Three-Dimensional CA-LBM Numerical Model and Experimental Verification of Cs2AgBiBr6 Perovskite Single Crystals Grown by Solution Method
Abstract
:1. Introduction
2. Preparation of Cs2AgBiBr6 Single Crystals by the Solution Method
3. Numerical Model of Cs2AgBiBr6 Single-Crystal Growth Based on the CA Method
3.1. LBM Model
3.2. CA Model
3.2.1. Model for the Nucleation
3.2.2. Crystal Growth Model
3.2.3. Model for the Capture
4. Results and Discussion
4.1. The Physical Parameters of Cs2AgBiBr6 Precursor Solution
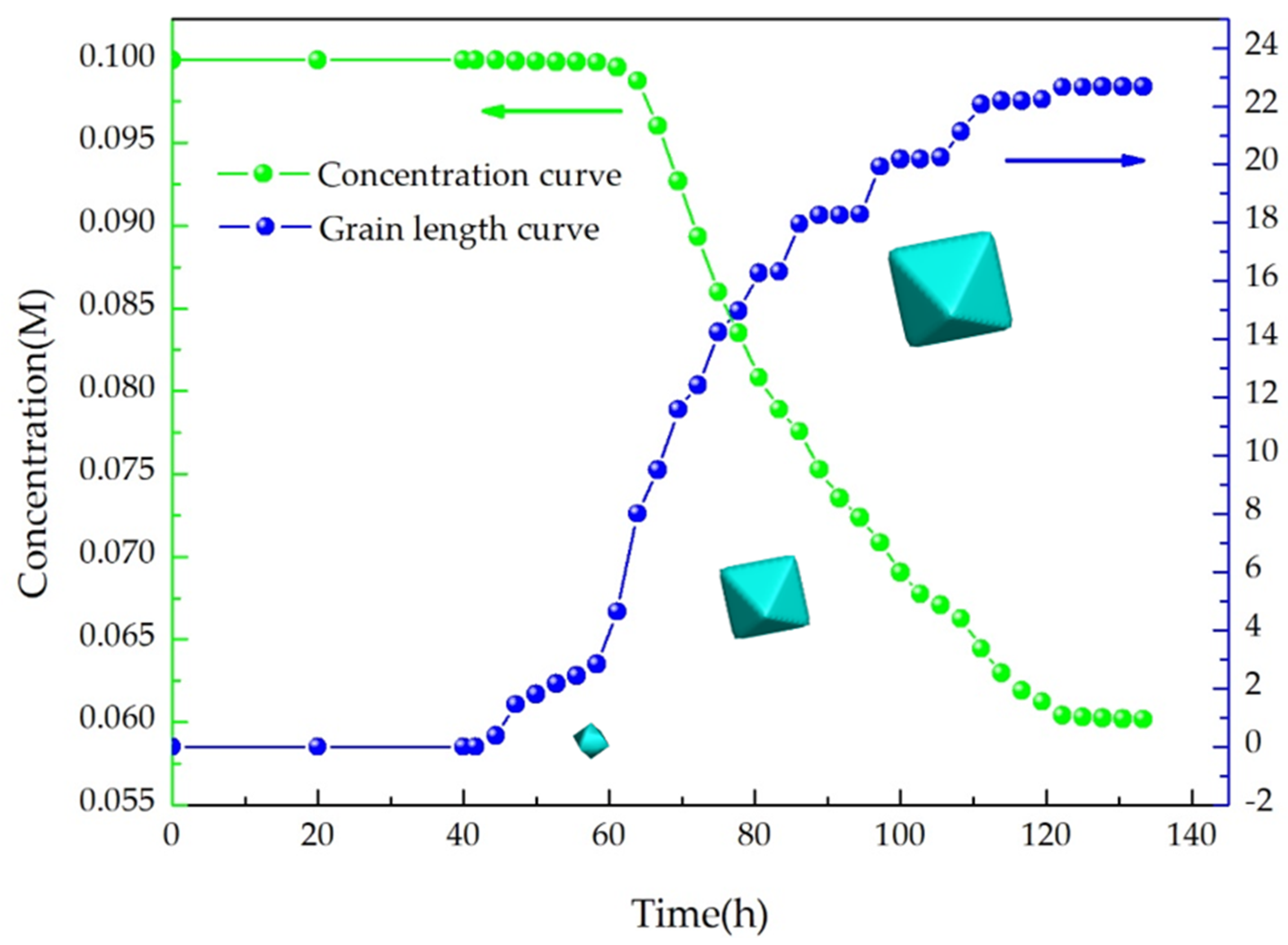
4.2. Simulation and Verification of Facet Growth
4.3. Simulation and Verification of Controlled Cooling Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canil, L.; Cramer, T.; Fraboni, B.; Ricciarelli, D.; Meggiolaro, D.; Singh, A.; Liu, M.; Rusu, M.; Wolff, C.M.; Phung, N.; et al. Tuning halide perovskite energy levels. Energy Environ. Sci. 2021, 14, 1429–1438. [Google Scholar] [CrossRef]
- Rong, S.S.; Faheem, M.B.; Li, Y.B. Perovskite single crystals: Synthesis, properties, and applications. J. Electron. Sci. Technol. 2021, 19, 100081. [Google Scholar] [CrossRef]
- Ma, L.; Yan, Z.; Zhou, X.; Pi, Y.; Du, Y.; Huang, J.; Wang, K.; Wu, K.; Zhuang, C.; Han, X. A polymer controlled nucleation route towards the generalized growth of organic-inorganic perovskite single crystals. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Yan, Q.; Gao, T.; Ding, J.; Lv, Q.; Ning, C.; Li, Q.; Sun, J.L. Perovskite CH3NH3PbI3(Cl) Single Crystals: Rapid Solution Growth, Unparalleled Crystalline Quality, and Low Trap Density toward 108 cm−3. J. Am. Chem. Soc. 2016, 138, 9409–9412. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Chang, J.; Liu, S. Recent progress of two-dimensional lead halide perovskite single crystals: Crystal growth, physical properties, and device applications. Eco. Mat. 2020, 2, 12036. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide—Based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [Green Version]
- NREL. Transforming Energy. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 4 August 2021).
- Lin, Q.; Armin, A.; Burn, P.L.; Meredith, P. Near infrared photodetectors based on sub-gap absorption in organohalide perovskite single crystals. Laser Photonics Rev. 2016, 10, 1047–1053. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Xu, Q.; Wei, H.; Fang, Y.; Wang, Q.; Deng, Y.; Li, T.; Gruverman, A.; Cao, L.; et al. Monolithic integration of hybrid perovskite single crystals with heterogenous substrate for highly sensitive X-ray imaging. Nat. Photonics 2017, 11, 315–321. [Google Scholar] [CrossRef]
- Yakunin, S.; Dirin, D.N.; Shynkarenko, Y.; Morad, V.; Cherniukh, I.; Nazarenko, O.; Kreil, D.; Nauser, T.; Kovalenko, M.V. Detection of gamma photons using solution-grown single crystals of hybrid lead halide perovskites. Nat. Photonics 2016, 10, 585–589. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Cortecchia, D.; Drozdowski, W.; Brylew, K.; Lachmanski, W.; Bruno, A.; Soci, C. X-ray Scintillation in Lead Halide Perovskite Crystals. Sci. Rep. 2016, 6, 37254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangyang, P.; Sun, H.; Zhu, X.; Yang, D.; Gao, X.; Liu, W.; Chen, Y.; Tian, H.; Huanglong, S. Solution-Processable Methyl Ammonium Lead Iodide Single Crystal Photodetectors for Visible Light and X-ray. Phys. Status Solidi A 2017, 214, 1700538. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite light-emitting di-odes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Huang, Y.-Q.; Chen, H.; Huang, J. Solution Growth and Performance Study of Cs2AgBiBr6 Single Crystal. Cryst. Res. Technol. 2020, 55, 1900222. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Erbing, A.; Johansson, M.B.; Wang, J.; Kamal, C.; Odelius, M.; Johansson, E.M.J. Mixed-Halide Double Perovskite Cs2 AgBiX6 (X = Br, I) with Tunable Optical Properties via Anion Exchange. Chem. Sus. Chem. 2021. [Google Scholar] [CrossRef]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S.; et al. The Dawn of Lead-Free Perovskite Solar Cell: Highly Stable Double Perovskite Cs2AgBiBr6 Film. Adv. Sci. 2018, 5, 1700759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Hassan, I.; Regue, M.; Gonzalez-Carrero, S.; Rattner, E.; Isaacs, M.A.; Eslava, S. Mechanochemically synthesized Pb-free halide perovskite-based Cs2AgBiBr6–Cu–RGO nanocomposite for photocatalytic CO2 reduction. J. Mater. Chem. A 2021, 9, 12179–12187. [Google Scholar] [CrossRef]
- Keshavarz, M.; Debroye, E.; Ottesen, M.; Martin, C.; Zhang, H.; Fron, E.; Küchler, R.; Steele, J.A.; Bremholm, M.; Van De Vondel, J.; et al. Tuning the Structural and Optoelectronic Properties of Cs2AgBiBr6 Double-Perovskite Single Crystals through Alkali-Metal Substitution. Adv. Mater. 2020, 32, 2001878. [Google Scholar] [CrossRef]
- Yin, L.; Wu, H.; Pan, W.; Yang, B.; Li, P.; Luo, J.; Niu, G.; Tang, J. Controlled cooling for synthesis of Cs2AgBiBr6 single crystals and its application for X-ray detection. Adv. Opt. Mater. 2019, 7, 1900491. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, Z.; Pan, S.; Zhang, Y.; Chen, D.; Pan, J. Growth and photodetection properties of Cs2AgBiBr6 crystals with large flat (1 1 1) plane grown from the solution by adding toluene. J. Cryst. Growth 2020, 552, 125922. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Pan, S.; Li, H.; Zhang, J.; Gong, Z.; Zhang, Y.; Pan, J. Growth and properties of centimeter-sized lead free all inorganic perovskite Cs2AgBiBr6 crystal by additive CH3COONa. J. Cryst. Growth 2020, 532, 125440. [Google Scholar] [CrossRef]
- Dang, Y.; Tong, G.; Song, W.; Liu, Z.; Qiu, L.; Ono, L.K.; Qi, Y. Interface engineering strategies towards Cs2AgBiBr6 single-crystalline photodetectors with good Ohmic contact behaviours. J. Mater. Chem. C 2020, 8, 276–284. [Google Scholar] [CrossRef]
- Lian, Q.; Liu, W.; Li, R.; Yan, W.; Liu, C.; Zhang, Y.; Wang, L.; Chen, H. Numerical Simulation of Multi-Crystalline Silicon Crystal Growth Using a Macro–Micro Coupled Method during the Directional Solidification Process. Appl. Sci. 2016, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, R.; Li, N.; Yan, W.; Ma, W.; Chen, H. Cellular Automaton Modeling of Silicon Facet Formation during Directional Solidification. Crystals 2018, 8, 399. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, R.; Chen, H. Three-Dimensional CA-LBM Model of Silicon Facet Formation during Directional Solidification. Crystals 2020, 10, 669. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, P.; Gong, H.; Duan, P.; Hao, L.; Jin, K. Phase field method simulation of faceted dendrite growth with arbitrary symmetries. Trans. Nonferrous Met. Soc. China 2018, 28, 290–297. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Liu, B. Cellular automaton simulation of three-dimensional dendrite growth in Al–7Si–Mg ternary aluminum alloys. Comput. Mater. Sci. 2015, 105, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Rappaz, M.; Gandin, C.-A. Probabilistic modelling of microstructure formation in solidification processes. Acta Met. Mater. 1993, 41, 345–360. [Google Scholar] [CrossRef]
- Pan, S.; Zhu, M. A three-dimensional sharp interface model for the quantitative simulation of solutal dendritic growth. Acta Mater. 2010, 58, 340–352. [Google Scholar] [CrossRef]





| Property | Value |
|---|---|
| Initial solution concentration, C (M) | 0.1 |
| Initial temperature, T (K) | 393.0 |
| Solid density, ρ (g·cm−3) | 4.915 |
| Liquid solute diffusion coefficient, D (m2·s−1) | 3.0 × 10−9 |
| Gibbs–Thomson coefficient, Γ (mK) | 2.6 × 10−7 |
| Anisotropic coefficient of interface energy, ε | 1.5 × 10−2 |
| Maximum nucleation density, nmax (m−3) | 8.4 × 1010 |
| Standard deviation supersaturation, Sσ (M) | 1.0 × 10−3 |
| Maximum nucleation supersaturation, Smax (M) | 1.0 × 10−2 |
| Cell size, a (m) | 1.0 × 10−6 |
| Time step, ∆t (s) | 5.0 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wu, C.; Li, R.; Chen, H. Three-Dimensional CA-LBM Numerical Model and Experimental Verification of Cs2AgBiBr6 Perovskite Single Crystals Grown by Solution Method. Crystals 2021, 11, 1101. https://doi.org/10.3390/cryst11091101
Chen H, Wu C, Li R, Chen H. Three-Dimensional CA-LBM Numerical Model and Experimental Verification of Cs2AgBiBr6 Perovskite Single Crystals Grown by Solution Method. Crystals. 2021; 11(9):1101. https://doi.org/10.3390/cryst11091101
Chicago/Turabian StyleChen, Hui, Cuncun Wu, Ri Li, and Hongjian Chen. 2021. "Three-Dimensional CA-LBM Numerical Model and Experimental Verification of Cs2AgBiBr6 Perovskite Single Crystals Grown by Solution Method" Crystals 11, no. 9: 1101. https://doi.org/10.3390/cryst11091101
APA StyleChen, H., Wu, C., Li, R., & Chen, H. (2021). Three-Dimensional CA-LBM Numerical Model and Experimental Verification of Cs2AgBiBr6 Perovskite Single Crystals Grown by Solution Method. Crystals, 11(9), 1101. https://doi.org/10.3390/cryst11091101






