Development of a Novel Low-Silver Cu-P Brazing Filler Metal Bearing Sn
Abstract
:1. Introduction
2. Materials and Methods
3. Results
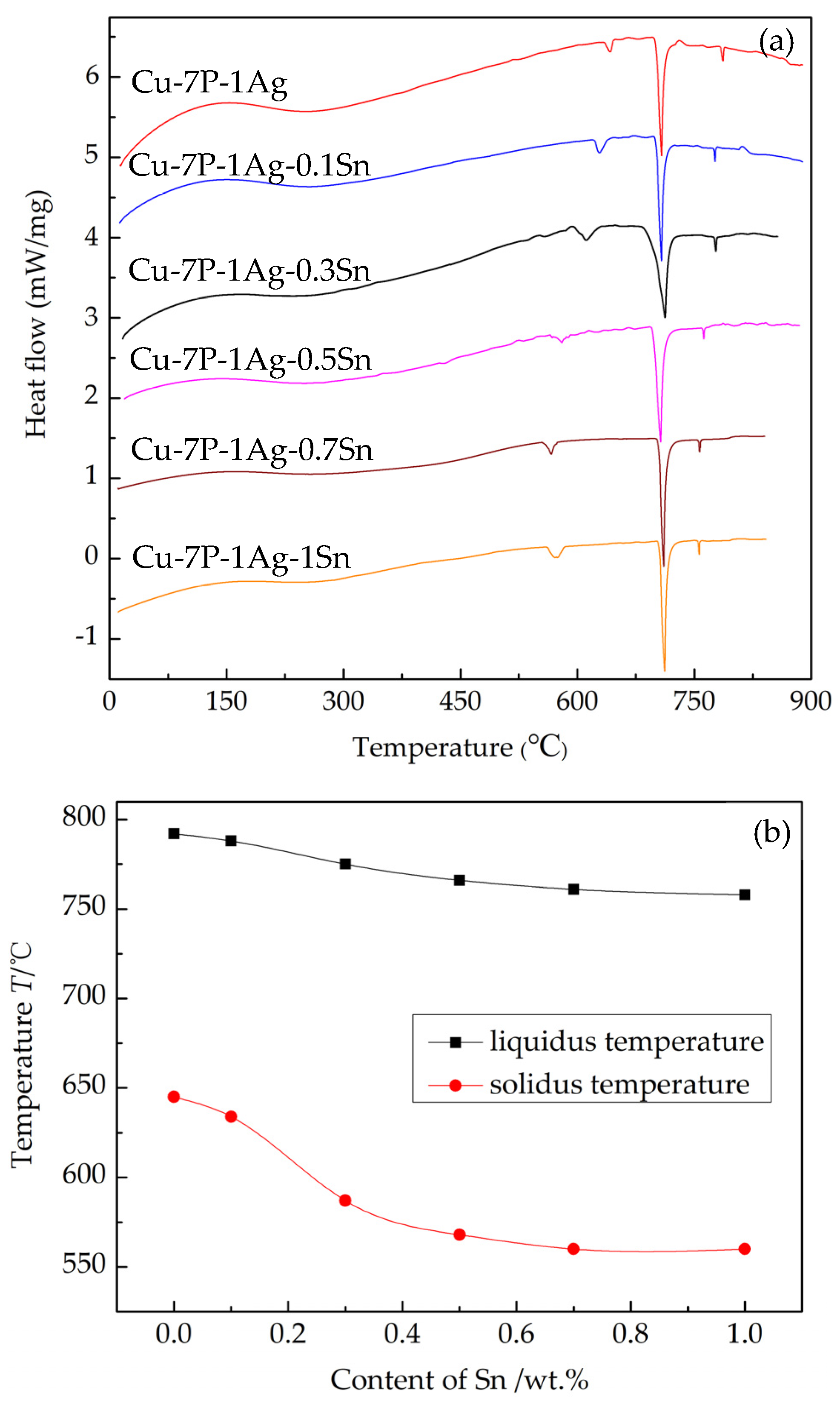
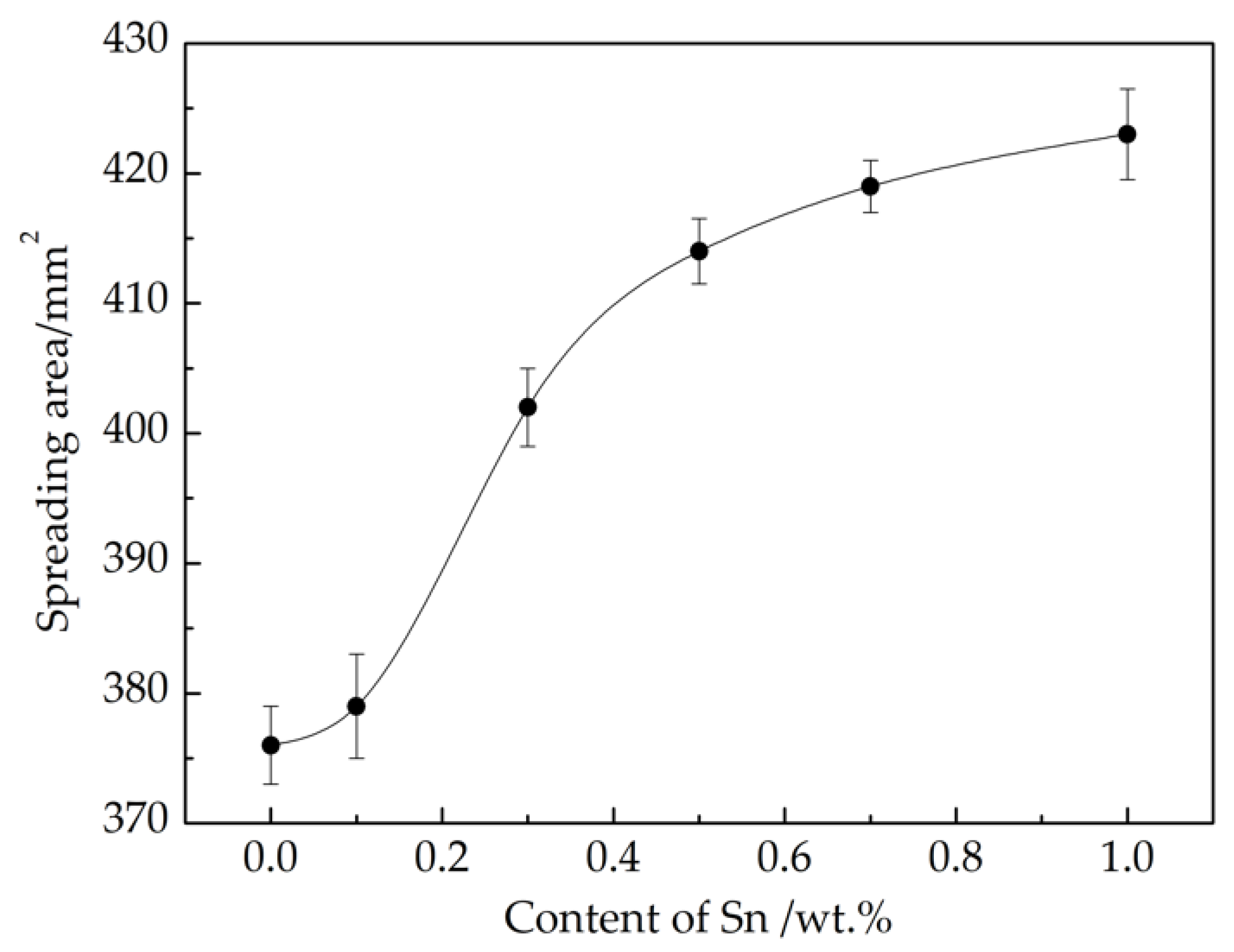
3.1. Thermal Properties and Spreading Performance of Cu-7P-1Ag-xSn Filler Metals
3.2. Microstructure of Cu-7P-1Ag-xSn Filler Metals
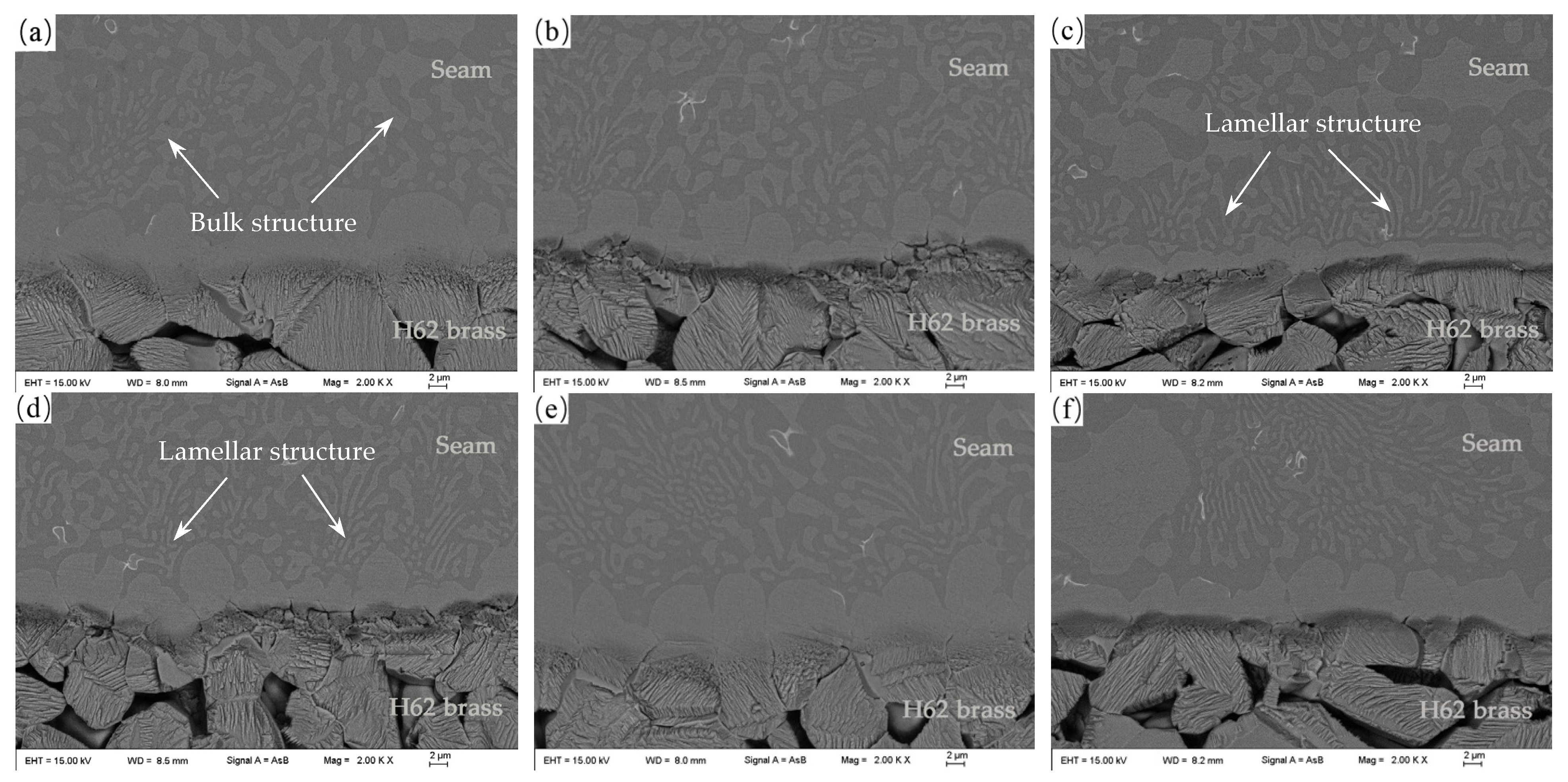
3.3. Microstructure of the Brazed Joints
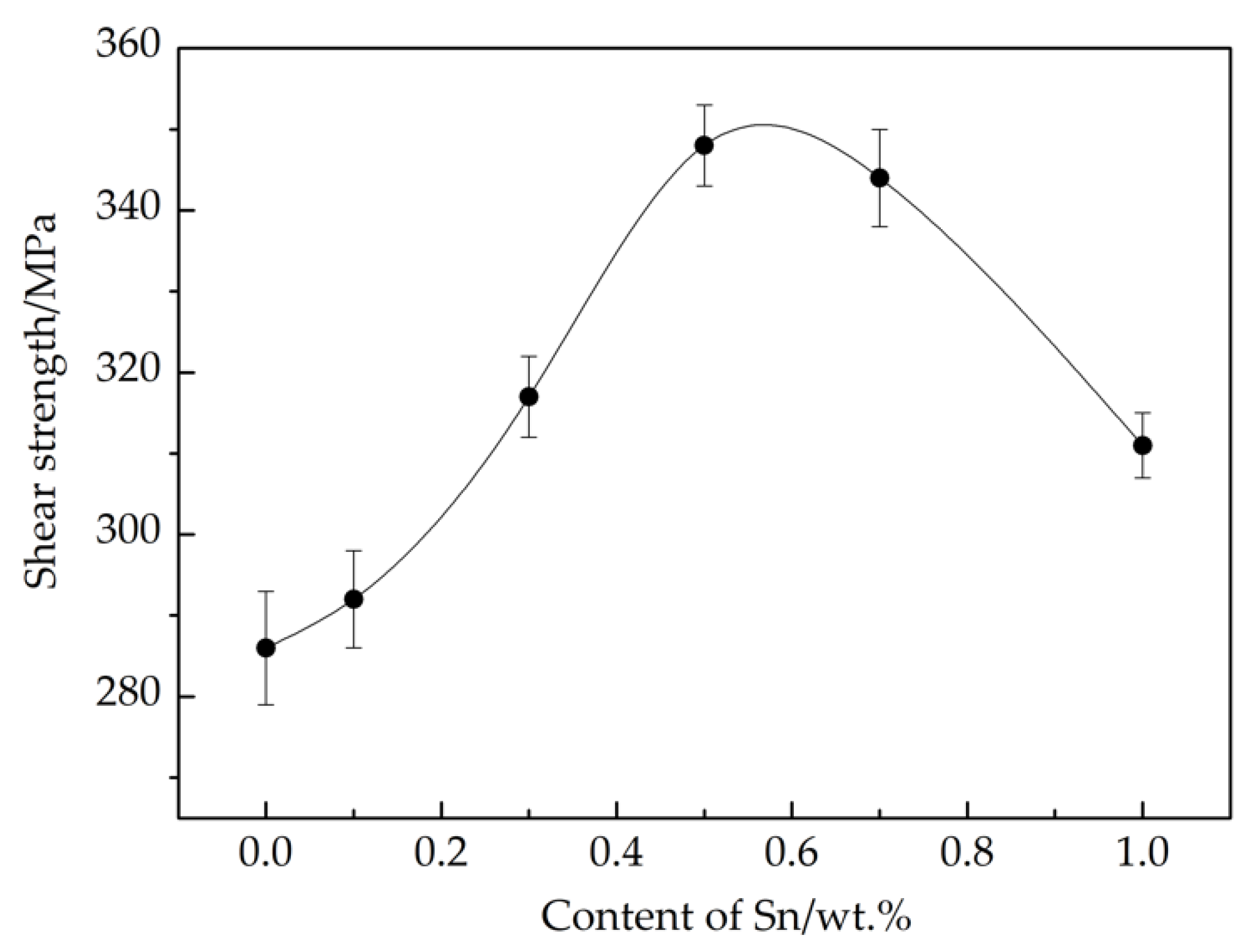
3.4. Mechanical Property of the Brazed Joints
4. Conclusions
- (1)
- The solidus and liquidus temperatures of Cu-7P-1Ag-xSn filler metals decrease with the addition of Sn. Meanwhile, the spreadability of the filler metals on H62 brass substrates is significantly improved.
- (2)
- Cu-7P-1Ag-xSn filler metals are mainly comprised of α-Ag solid solution, α-Cu solid solution and Cu3P. Cu20Sn6 compound is formed in the matrix of Cu-7P-1Ag-1Sn filler metal. With the addition of Sn, the microstructure of the filler metal gradually changes from thick dendrites into tiny worm-like structures.
- (3)
- With increasing Sn content, the microstructure of the brazed joints gradually changes from a bulk to a lamellar structure. Ag and Sn have higher diffusion coefficients into H62 brass substrate than P.
- (4)
- The strength of H62 brass brazed joints presents a parabolic trend according to Sn content. A peak value of 348 MPa was achieved using Cu-7P-1Ag-0.5Sn filler metal. The fracture morphology of the joint brazed with Cu-7P-1Ag-0.5Sn showed quasi-cleavage fractures with secondary cracks and tearing edges, whereas when Cu-7P-1Ag-1Sn was studied, the fracture surface showed typical brittle fracture characteristics.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xue, S.B.; Wang, B.; Zhang, L.; Long, W.M. Development of green welding technology in China during the past decade. Mater. Rep. 2019, 33, 2813–2830. [Google Scholar]
- Xu, X.P.; Ma, Q.J.; Xia, C.Z. Micromorphology change and microstructure of Cu-P based amorphous filler during heating process. High Temp. Mater. Process. 2019, 38, 651–661. [Google Scholar] [CrossRef]
- Luo, Q.C.; Xue, S.B.; Wu, J. Influences of Sn on properties of Ag-based and Cu-based brazing filler metals. Crystals 2021, 11, 1403. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, C.W.; Peng, Z.L.; Yan, J.C.; Liu, X.S. Dissolution behavior of Cu in Cu-Ag and Cu-P brazing alloys using weld brazing. Trans. Nonferrous Met. Soc. China 2011, 21, 394–399. [Google Scholar] [CrossRef]
- Xie, W.H.; Yang, Y.Z.; Chen, W.L.; Liu, M. Effects of Ni element on properties of Cu-P amorphous filler metal. Hot Work. Technol. 2015, 44, 23–25. [Google Scholar]
- Terasaki, T.; Kon, N.; Chiba, H.; Ohashi, T.; Nagatomo, Y.; Kuromitsu, Y.; Knowles, K.M. Interfacial structure between aluminum nitride and Cu-P-Sn-Ni brazing alloy with Ti film. J. Mater. Sci. 2021, 56, 8778–8788. [Google Scholar] [CrossRef]
- Jattakul, P.; Kanlayasiri, K. Influences of solid-state brazing conditions on microstructure and tensile shear force of Ag-Cu-P brazed copper sheets. IOP Conf. Ser. Mater. Sci. Eng. 2018, 361, 012001. [Google Scholar] [CrossRef]
- Sami, M.M.; Zaharinie, T.; Yusof, F.; Ariga, T. Investigation on strength and microstructural evolution of porous Cu/Cu brazed joints using Cu-Ni-Sn-P filler. Metals 2020, 10, 416. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.Z.; Liu, Z.L.; Liu, F.M.; Liu, S.T. Effects of Sn on properties of Cu-P filler metals. Hot Work. Technol. 2011, 40, 41–43. [Google Scholar]
- Gao, F.; Xu, W.L.; Wang, C.; Zou, J.S. Strength and microstructure of Cu joints brazed with Cu-P brazed amorphous filler metal contained Zr. Trans. China Weld. Inst. 2011, 32, 53–56. [Google Scholar]
- Jiang, F.; Liu, H.; Wen, K.; Xu, H.H. Effects of La, Ce and Si co-addition on wettability of copper phosphorus brazing filler metal and microstructure of brazing seam. Hot Work. Technol. 2013, 42, 202–205. [Google Scholar]
- Zhai, Z.M. The Effect of Alloy Element and Brazing Process on Cu-P-Ag Brazing Performance. Master’s Thesis, Central South University, Changsha, China, 2014. [Google Scholar]
- Li, Y.; Lu, J.B.; Mu, Y.C.; Xu, S.; Qiu, X.K. Effect of Cu-P-Sn on vacuum brazing of diamond by Ni based filler metal. Hot Work. Technol. 2018, 47, 58–61. [Google Scholar]
- Huang, J.L.; Long, W.M.; Zhang, G.X. Effects of Sn on properties and microstructures of Cu-P filler metal. JWJ 2012, 3, 57–60. [Google Scholar]
- GB/T 11364-2008. Test Method of Wettability for Brazing Filler Metals; Standardization Administration: Beijing, China, 2008.
- GB/T 11363-2008. Test Method of the Strength for Brazed and Soldered Joint; Standardization Administration: Beijing, China, 2008.
- Shalaby, R.M.; Kamal, M.; Ali, E.A.M.; Gumaan, M.S. Microstructure and mechanical characterization of melt spun process Sn-3.5Ag and Sn-3.5Ag-xCu lead-free solder for low cost electronic assembly. Mater. Sci. Eng. A 2017, 690, 446–452. [Google Scholar] [CrossRef]
- Jubair, M.M.; Gumaan, M.S.; Shalaby, R.M. Reliable Sn-Ag-Cu lead-free melt-spun material required for high-performance applications. Z. Kristallogr. Cryst. Mater. 2019, 234, 757–767. [Google Scholar] [CrossRef]
- Abbas, S.R.; Gumaan, M.S.; Shalaby, R.M. Chromium effects on the microstructure, mechanical and thermal properties of a rapidly solidified eutectic Sn-Ag alloy. Solder. Surf. Mt. Technol. 2020, 32, 137–146. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhuang, H.S. Handbook of Brazing and Soldering, 3rd ed.; China Machine Press: Beijing, China, 2018; pp. 109–134. [Google Scholar]
- Zhao, Z.Y.; Li, T.; Duan, Y.R.; Wang, Z.C.; Li, H. Wetting and coalescence of the liquid metal on the metal substrate. Chin. Phys. B 2017, 26, 140–146. [Google Scholar] [CrossRef]
- Lai, Z.M.; Xue, S.B.; Han, X.P.; Gu, L.Y.; Gu, W.H. Study on microstructure and property of brazed joint of AgCuZn-X (Ga, Sn, In, Ni) brazing alloy. Rare Met. Mater. Eng. 2010, 39, 397–400. [Google Scholar]
- Hissyam, W.N.W.M.N.; Halil, A.M.; Kurniawan, T.; Ishak, M.; Ariga, T. Effect of copper-based fillers composition on spreading and wetting behaviour. IOP Conf. Ser. Mater. Sci. Eng. 2017, 238, 012020. [Google Scholar] [CrossRef] [Green Version]
- Wang, L. Study on Properties and Brazing Mechanism of Cu-P Based Amorphous Filler Metal. Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2010. [Google Scholar]
- Ji, F.; Xue, S.B.; Dai, W. Effects of Ti on the brazability of Zn-22Al-xTi filler metals as well as properties of Cu/Al brazing joints. Rare Met. Mater. Eng. 2013, 42, 2453–2457. [Google Scholar]
- Zhao, Z.X.; Zeng, P.; Xie, G.R.; Zhong, G.M.; Chen, D.C.; Xu, X.D. Study on composition control and performance of brush plated copper-tin alloy deposits. Electroplat. Met. Finish. 2015, 34, 595–601. [Google Scholar]





| No. | Cu | P | Ag | Sn | |||
|---|---|---|---|---|---|---|---|
| Designed | Actual | Designed | Actual | Designed | Actual | ||
| 1 | Bal. | 7.00 | 6.96 | 1.00 | 1.02 | 0 | 0 |
| 2 | Bal. | 7.00 | 6.99 | 1.00 | 0.98 | 0.10 | 0.11 |
| 3 | Bal. | 7.00 | 7.03 | 1.00 | 1.00 | 0.30 | 0.31 |
| 4 | Bal. | 7.00 | 6.95 | 1.00 | 0.98 | 0.50 | 0.52 |
| 5 | Bal. | 7.00 | 6.93 | 1.00 | 1.05 | 0.70 | 0.70 |
| 6 | Bal. | 7.00 | 7.06 | 1.00 | 1.03 | 1.00 | 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Xue, S.; Luo, Q. Development of a Novel Low-Silver Cu-P Brazing Filler Metal Bearing Sn. Crystals 2022, 12, 66. https://doi.org/10.3390/cryst12010066
Wu J, Xue S, Luo Q. Development of a Novel Low-Silver Cu-P Brazing Filler Metal Bearing Sn. Crystals. 2022; 12(1):66. https://doi.org/10.3390/cryst12010066
Chicago/Turabian StyleWu, Jie, Songbai Xue, and Qingcheng Luo. 2022. "Development of a Novel Low-Silver Cu-P Brazing Filler Metal Bearing Sn" Crystals 12, no. 1: 66. https://doi.org/10.3390/cryst12010066
APA StyleWu, J., Xue, S., & Luo, Q. (2022). Development of a Novel Low-Silver Cu-P Brazing Filler Metal Bearing Sn. Crystals, 12(1), 66. https://doi.org/10.3390/cryst12010066






