Graphene Synthesis: Method, Exfoliation Mechanism and Large-Scale Production
Abstract
:1. Introduction
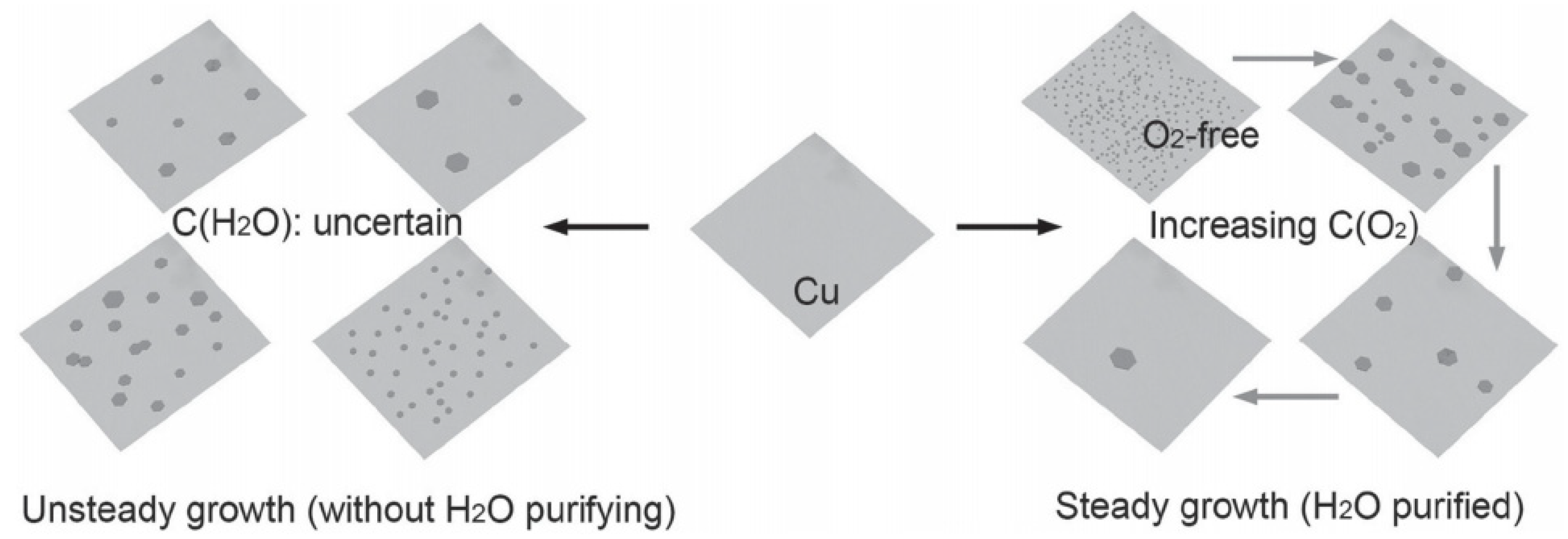
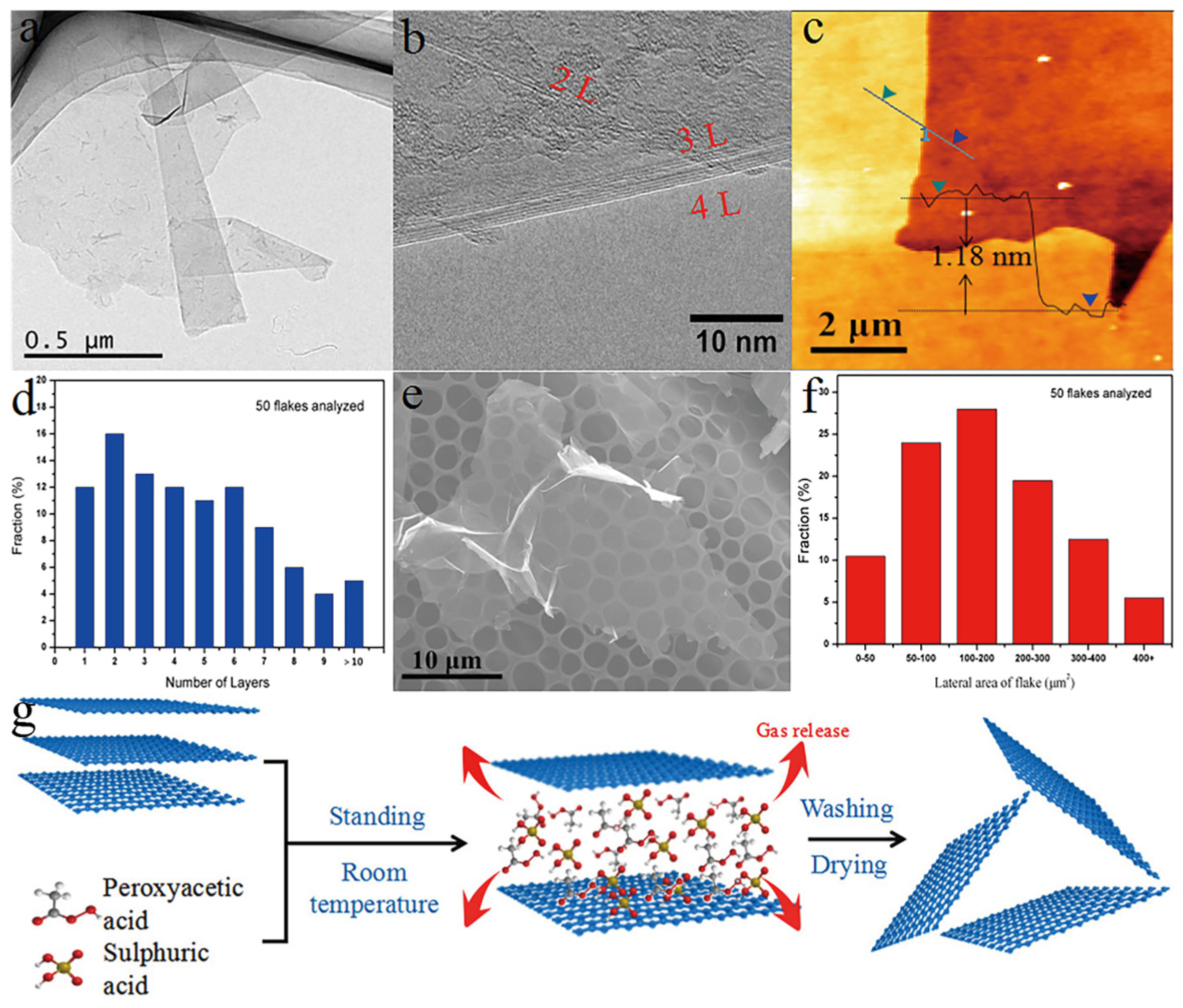
2. Preparation Method of Graphene
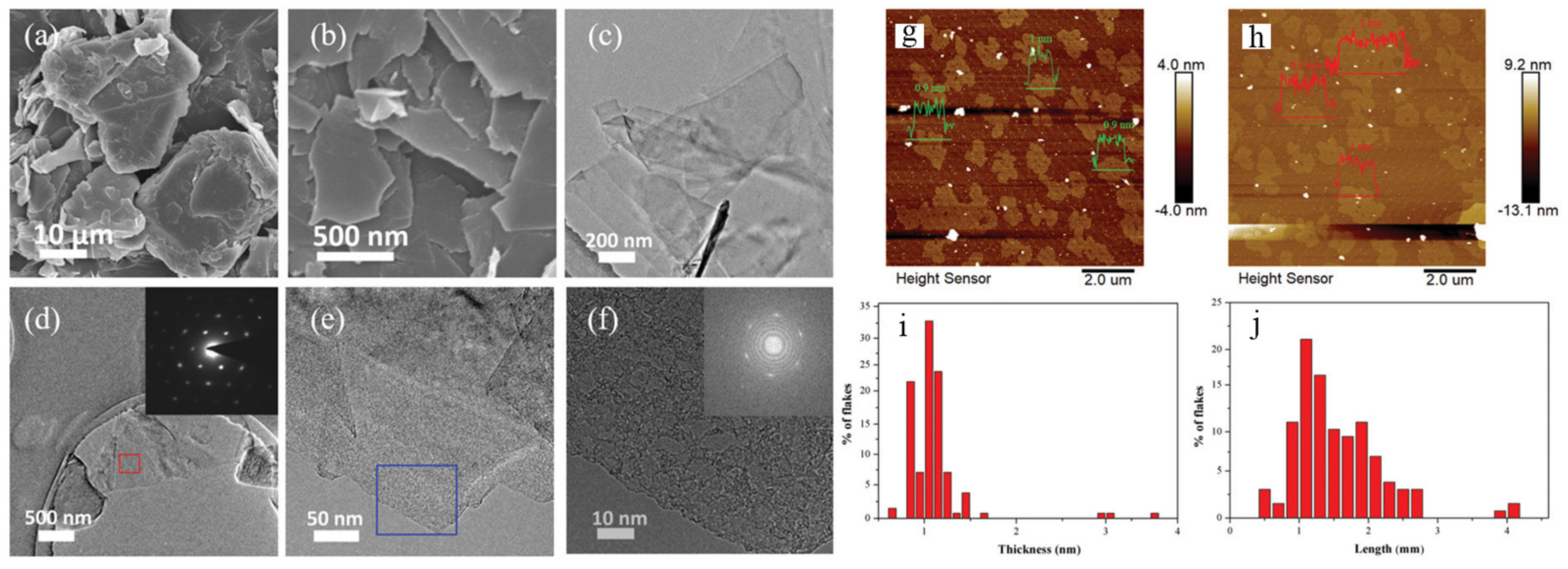
3. Exfoliation Mechanism of Graphene
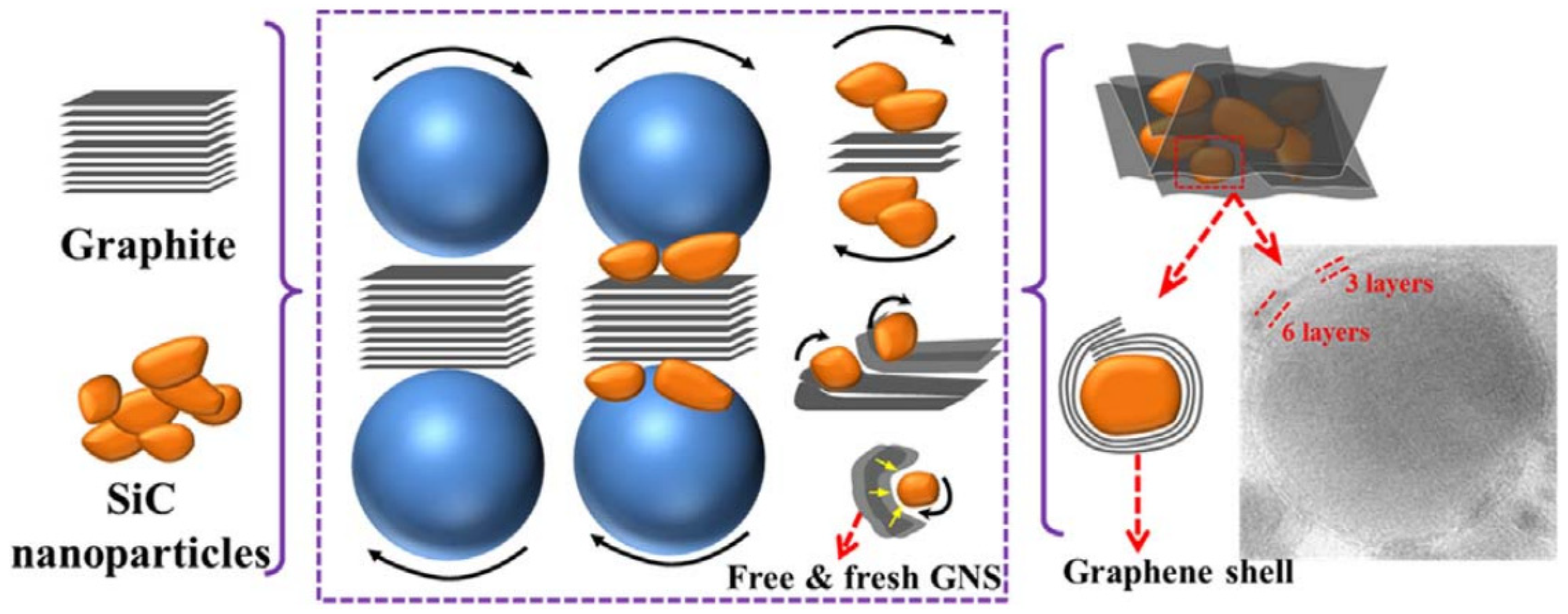
4. Large-Scale Exfoliation of Graphite Using Polymer Materials
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef]
- Yaragallaa, S.; Meera, A.P.; Kalarikkal, N.; Thomas, S. Chemistry associated with natural rubber–graphene nanocomposites and its effect on physical and structural properties. Ind. Crops Prod. 2015, 74, 792–802. [Google Scholar] [CrossRef]
- Tung, T.T.; Yoo, J.; Alotaibi, F.K.; Nine, M.J.; Karunagaran, R.; Krebsz, M.; Nguyen, G.T.; Tran, D.N.H.; Feller, J.F.; Losic, D. Graphene oxide-assisted liquid phase exfoliation of graphite into graphene for highly conductive film and electromechanical sensors. ACS Appl. Mater. Interfaces 2016, 8, 16521–16532. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Crespo, M.; Porwal, H.; Picot, O.; Santagiuliana, G.; Huang, Z.; Barbieri, E.; Pugno, N.M.; Peijs, T.; et al. In situ exfoliation of graphene in epoxy resins: A facile strategy to efficient and large scale graphene nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 24112–24122. [Google Scholar] [CrossRef]
- Cai, W.; Feng, X.; Hu, W.; Pan, Y.; Hu, Y.; Gong, X. Functionalized graphene from electrochemical exfoliation for thermoplastic polyurethane: Thermal stability, mechanical properties, and flame retardancy. Ind. Eng. Chem. Res. 2016, 55, 10681–10689. [Google Scholar] [CrossRef]
- Wipatkrut, P.; Poompradub, S. Exfoliation approach for preparing high conductive reduced graphite oxide and its application in natural rubber composites. Mater. Sci. Eng. B 2017, 218, 74–83. [Google Scholar] [CrossRef]
- Aliprandi, A.; Eredia, M.; Anichini, C.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Samori, P. Persian waxing of graphite: Towards green large-scale production of graphene. Chem. Commun. 2019, 55, 5331–5334. [Google Scholar] [CrossRef]
- Kang, H.G.; Jeong, J.M.; Hong, S.B.; Lee, G.Y.; Kim, D.H.; Kim, J.W.; Choi, B.G. Scalable exfoliation and activation of graphite into porous graphene using microwaves for high–performance supercapacitors. J. Alloys Compd. 2019, 770, 458–465. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, Y.; Nie, W.; Chen, P. Functionalized graphene nanosheets with fewer defects prepared via sodium alginate assisted direct exfoliation of graphite in aqueous media for lithium-ion batteries. ACS Appl. Nano Mater. 2018, 1, 1985–1994. [Google Scholar] [CrossRef]
- Shukla, A.; Dhanasekaran, P.; Nagaraju, N.; Bhat, S.D.; Pillai, V.K. A facile synthesis of graphene nanoribbon-quantum dot hybrids and their application for composite electrolyte membrane in direct methanol fuel cells. Electrochim. Acta 2019, 297, 267–280. [Google Scholar] [CrossRef]
- Kiani, F.; Razzaghi, Z.; Ghadiani, B.; Mohmmadi, M.T.M.H.; Simchi, A. Self-limited growth of large-area monolayer graphene films by low pressure chemical vapor deposition for graphene-based field effect transistors. Cera. Int. 2017, 43, 15010–15017. [Google Scholar] [CrossRef]
- Wang, L.; Tian, C.; Wang, H.; Ma, Y.; Wang, B.; Fu, H. Mass production of graphene via an in situ self-generating template route and its promoted activity as electrocatalytic support for methanol electroxidization. J. Phys. Chem. C 2010, 114, 8727–8733. [Google Scholar] [CrossRef]
- Liu, G.; Qin, H.; Amano, T.; Murakami, T.; Komatsu, N. Direct fabrication of the graphene-based composite for cancer phototherapy through graphite exfoliation with a photosensitizer. ACS Appl. Mater. Interfaces 2015, 7, 23402–23406. [Google Scholar] [CrossRef]
- Xu, H.; Meng, L.; Li, Y.; Yang, T.Z.; Bao, L.H.; Liu, G.D.; Zhao, L.; Liu, T.S.; Xing, J.; Gao, H.J.; et al. Applications of new exfoliation technique in study of two-dimensional materials. Acta Phys. Sin. 2018, 67, 21. [Google Scholar]
- Barberio, M.; Antici, P. Laser-plasma driven Synthesis of Carbon-based Nanomaterials. Sci. Rep. 2017, 7, 1–8. [Google Scholar]
- Li, W.; Li, M.; Liu, Y.; Pan, D.; Li, Z.; Wang, L.; Wu, M. Three minute ultrarapid microwave-assisted synthesis of bright fluorescent graphene quantum dots for live cell staining and white LEDs. ACS Appl. Nano Mater. 2018, 1, 1623–1630. [Google Scholar] [CrossRef]
- Bekduz, B.; Kaya, U.; Langer, M.; Mertin, W.; Bacher, G. Direct growth of graphene on Ge (100) and Ge (110) via thermal and plasma enhanced CVD. Sci. Rep. 2020, 10, 12938. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.; Maddi, C.; Donnet, C.; Garrelie, F. Review of graphene growth from a solid carbon source by pulsed laser deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef] [Green Version]
- Bleu, Y.; Bourquard, F.; Gartiser, V.; Loir, A.; Caja-Munoz, B.; Avila, J.; Barnier, V.; Garrelie, F.; Donnet, C. Graphene synthesis on SiO2 using pulsed laser deposition with bilayer predominance. Mater. Chem. Phys. 2019, 238, 121905. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Farre, C.; Chaix, C.; Galipaud, J.; Loir, A.; Barnier, V.; Garrelie, F.; Donnet, C. Boron doped graphene synthesis using pulsed laser deposition and its electrochemical characterization. Diamond Relat. Mater. 2021, 115, 108382. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Xu, C.; Wang, Y.; Wang, Y.; Li, P.; Sun, H.; Wang, M.; Xia, Y.; Lin, C.T.; et al. Chemical vapor deposition growth of scalable monolayer polycrystalline graphene films with millimeter-sized domains. Mater. Lett. 2018, 215, 259–262. [Google Scholar] [CrossRef]
- Guo, W.; Wu, B.; Wang, S.; Liu, Y. Controlling fundamental fluctuations for reproducible growth of large single-crystal graphene. ACS Nano 2018, 12, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lin, Q.; Zhou, T.; Chen, T.; Lin, S.; Dong, S. Facile preparation of graphene nanosheets by pyrolysis of coal-tar pitch with the presence of aluminum. J. Anal. Appl. Pyrolysis 2014, 110, 481–485. [Google Scholar] [CrossRef]
- Munuera, J.M.; Paredes, J.I.; Enterria, M.; Pagan, A.; Villar-Rodil, S.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L.; Cenis, J.L.; Martinez-Alonso, A.; et al. Electrochemical exfoliation of graphite in aqueous sodium halide electrolytes toward low oxygen content graphene for energy and environmental applications. ACS Appl. Mater. Interfaces 2017, 9, 24085–24099. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Jing, F.; Xiao, J.; Zhou, C.; Lin, Y.; Wang, S. Oxidative-Etching-Assisted Synthesis of Centimeter-Sized Single-Crystalline Graphene. Adv. Mater. 2016, 28, 3152–3158. [Google Scholar] [CrossRef]
- Auchter, E.; Marquez, J.; Yarbro, S.L.; Dervishi, E. A facile alternative technique for large-area graphene transfer via sacrificial polymer. AIP Adv. 2017, 7, 125306. [Google Scholar] [CrossRef]
- Auchter, E.; Marquez, J.; Stevens, G.; Silva, R.; Mcculloch, Q.; Guengerich, Q.; Blair, A.; Litchfield, S.; Li, N.; Sheehan, C. Ultra-thin and strong formvar-based membranes with controlled porosity for micro-and nano-scale systems. Nanotechnology 2018, 29, 215712. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Irzhak, A.; Irzhak, D.; Kang, T.; Kononenko, O.; Matveev, V.; Panin, G.; Roshchupkin, D. Direct growth of graphene film on piezoelectric La3Ga5.5Ta0.5O14 crystal. Phys. Status Solidi RRL 2016, 10, 639–644. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, J.; Ding, F. Strategies, Status, and Challenges in Wafer Scale Single Crystalline Two-Dimensional Materials Synthesis. Chem. Rev. 2021, 121, 6321–6372. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J.; et al. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Huang, M.; Luo, D.; Li, Y.; Choe, M.; Seong, W.; Kim, M.; Jin, S.; Wang, M.; Chatterjee, S.; et al. Single-crystal, large-area, fold-free monolayer graphene. Nature 2021, 596, 519–524. [Google Scholar] [CrossRef]
- Huang, Z.; Alharbi, A.; Mayer, W.; Cuniberto, E.; Taniguchi, T.; Watanabe, K.; Shabani, J.; Shahrjerdi, D. Versatile construction of van der Waals heterostructures using a dual-function polymeric film. Nat. Commun. 2020, 11, 3029. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, N.; Alimadadi, M.; Hummelgard, M.; Dahlstrom, C.; Olsen, M.; Olin, H. Effects of geometry on large-scale tube-shear exfoliation of graphite to multilayer graphene and nanographite in water. Sci. Rep. 2019, 9, 8966. [Google Scholar] [CrossRef]
- Ptak, F.; Almeida, C.M.; Prioli, R. Velocity-dependent friction enhances tribomechanical differences between monolayer and multilayer graphene. Sci. Rep. 2019, 9, 14555. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-B.; Yin, J.-Z.; Xu, Q.-Q.; Zhi, J.-T. Insightful Understanding of Shear-Assisted Supercritical CO2 Exfoliation for Fabricating Graphene Nanosheets through the Combination of Kinetics and Process Parameters. Ind. Eng. Chem. Res. 2020, 59, 10967–10975. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Bandgar, S.B.; Kolekar, S.S.; Chang, J.-Y.; Ling, Y.-C.; Ye, Z.; Ghule, A.V. Holey C@ZnFe2O4 Nanoflakes by Carbon Soot Layer Blasting Approach for High Performance Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 6693–6704. [Google Scholar] [CrossRef]
- Szirmai, P.; Markus, B.G.; Chacon-Torres, J.C.; Eckerlein, P.; Edelthalhammer, K.; Englert, J.M.; Mundloch, U.; Hirsch, A.; Hauke, F.; Nafradi, B.; et al. Characterizing the maximum number of layers in chemically exfoliated graphene. Sci. Rep. 2019, 9, 19480. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yu, B. Corn Flour Nano-Graphene Prepared by the Hummers Redox Method. ACS Omega 2020, 5, 30252–30256. [Google Scholar] [CrossRef] [PubMed]
- Ejigu, A.; le Fevre, L.W.; Fujisawa, K.; Terrones, M.; Forsyth, A.J.; Dryfe, R.A.W. Electrochemically exfoliated graphene electrode for high-performance rechargeable chloroaluminate and dual-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 23261–23270. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Mohamed, A.; Tatariants, M. Mass production of graphene nanosheets by multi-roll milling technique. Tribol. Int. 2018, 121, 54–63. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Valverde, J.L.; Sanchez-Silva, L.; Romero, A. Solvent-based exfoliation via sonication of graphitic materials for graphene manufacture. Ind. Eng. Chem. Res. 2016, 55, 845–855. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, J.; Yuan, J.; Li, J.; Sun, Y.; Matsuba, Y.; Zhu, D.-M.; Qin, L.-C. Production of few-layer graphene via enhanced high-pressure shear exfoliation in liquid for supercapacitor applications. ACS Appl. Nano Mater. 2019, 1, 2877–2884. [Google Scholar] [CrossRef]
- Badri, M.A.S.; Salleh, M.M.; Noor, N.F.M.; Rahman, M.Y.A.; Umar, A.A. Green synthesis of few-layered graphene from aqueous processed graphite exfoliation for graphene thin film preparation. Mater. Chem. Phys. 2017, 193, 212–219. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Rafieerad, A.R.; Rashidi, M.M.; Zaharinie, T.; Zubir, M.N.M.; Kazi, S.N.; Chew, B.T. Functionalization and exfoliation of graphite into mono layer graphene for improved heat dissipation. J. Taiwan Inst. Chem. Eng. 2017, 71, 480–493. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, Y.; Xu, H. High-yield production of high-quality graphene by novel electrochemical exfoliation at air-electrolyte interface. Mater. Lett. 2019, 235, 153–156. [Google Scholar] [CrossRef]
- Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen, D.; Ruoff, R.S. Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano 2010, 4, 1227–1233. [Google Scholar] [CrossRef]
- Potts, J.R.; Shankar, O.; Murali, S.; Du, L.; Ruoff, R.S. Latex and two-roll mill processing of thermally-exfoliated graphite oxide/natural rubber nanocomposites. Compos. Sci. Technol. 2013, 74, 166–172. [Google Scholar] [CrossRef]
- Cheng, H.; Ye, M.; Zhao, F.; Hu, C.; Zhao, Y.; Liang, Y.; Chen, N.; Chen, S.; Jiang, L.; Qu, L. A general and extremely simple remote approach toward graphene bulks with in situ multifunctionalization. Adv. Mater. 2016, 28, 3305–3312. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wu, W.; Liu, T.; Liu, Y.; Guo, B.; Zhang, R. One-step chemical exfoliation of graphite to ∼100% few-layer graphene with high quality and large size at ambient temperature. Chem. Eng. J. 2019, 355, 181–185. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Wang, S.; Chen, G. Microwave-assisted rapid exfoliation of graphite into graphene by using ammonium bicarbonate as the intercalation agent. Ind. Eng. Chem. Res. 2017, 56, 9341–9346. [Google Scholar] [CrossRef]
- Yoon, G.; Seo, D.-H.; Ku, K.; Kim, J.; Jeon, S.; Kang, K. Factors affecting the exfoliation of graphite intercalation compounds for graphene synthesis. Chem. Mater. 2015, 27, 2067–2073. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, J.M.; Leon, V.; Lucio, M.I.; Prato, M.; Vazquez, E. Production of ready-to-use few-layer graphene in aqueous suspensions. Nat. Protoc. 2018, 13, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Mizuno, Y.; Araki, K.; Kubouchi, M. Mass production of high-aspect-ratio few-layer-graphene by high-speed laminar flow. Carbon 2016, 102, 330–338. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wu, Z.; Li, Y.; Yang, W.; Li, Y. High-Efficiency Production of Graphene by Supercritical CO2 Exfoliation with Rapid Expansion. Langmuir 2018, 34, 7797–7804. [Google Scholar] [CrossRef]
- Bellani, S.; Petroni, E.; Castillo, A.E.D.R.; Curreli, N.; Martin-Garcia, B.; Oropesa-Nunez, R.; Prato, M.; Bonaccorso, F. Scalable production of graphene inks via wet-jet milling exfoliation for screen-printed micro-supercapacitors. Adv. Funct. Mater. 2019, 29, 1807659. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Jin, H.; Miao, X.; Ju, T.; Li, Y.; Ji, J. Gas-driven exfoliation for producing high-quality graphene. Chem. Commun. 2019, 55, 7749–7751. [Google Scholar] [CrossRef]
- Chuan, X.-Y. Microstructure Design of Graphite in Nanoscale. J. Inorg. Mater. 2017, 32, 1121–1127. [Google Scholar]
- Zhang, J.; Yang, S.; Chen, Z.; Yan, Y.; Zhao, J.; Li, J.; Jiang, Z. In Situ synthesis of SiC-graphene core-shell nanoparticles using wet ball milling. Ceram. Int. 2018, 44, 8283–8289. [Google Scholar] [CrossRef]
- van Engers, C.D.; Cousens, N.E.A.; Babenko, V.; Britton, J.; Zappone, B.; Grobert, N.; Perkin, S. Direct measurement of the surface energy of graphene. Nano Lett. 2017, 17, 3815–3821. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. High-yield organic dispersions of unfunctionalized graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Wang, G.; Liu, L.; Chen, Y.; Zhang, Z.; Shi, X. Bending induced interlayer shearing, rippling and kink buckling of multilayered graphene sheets. J. Mech. Phys. Solids 2019, 122, 340–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.; Dong, B.; Wang, F.; Tang, J.; Stadler, F.J.; Yang, G.; Hong, S.; Xing, F. Interfacial jamming reinforced Pickering emulgel for arbitrary architected nanocomposite with connected nanomaterial matrix. Nat. Commun. 2021, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, L.; Mateos, A.; Kudo, A.; Vyatskikh, A.; Gao, H.; Greer, J.R.; Li, X. Theoretical strength and rubber-like behaviour in micro-sized pyrolytic carbon. Nat. Nanotechnol. 2019, 14, 762–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritikos, G.; Rissanou, A.N.; Harmandaris, V.; Karatasos, K. Bound layer polymer behavior on graphene and graphene oxide nanosheets. Macromolecules 2020, 53, 6190–6203. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, G.; Zong, L.; Jiang, M.; Song, Z.; Ma, C.; Zhang, T.; Duan, Y.; Zhang, J. Tough, ultralight, and water-adhesive graphene/natural rubber latex hybrid aerogel with sandwichlike cell wall and biomimetic rose-petal-like surface. ACS Appl. Mater. Interfaces 2020, 12, 1378–1386. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, N.; Liang, X.; Liang, J. Method for Preparing Graphene by Mechanically Driving Rubber Molecules to Strip Flake Graphite. China Patent 201910640615.3, 6 October 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Tang, Q.; Huang, B.; Wang, Y. Graphene Synthesis: Method, Exfoliation Mechanism and Large-Scale Production. Crystals 2022, 12, 25. https://doi.org/10.3390/cryst12010025
Liu N, Tang Q, Huang B, Wang Y. Graphene Synthesis: Method, Exfoliation Mechanism and Large-Scale Production. Crystals. 2022; 12(1):25. https://doi.org/10.3390/cryst12010025
Chicago/Turabian StyleLiu, Naixu, Qingguo Tang, Bin Huang, and Yaping Wang. 2022. "Graphene Synthesis: Method, Exfoliation Mechanism and Large-Scale Production" Crystals 12, no. 1: 25. https://doi.org/10.3390/cryst12010025
APA StyleLiu, N., Tang, Q., Huang, B., & Wang, Y. (2022). Graphene Synthesis: Method, Exfoliation Mechanism and Large-Scale Production. Crystals, 12(1), 25. https://doi.org/10.3390/cryst12010025






