Comparative Study of Bulk and Nanoengineered Doped ZnSe
Abstract
1. Introduction
2. Experimental Methods and Results
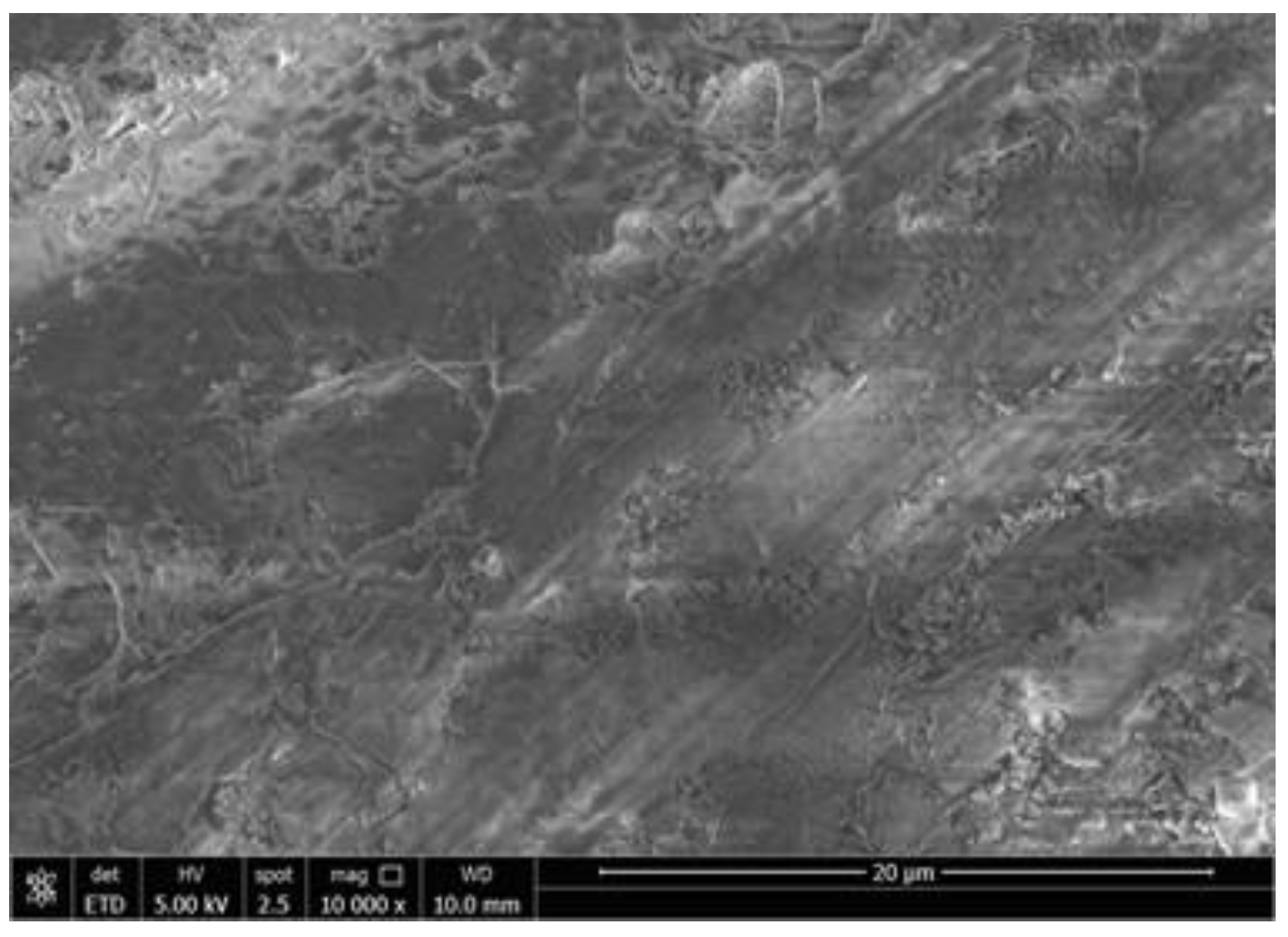
2.1. Growth of Bulk ZnSe and Morphology
2.2. Synthesis and Morphological Characteristics of Cr-Doped ZnSe Nanoparticles
2.3. Synthesis and Morphological Characteristics of Co-ZnSe Nanoparticles
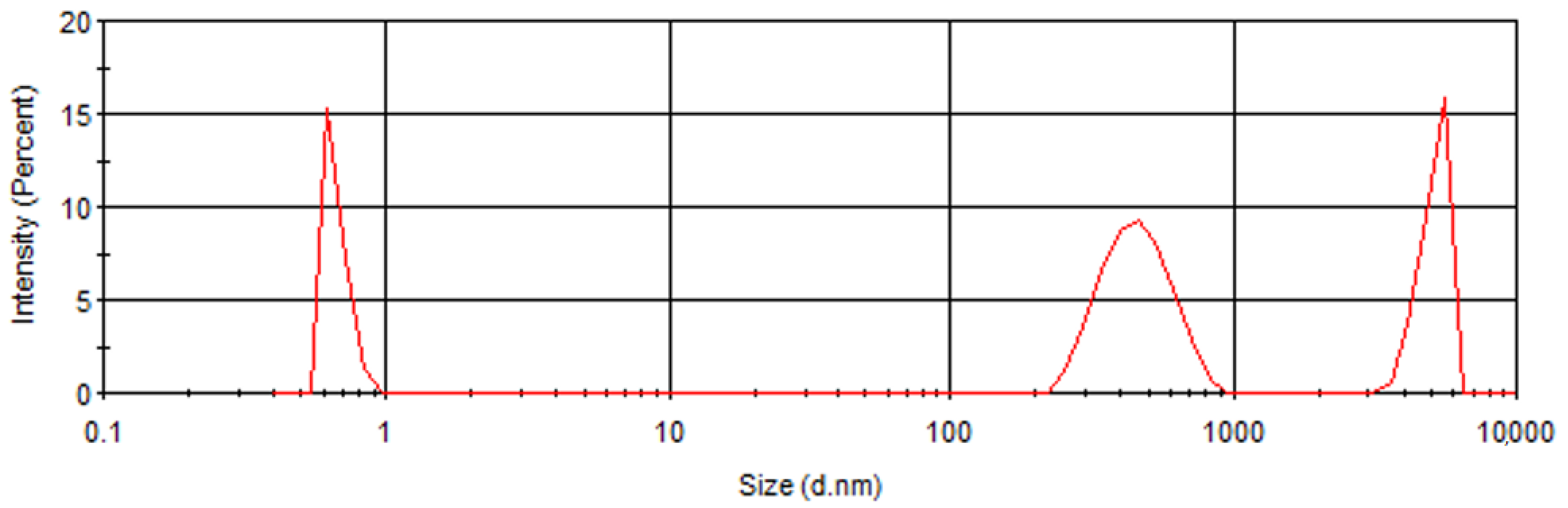
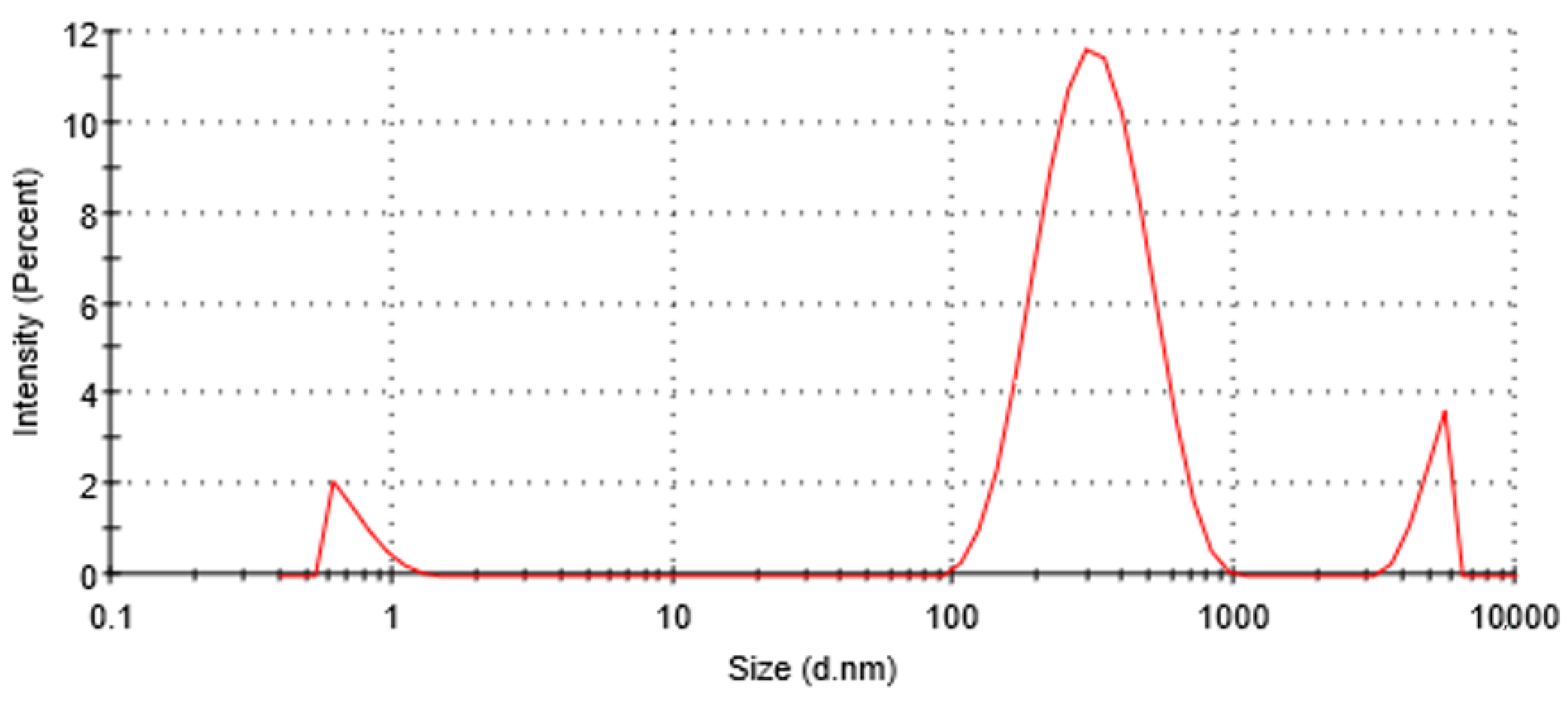
2.4. Measurement of Particle Size
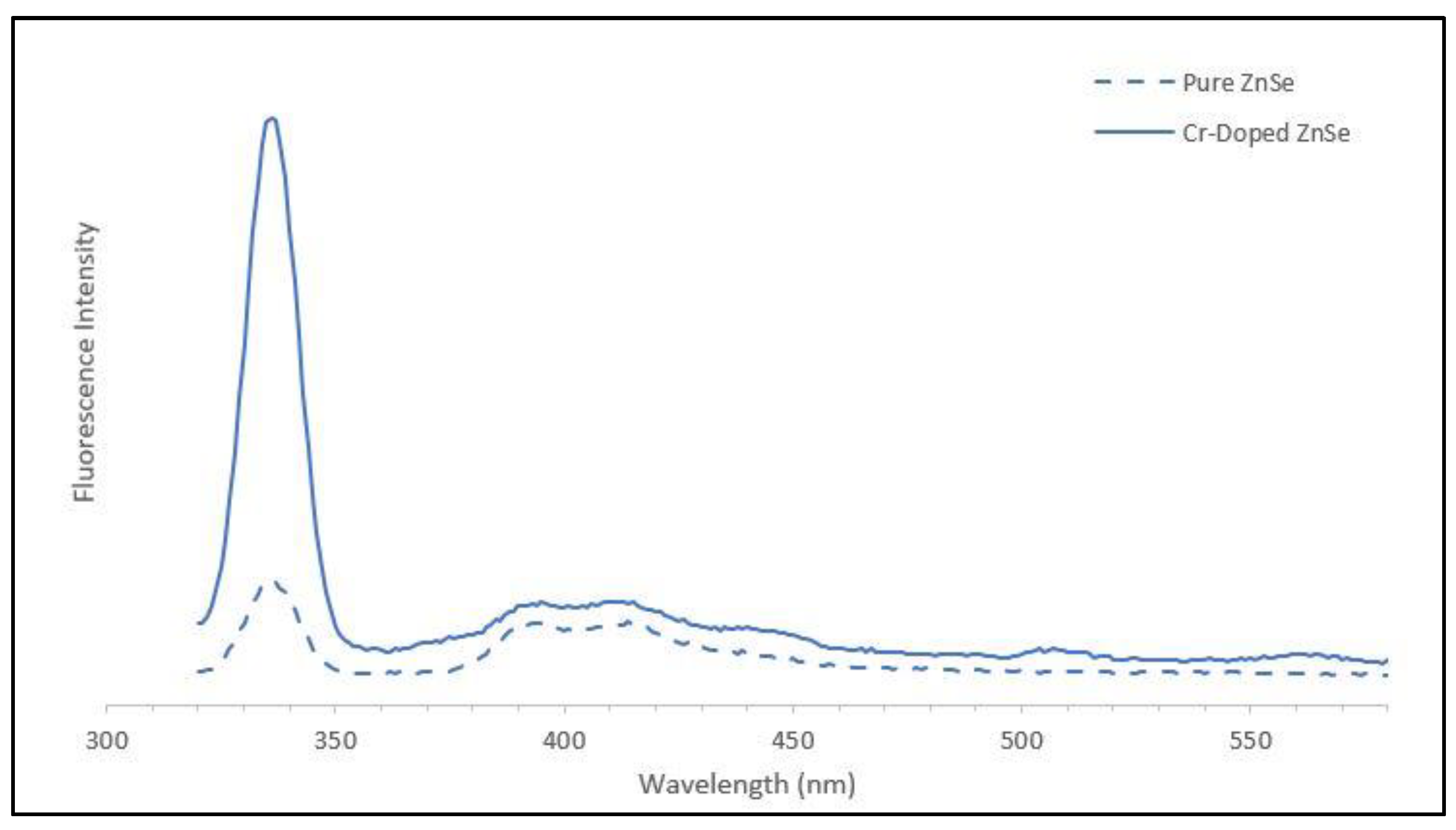
2.5. Optical Characterization
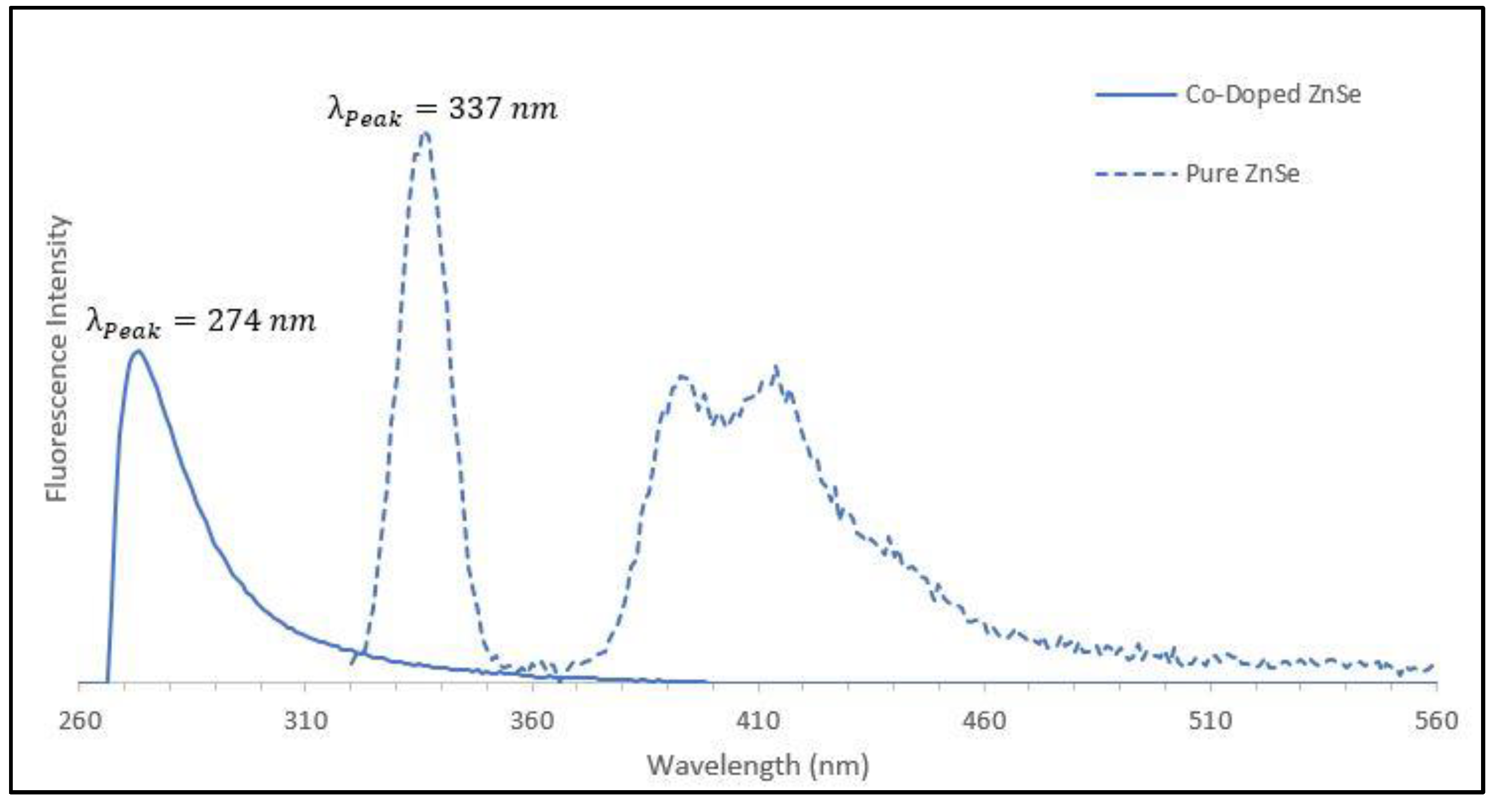
2.6. Optical Characteristics of Co-ZnSe Nanocrystals
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamaguchi, M.; Shigematsu, T. Behavior of Copper Impurity in ZnSe. Jpn. J. Appl. Phys. 1978, 17, 335–340. [Google Scholar] [CrossRef]
- DeLoach, L.D.; Page, R.H.; Wilke, G.D.; Payne, S.A.; Krupke, W.F. Properties of Transition Metal-Doped Zinc Chalcogenide Crystals for Tunable IR Laser Radiation. Adv. Solid State Lasers 1995, LM, LM4. Available online: https://www.osapublishing.org/abstract.cfm?uri=ASSL-1995-LM4 (accessed on 10 December 2021).
- DeLoach, L.D.; Page, R.H.; Wilke, G.D.; Payne, S.A.; Krupke, W.F. Transition metal-doped zinc chalcogenides: Spectroscopy and laser demonstration of a new class of gain media. IEEE J. Quantum Electron. 1996, 32, 885–895. [Google Scholar] [CrossRef]
- Toma, O.; Ion, L.; Iftimie, S.; Antohe, V.A.; Radu, A.; Raduta, A.M.; Manica, D.; Antohe, S. Physical properties of rf-sputtered ZnS and ZnSe thin films used for double-heterojunction ZnS/ZnSe/CdTe photovoltaic structures. Appl. Surf. Sci. 2019, 478, 831–839. [Google Scholar] [CrossRef]
- Manica, D.; Antohe, V.A.; Moldovan, A.; Pascu, R.; Iftimie, S.; Ion, L.; Suchea, M.P.; Antohe, Ş. Thickness Effect on Some Physical Properties of RF Sputtered ZnTe Thin Films for Potential Photovoltaic Applications. Nanomaterials 2021, 11, 2286. [Google Scholar] [CrossRef] [PubMed]
- Iftimie, S.; Radu, A.; Antohe, V.; Baiasu, F. On the structural, optical and morphological properties of ZnSe1−xOx thin films grown by RF-magnetron sputtering. Chalcogenide Lett. 2018, 15, 389–394. [Google Scholar]
- Toma, O.; Antohe, V.A.; Panaitescu, A.M.; Iftimie, S.; Răduţă, A.M.; Radu, A.; Ion, L.; Antohe, Ş. Effect of RF Power on the Physical Properties of Sputtered ZnSe Nanostructured Thin Films for Photovoltaic Applications. Nanomaterials 2021, 11, 2841. [Google Scholar] [CrossRef]
- Su, C.-H.; Sha, Y.-G.; Volz, M.P.; Carpenter, P.; Lehoczky, S.L. Vapor growth and characterization of ZnSeTe solid solutions. J. Cryst. Growth 2000, 216, 104–112. [Google Scholar] [CrossRef]
- Su, C.H.; George, M.A.; Palosz, W.; Feth, S.; Lehoczky, S.L. Contactless growth of ZnSe single crystals by physical vapor transport. J. Cryst. Growth 2000, 213, 267–275. [Google Scholar] [CrossRef][Green Version]
- Su, C.-H.; Feth, S.; Wang, L.J.; Lehoczky, S.L. Photoluminescence studies of ZnSe starting materials and vapor grown bulk crystals. J. Cryst. Growth 2001, 224, 32–40. [Google Scholar] [CrossRef]
- Su, C.-H.; Feth, S.; Zhu, S.; Lehoczky, S.L.; Wang, L.J. Optical characterization of bulk ZnSeTe solid solutions. J. Appl. Phys. 2000, 88, 5148–5152. [Google Scholar] [CrossRef]
- Singh, N.B.; Su, C.-H.; Arnold, B.; Choa, F.-S. Optical and morphological characteristics of zinc selenide-zinc sulfide solid solution crystals. Opt. Mater. 2016, 60, 474–480. [Google Scholar] [CrossRef]
- Singh, N.B.; Su, C.H.; Arnold, B.; Choa, F.S.; Cullum, B.; Sova, S.; Cooper, C. Morphological and Optical Characteristics of Transition Metal Doped PVT Grown Zinc Selenide Single Crystal. Cryst. Res. Technol. 2019, 54, 1800231. [Google Scholar] [CrossRef]
- Deshpande, A.C.; Singh, S.B.; Abyaneh, M.K.; Pasricha, R.; Kulkarni, S.K. Low temperature synthesis of ZnSe nanoparticles. Mater. Lett. 2008, 62, 3803–3805. [Google Scholar] [CrossRef]
- Shakir, M.; Kushwaha, S.K.; Maurya, K.K.; Bhagavannarayana, G.; Wahab, M.A. Characterization of ZnSe nanoparticles synthesized by microwave heating process. Solid State Commun. 2009, 149, 2047–2049. [Google Scholar] [CrossRef]
- Singh, N.B.; Su, C.H.; Arnold, B.; Choa, F.S.; Setera, B.; Sachs, D.; Cooper, C.E.; Kelly, L.; Mandal, K.D. Performance of Chromium Doped Zinc Selenide Nanocrystals: Morphological and Fluorescence Characteristics. In TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2021; p. 980. [Google Scholar] [CrossRef]
- Li, M.; Li, J.C. Size effects on the band-gap of semiconductor compounds. Mater. Lett. 2006, 60, 2526–2529. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setera, B.; Su, C.-H.; Arnold, B.; Choa, F.-S.; Kelly, L.; Sood, R.; Singh, N.B. Comparative Study of Bulk and Nanoengineered Doped ZnSe. Crystals 2022, 12, 71. https://doi.org/10.3390/cryst12010071
Setera B, Su C-H, Arnold B, Choa F-S, Kelly L, Sood R, Singh NB. Comparative Study of Bulk and Nanoengineered Doped ZnSe. Crystals. 2022; 12(1):71. https://doi.org/10.3390/cryst12010071
Chicago/Turabian StyleSetera, Brett, Ching-Hua Su, Bradley Arnold, Fow-Sen Choa, Lisa Kelly, Rachit Sood, and N. B. Singh. 2022. "Comparative Study of Bulk and Nanoengineered Doped ZnSe" Crystals 12, no. 1: 71. https://doi.org/10.3390/cryst12010071
APA StyleSetera, B., Su, C.-H., Arnold, B., Choa, F.-S., Kelly, L., Sood, R., & Singh, N. B. (2022). Comparative Study of Bulk and Nanoengineered Doped ZnSe. Crystals, 12(1), 71. https://doi.org/10.3390/cryst12010071






