurter, P.; Mueller, P. Discovery and Development of Telaprevir: An NS3-4A Protease Inhibitor for Treating Genotype 1 Chronic Hepatitis C Virus. Nat. Biotechnol. 2011, 29, 993–1003. [Google Scholar] [CrossRef]
- Anderson, J. Degarelix: A Novel Gonadotropin-Releasing Hormone Blocker for the Treatment of Prostate Cancer. Futur. Oncol. 2009, 5, 433–443. [Google Scholar] [CrossRef]
- Khan, M.L.; Stewart, A.K. Carfilzomib: A Novel Second-Generation Proteasome Inhibitor. Futur. Oncol. 2011, 7, 607–612. [Google Scholar] [CrossRef]
- So, S.M.; Kim, H.; Mui, L.; Chin, J. Mimicking Nature to Make Unnatural Amino Acids and Chiral Diamines. Eur. J. Org. Chem. 2012, 2012, 229–241. [Google Scholar] [CrossRef]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Keßeler, M.; Stürmer, R.; Zelinski, T. Industrial Methods for the Production of Optically Active Intermediates. Angew. Chem. Int. Ed. Engl. 2004, 43, 788–824. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T. Unusual Amino Acids in Medicinal Chemistry. J. Med. Chem. 2016, 59, 10807–10836. [Google Scholar] [CrossRef]




| [Cu(Citr)2]n | |
|---|---|
| Chemical formula | C12H24CuN6O6 |
| Formula weight (g mol−1) | 411.91 |
| CCDC | 2,172,421 |
| Crystal system | Monoclinic |
| Space group | P21 |
| Temperature (K) | 300.7 |
| a, b, c (Å) | 5.1270 (2), 9.2086 (4), 17.1099 (7) |
| β (°) | 93.972 (2) |
| V (Å3) | 805.86 (6) |
| Z | 2 |
| Radiation type | Cu Kα (1.54178 λ) |
| ρcalcd (g cm−3) | 1.698 |
| µ (mm−1) | 2.339 |
| Crystal size (mm) | 0.12 × 0.1 × 0.1 |
| GoF on F2 | 1.147 |
| R1 [I > 2σ(I)] | 0.0497 |
| R1 [all data] | 0.0512 |
| wR2 [I > 2σ(I)] | 0.1469 |
| wR2 [all data] | 0.1520 |
| D-H⋯A | D-H (Å) | H⋯A (Å) | D-H⋯A (Å) | Angle (°) |
|---|---|---|---|---|
| N2A-H2A⋯O2A i | 0.86 | 2.17 | 2.955(14) | 150.9 |
| N3A-H3AB⋯O2A i | 0.87 | 2.14 | 2.935(16) | 151.6 |
| N2B-H2B⋯O2B ii | 0.86 | 2.02 | 2.840(14) | 159.1 |
| N3B-H3BA⋯O2B ii | 0.86 | 2.26 | 2.997 | 144.1 |
| N1A iii-H1AA⋯O1A | 0.89 | 2.34 | 3.153 | 151.7 |
| N1A iv-H1AB⋯O3A | 0.89 | 2.22 | 2.996 | 145.6 |
| N3B v-H3BB⋯O1A | 0.86 | 2.07 | 2.928(12) | 171.4 |
| N3A-H3AA⋯O1B iv | 0.88 | 2.26 | 3.047 | 148.7 |
| Parameter | X-ray Data | Calculated |
|---|---|---|
| Cu1–O1A/Cu1–O1B | 1.974/1.940 | 1.978/1.965 |
| Cu1–N1A/Cu1–N1B | 1.993/1.958 | 2.018/2.027 |
| O1A–C1A/O1B–C1B | 1.271/1.286 | 1.290/1.290 |
| C1A–O2A/C1B–O2B | 1.231/1.217 | 1.237/1.237 |
| C1A–C2A/C1B–C2B | 1.529/1.531 | 1.535/1.531 |
| N1A–C2A/N1B–C2B | 1.502/1.461 | 1.474/1.474 |
| C2A–C3A/C2B–C3B | 1.528/1.541 | 1.523/1.533 |
| C3A–C4A/C3B–C4B | 1.501/1.516 | 1.525/1.527 |
| C4A–C5A/C4B–C5B | 1.525/1.477 | 1.520/1.522 |
| C5A–N2A/C5B–N2B | 1.476/1.429 | 1.450/1.447 |
| O1A–Cu1–N1B/O1B–Cu1–N1A | 96.91/94.43 | 98.00/96.07 |
| C1A–O1A–Cu1/C1B–O1B–Cu1 | 113.46/115.04 | 114.95/115.43 |
| C2A–N1A–Cu1/C2B–N1B–Cu1 | 104.76/110.23 | 106.74/108.31 |
| O1A–C1A–C2A/O1B–C1B–C2B | 116.38/116.54 | 115.56/116.86 |
| O2A–C1A–O1A/O2B–C1B–O1B | 123.16/123.33 | 123.90/124.08 |
| C2A–C3A–C4A/C2B–C3B–C4B | 112.72/116.79 | 112.26/115.66 |
| C3A–C4A–C5A/C3B–C4B–C5B | 110.52/116.08 | 112.72/114.08 |
| C4A–C5A–N2A/C4B–C5B–N2B | 111.02/112.14 | 109.92/109.49 |
| C1A–O1A–Cu1–N1A/C1B–O1B–Cu1–N1B | 12.41/−10.77 | 13.22/−10.06 |
| O2A–C1A–O1A–Cu1/O2B–C1B–O1B–Cu1 | −173.90/−177.11 | −177.30/179.08 |
| C1A–C2A–N1A–Cu1/C1B–C2B–N1B–Cu1 | 40.41/−17.16 | 36.87/−26.97 |
| C1A–C2A–C3A–H3AC/C1B–C2B–C3B–H3BC | −53.01/−51.68 | −48.62/−50.53 |
| C2A–C3A–C4A–C5A/C2B–C3B–C4B–C5B | 176.50/81.02 | 177.12/65.95 |
| C3A–C4A–C5A–N2A/C3B–C4B–C5B–N2B | 176.51/174.14 | −178.41/178.61 |
| λexp (nm) | λtheo (nm) | Eexc. (eV) | Osc. Strengths | Major Contributions |
|---|---|---|---|---|
| 720 | 665 | 1.87 | 0.0001 | H-6→L+1 (2%) |
| 651 | 1.90 | 0.0003 | H-8→L (4%) | |
| 642 | 1.93 | 0.0003 | H-4→L (3%) | |
| 600 | 600 | 2.06 | 0.0223 | H→L (95%) |
| 598 | 2.07 | 0.0105 | H-4→L (55%) | |
| 592 | 2.09 | 0.0009 | H-1→L (75%) | |
| 540 | 523 | 2.37 | 0.0003 | H-8→L+1 (3%) |
| 516 | 2.40 | 0.0005 | H-6→L+1 (2%) | |
| 511 | 2.43 | 0.0001 | H-2→L+1 (5%) | |
| 267 | 253 | 4.90 | 0.0436 | H→L (91%) |
| Experimental (cm−1) | mPW1PW91 (cm−1) | Assignments |
|---|---|---|
| 3450 | 3501 | νasym(NH2) amide |
| 3438 | νsym(NH2) amide + νsym(NH) amide | |
| 3416 | νsym(NH2) amide | |
| 3415 | νasym(NH2) amino | |
| 3150 | 3330 | νsym(NH2) amino |
2900 | 2951 2947 2909 | νasym(CH2) |
2800 | 2910 2904 | νsym(CH2) |
| 1652 | 1708 | ν(C=O) carbonylic group |
| 1636 | 1695 | νasym(O=C–O−) carboxylic group |
| 1575 | 1583 | δ(NH2) amide |
| 1549 | 1559 | δ(NH2) amino |
| 1385 | 1390 | νsym(O=C–O−) carboxylic group |
| 576 | ν(Cu–N) |
| Experimental | mPW1PW91 | Assignments |
|---|---|---|
| 3276 | 3330 | νsym(–NH2) amino |
| 2935–2929 | ν(CαH) | |
| 2924, 2910 | 2927, 2913 | ν(CH2) |
| 2895, 2881, 2874 | 2902–2894 | ν(CH) |
| 1684 | 1642–1619 | ν(C=O) amide |
| 1463–1460 | 1465–1436 | ν(C=O) carboxylic group |
| 1327–1297 | 1354–1307 | δ(CH2) + ν(O=C–O−) |
| 1127 | 1189–1163 | ν(C–N) amino |
| Nuclei | <Signal Assignment> | |||||
|---|---|---|---|---|---|---|
| 13C | 161.52 | 0.00000 | 0.00 | 151.32 | 151.32 | <C6A>, <C6B> |
| 57.40, 48.83 | 0.00014, 0.00005 | 16.45, 5.88 | 41.93, 39.54 | 58.38, 45.42 | <C5A>, <C5B> | |
| 38.94, 26.99 | 0.00004, -0.00005 | 4.70, −5.88 | 32.61, 32.39 | 37.31, 26.52 | <C3A>, <C3B> | |
| 24.78, 16.74 | −0.00003, −0.00007 | 3.53, −8.23 | 27.76, 24.85 | 24.24, 16.63 | <C4A>, <C4B> | |
| Not observed | −0.00367, −0.00363 | −431.23, −426.53 | 173.05, 176.55 | −258.17, −249.97 | <C1A>, <C1B> | |
| Not observed | −0.00313, −0.00319 | −367.78, −374.83 | 56.75, 53.63 | −311.02, −321.19 | <C2A>, <C2B> | |
| 1H | 3.96 | 0.00001 | 1.07 | 3.32 | 4.38 | <H2AA> |
| Not observed | 0.00101 | 107.56 | 3.19 | 110.75 | <H2BA> | |
| 3.05 | 0.00000 | 0.00 | 2.91, 3.03 | 2.91, 3.03 | <H5AA>, <H5BA> | |
| 3.05 | 0.00000 | 0.00 | 2.89, 2.89 | 2.89, 2.89 | <H5AB>, <H5BB> | |
| 1.87, 1.87 | 0.00057, 0.00000 | 60.70, 0.00 | 1.85, 1.77 | 62.55, 1.77 | <H3AD>, <H3BD> | |
| 1.87, 1.87 | 0.00001, 0.00000 | 1.07, 0.00 | 1.21, 2.01 | 2.27, 2.01 | <H3AC>, <H3BC> | |
| 1.51 | 0.00000 | 0.00 | 1.33, 1.68 | 1.33, 1.68 | <H4AA>, <H4BA> | |
| 1.51 | 0.00000 | 0.00 | 1.56, 1.57 | 1.56, 1.57 | <H4AB>, <H4BB> | |
| Not observed | −0.00118, −0.00108 | −125.66, −115.02 | 1.23, 1.68 | −124.44, −113.34 | <H1AA>, <H1BA> | |
| Not observed | −0.00126, −0.00136 | −134.18, −144.83 | 1.98, 1.87 | −132.20, −142.96 | <H1AB>, <H1BB> |
| BCP | ρ(r) | ∇2ρ(r) | EH⋯Y |
|---|---|---|---|
| N-H3AB/H3BA⋯O2A/O2B | 0.0225–0.0246 | 0.0760–0.0898 | 4.52–5.93 |
| N-H2A/H2B⋯O2A/O2B | 0.0172–0.0178 | 0.0770–0.0994 | 4.49–6.78 |
| Cu1⋯N1A/N1B | 0.0790–0.0807 | 0.4261–0.4349 | 39.28–40.47 |
| Cu1⋯O1A/O1B | 0.0749–0.0775 | 0.5133–0.5334 | 44.43–57.73 |
| Cu1⋯O3A/O3B | 0.0057–0.0082 | 0.0156–0.0208 | 1.26–1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Contreras, D.; García-García, A.; Sánchez-Gaytán, B.L.; Serrano-de la Rosa, L.E.; Melendez, F.J.; Choquesillo-Lazarte, D.; Rodríguez-Diéguez, A.; Castro, M.E.; González-Vergara, E. Bis-Citrullinato Copper(II) Complex: Synthesis, Crystal Structure, and Non-Covalent Interactions. Crystals 2022, 12, 1386. https://doi.org/10.3390/cryst12101386
Ramírez-Contreras D, García-García A, Sánchez-Gaytán BL, Serrano-de la Rosa LE, Melendez FJ, Choquesillo-Lazarte D, Rodríguez-Diéguez A, Castro ME, González-Vergara E. Bis-Citrullinato Copper(II) Complex: Synthesis, Crystal Structure, and Non-Covalent Interactions. Crystals. 2022; 12(10):1386. https://doi.org/10.3390/cryst12101386
Chicago/Turabian StyleRamírez-Contreras, Diego, Amalia García-García, Brenda L. Sánchez-Gaytán, Laura E. Serrano-de la Rosa, Francisco J. Melendez, Duane Choquesillo-Lazarte, Antonio Rodríguez-Diéguez, María Eugenia Castro, and Enrique González-Vergara. 2022. "Bis-Citrullinato Copper(II) Complex: Synthesis, Crystal Structure, and Non-Covalent Interactions" Crystals 12, no. 10: 1386. https://doi.org/10.3390/cryst12101386
APA StyleRamírez-Contreras, D., García-García, A., Sánchez-Gaytán, B. L., Serrano-de la Rosa, L. E., Melendez, F. J., Choquesillo-Lazarte, D., Rodríguez-Diéguez, A., Castro, M. E., & González-Vergara, E. (2022). Bis-Citrullinato Copper(II) Complex: Synthesis, Crystal Structure, and Non-Covalent Interactions. Crystals, 12(10), 1386. https://doi.org/10.3390/cryst12101386







