In Situ Evolution of Pores in Lithium Hydride at Elevated Temperatures Characterized by X-ray Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
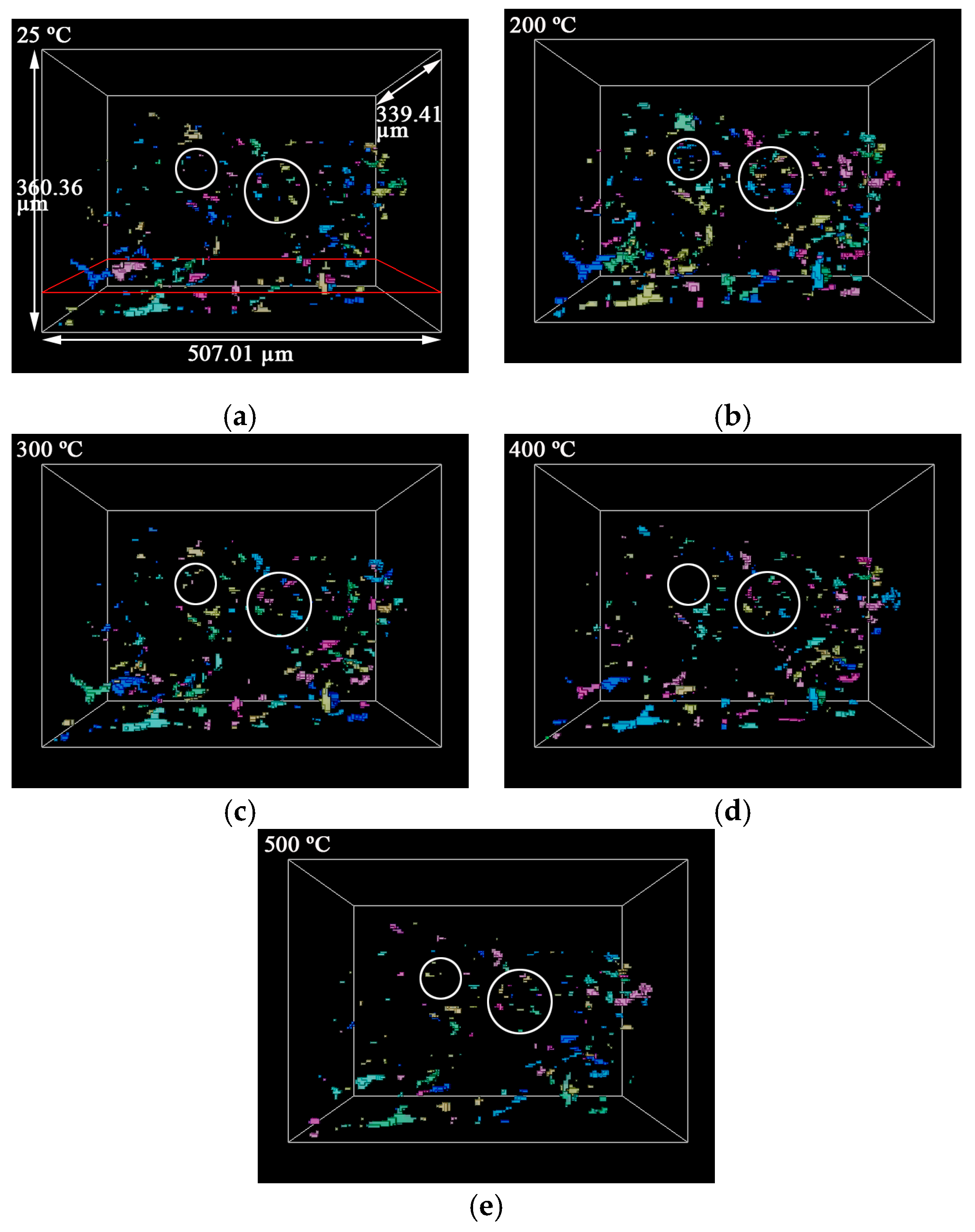
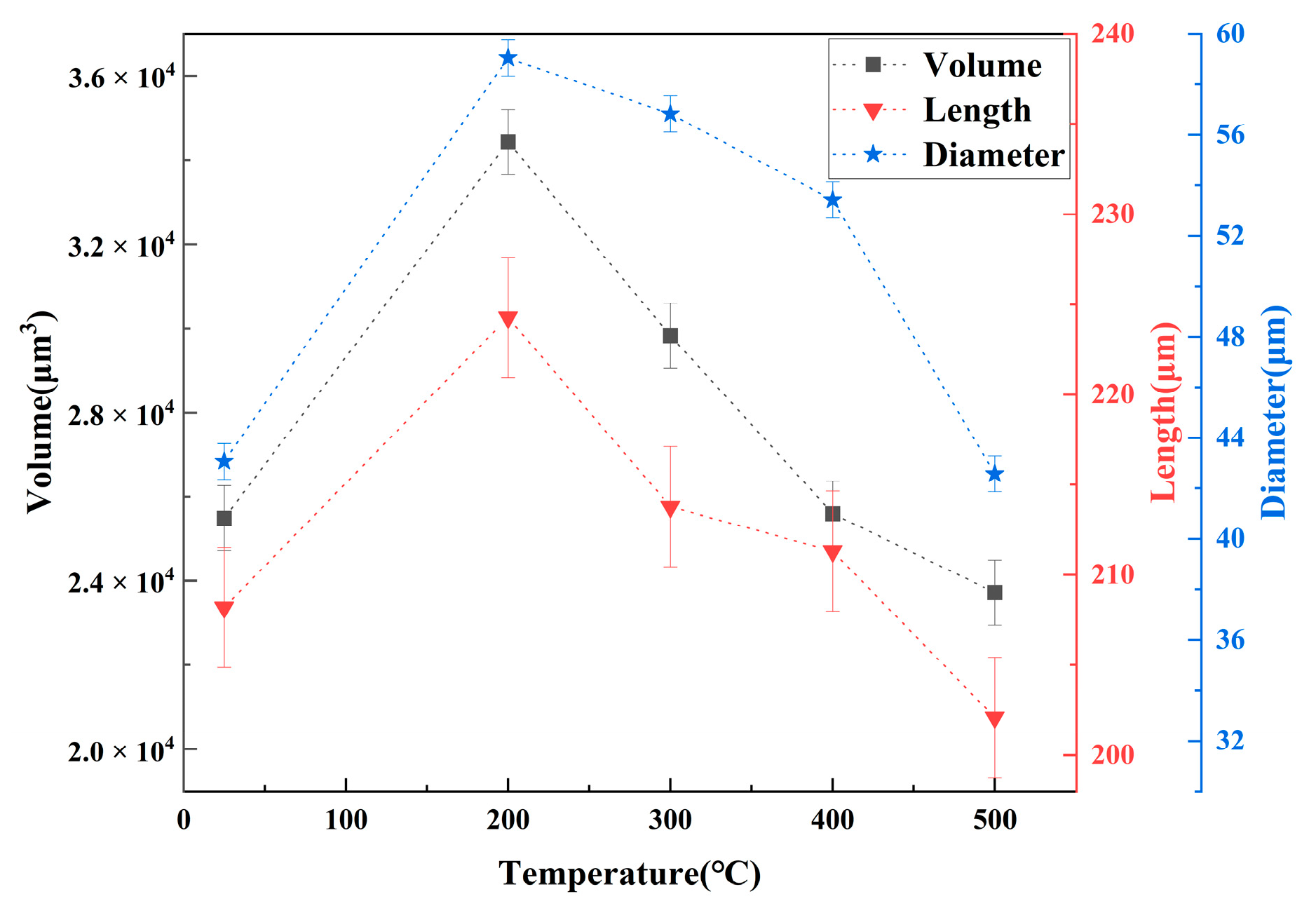
3.1. Statistics of Pores
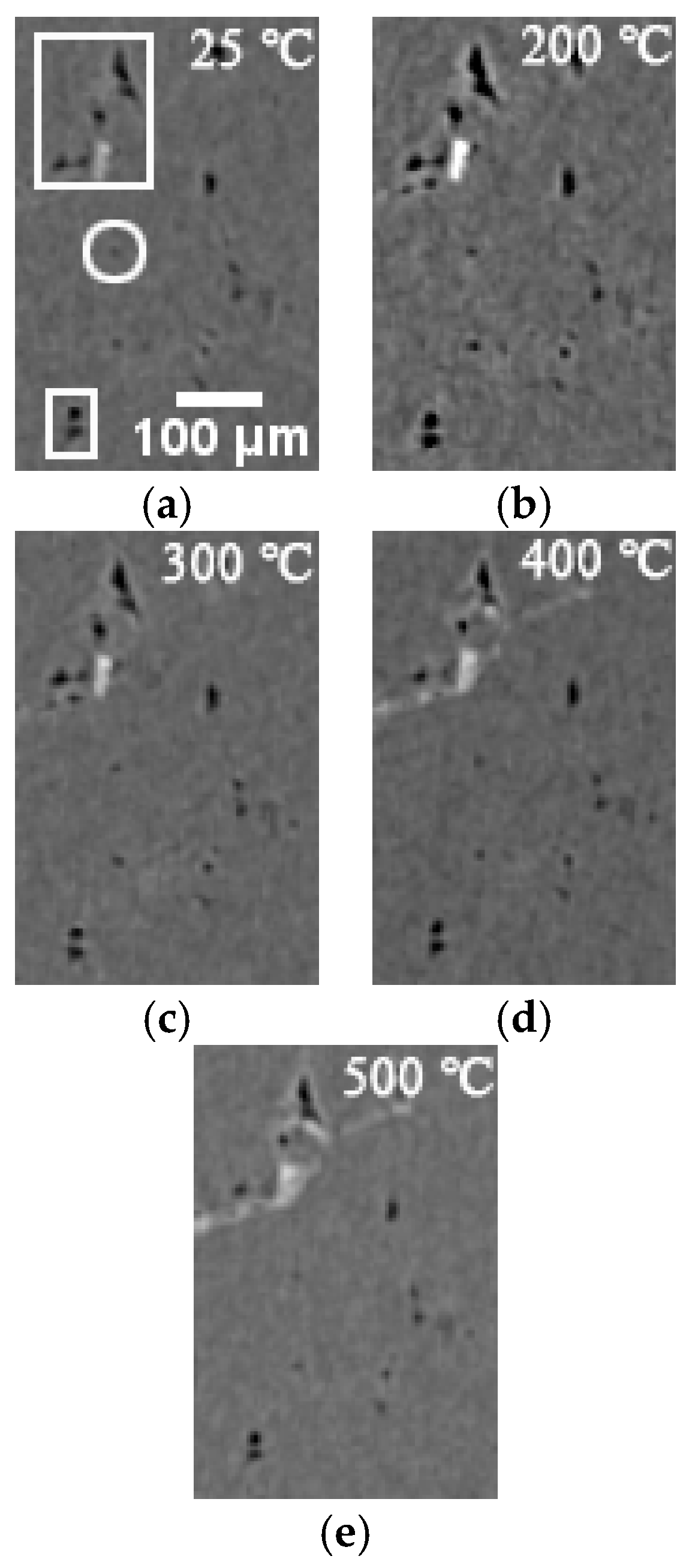
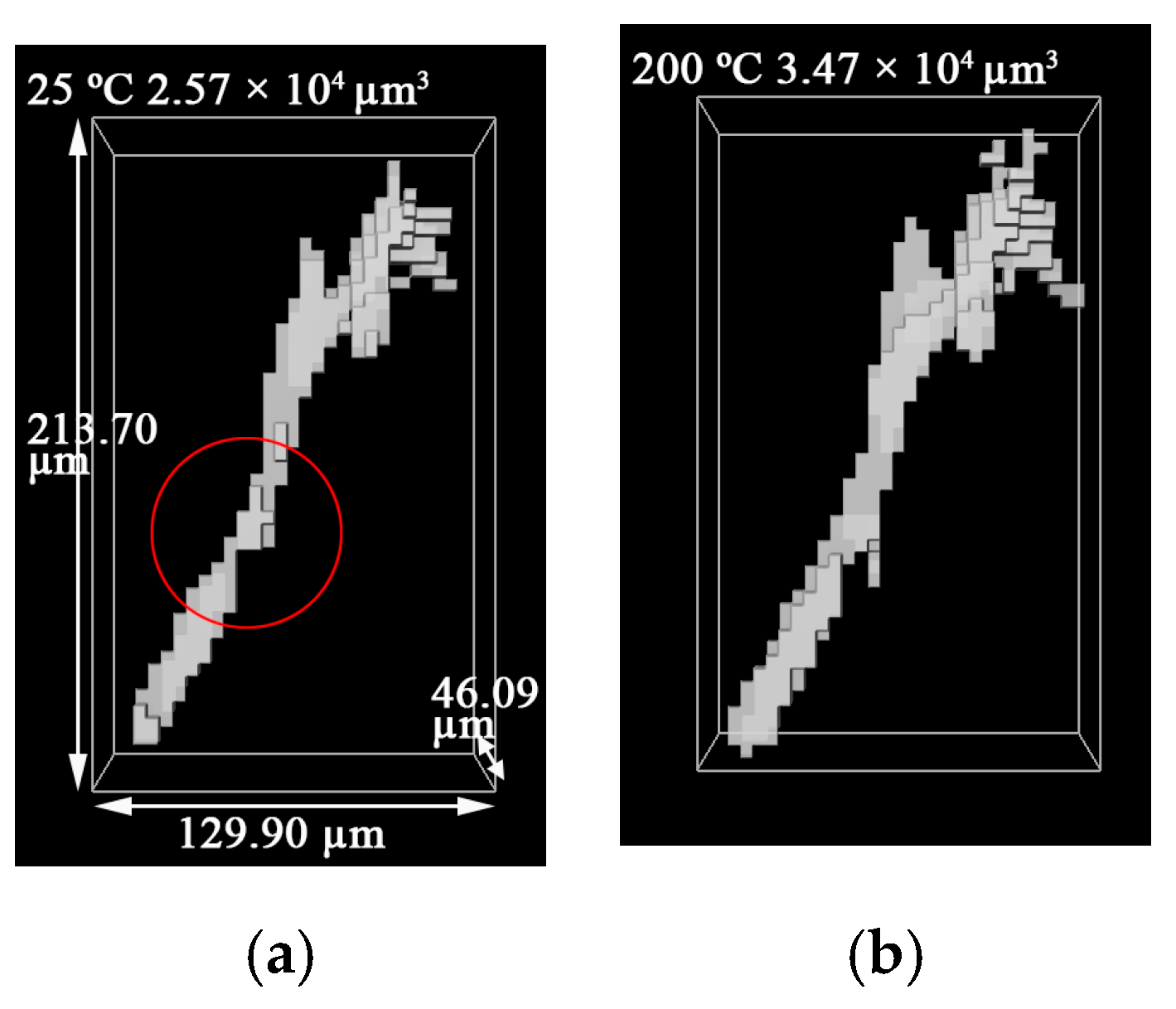
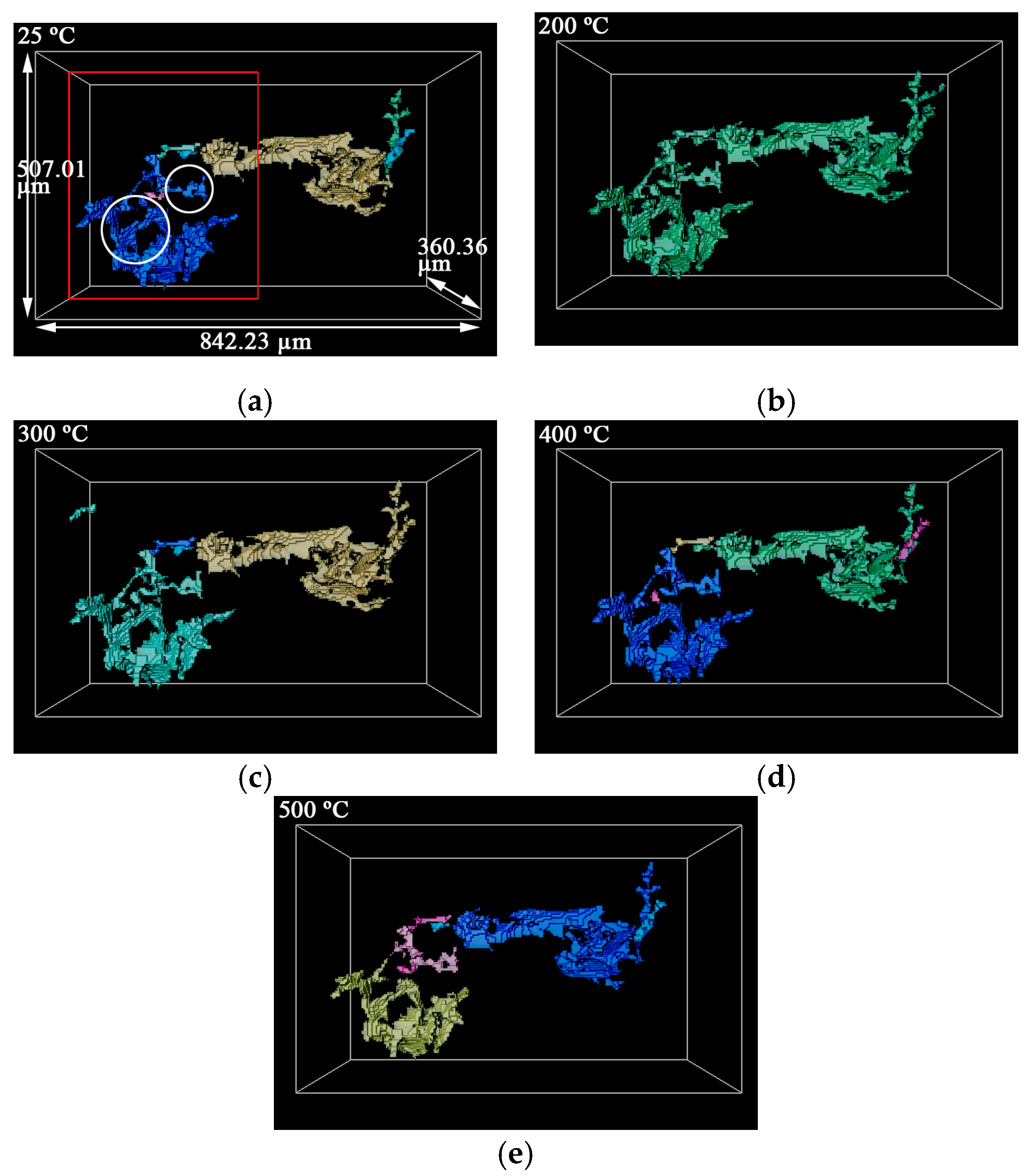
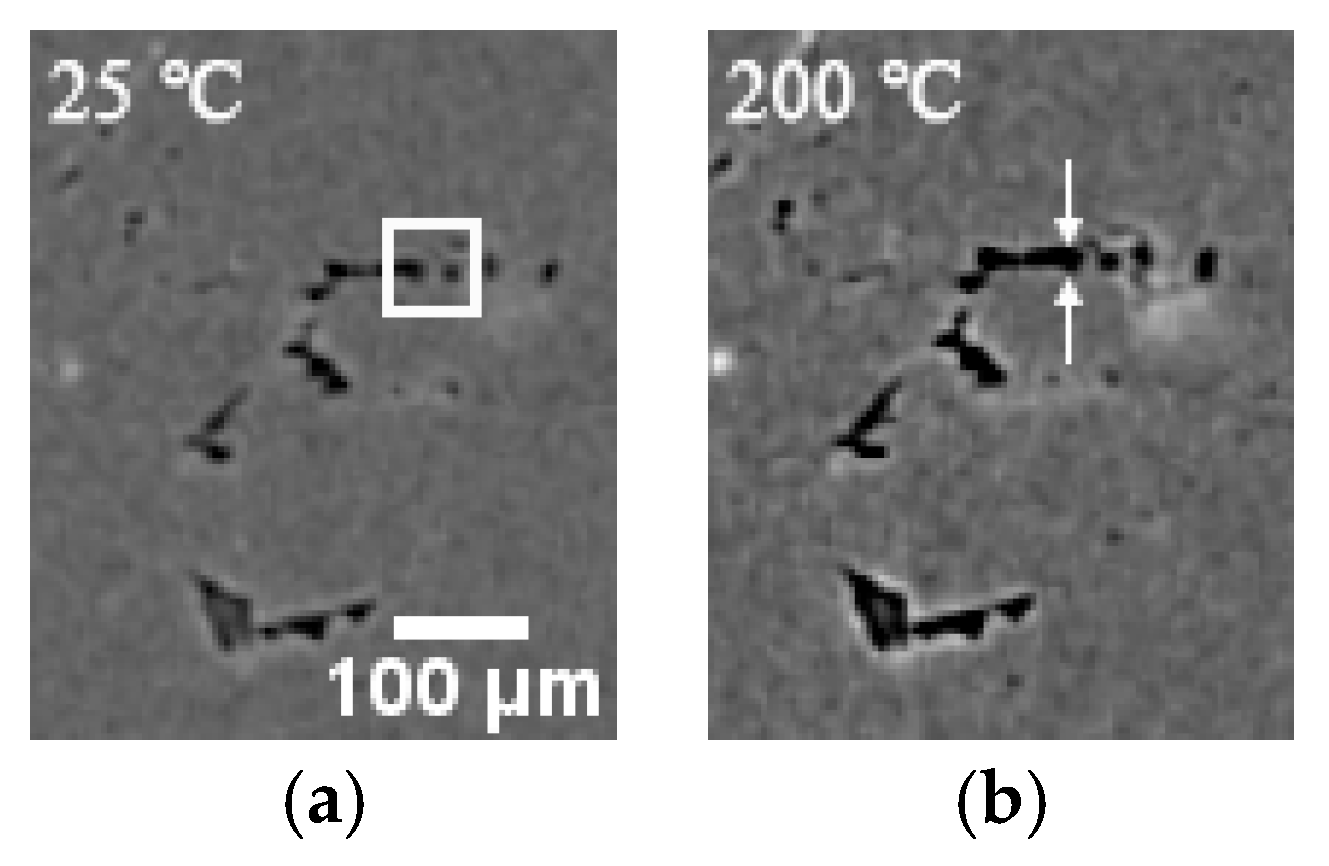
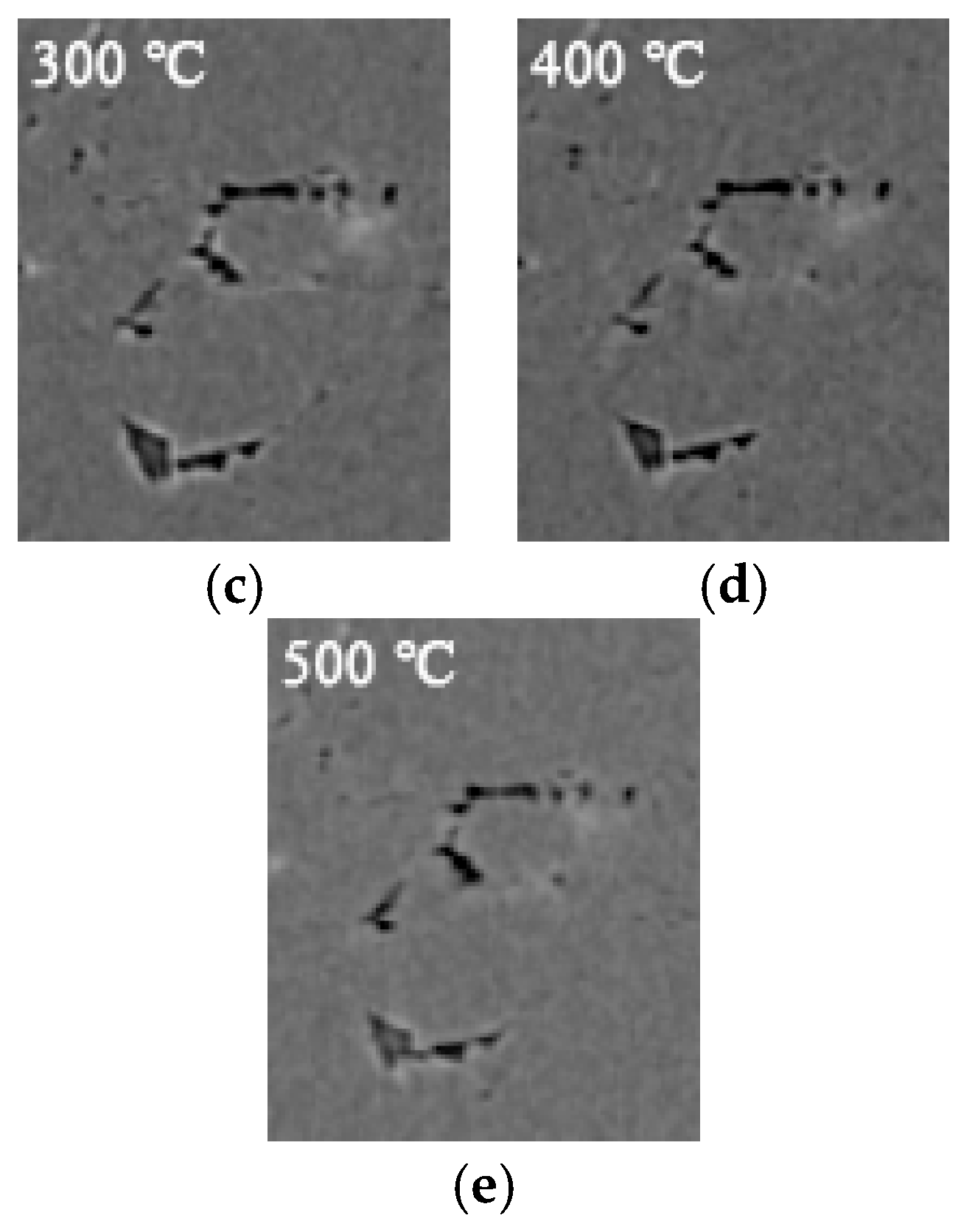
3.2. 3D Structure of Pores during Evolution
4. Conclusions
- (1)
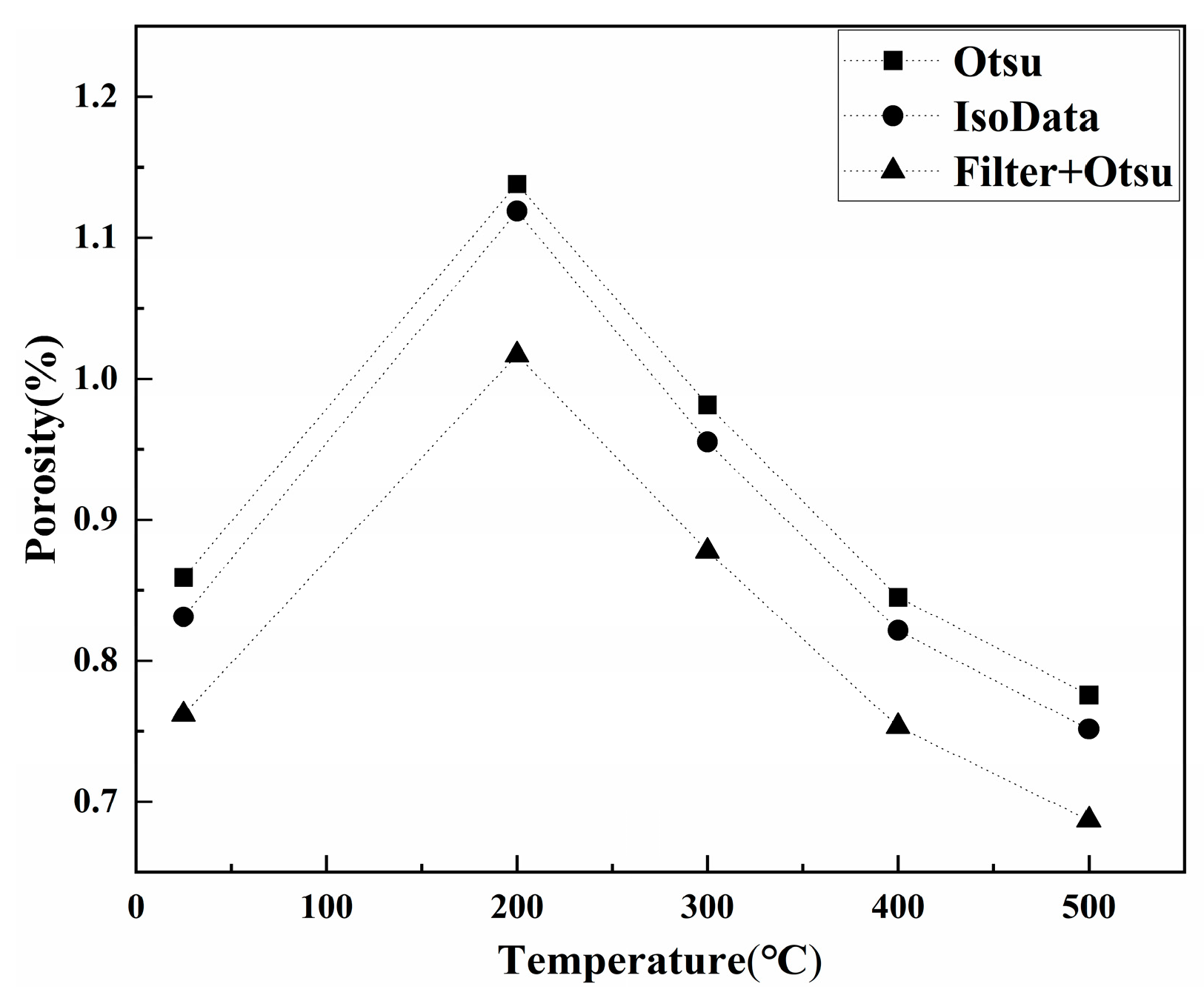
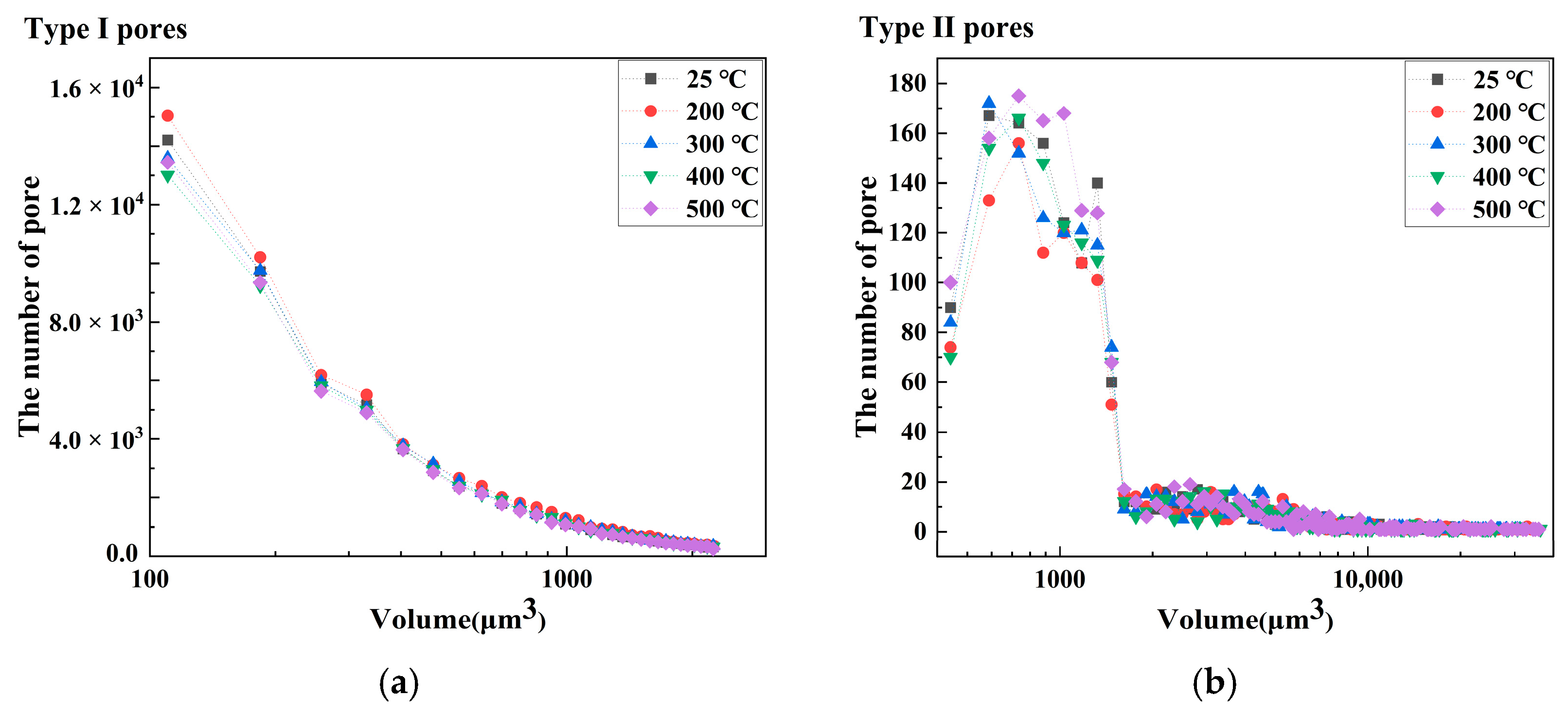
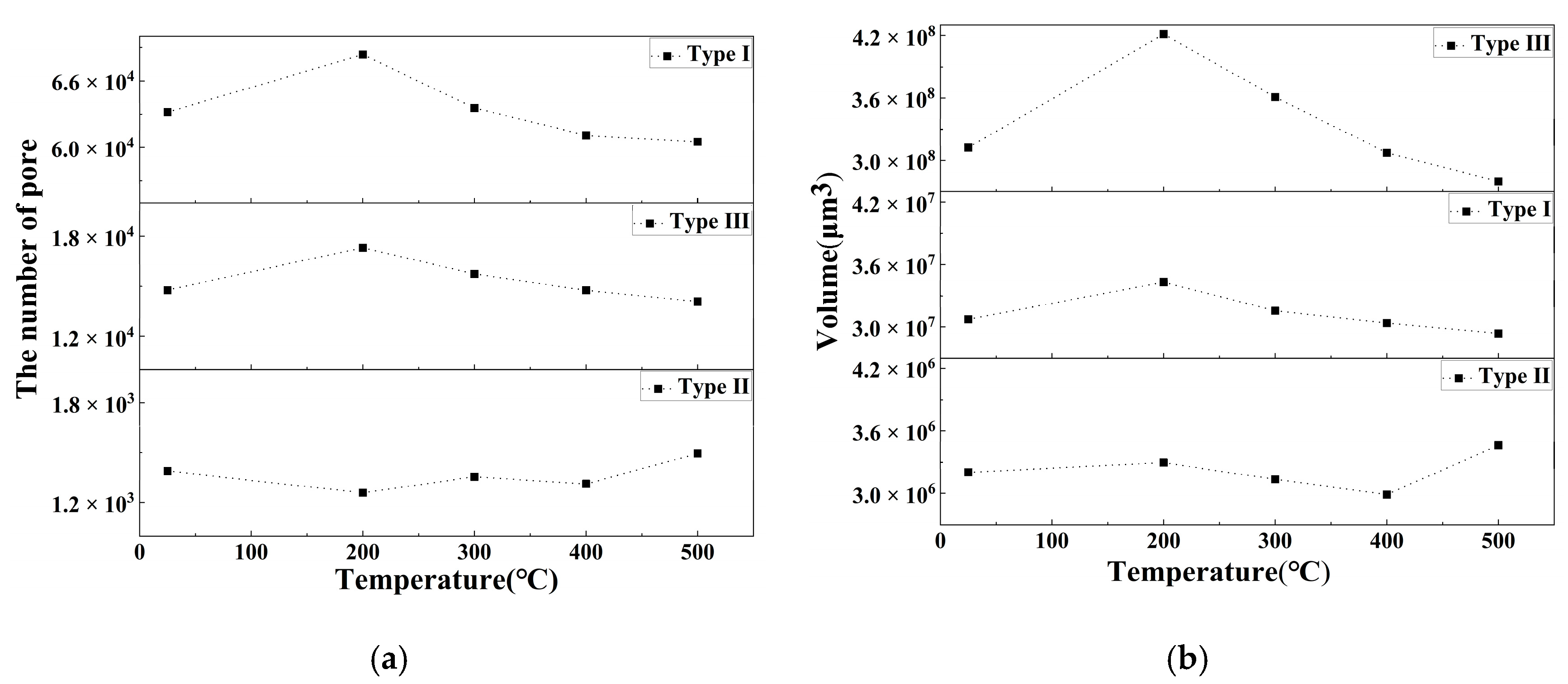
- The porosity of LiH significantly increased from 25 °C to 200 °C and decreased after 200 °C because of the significant change in the number and total volume of Type I and III pores with an increasing temperature. The amount and total volume of Type II pores slightly changed with the temperature.
- (2)
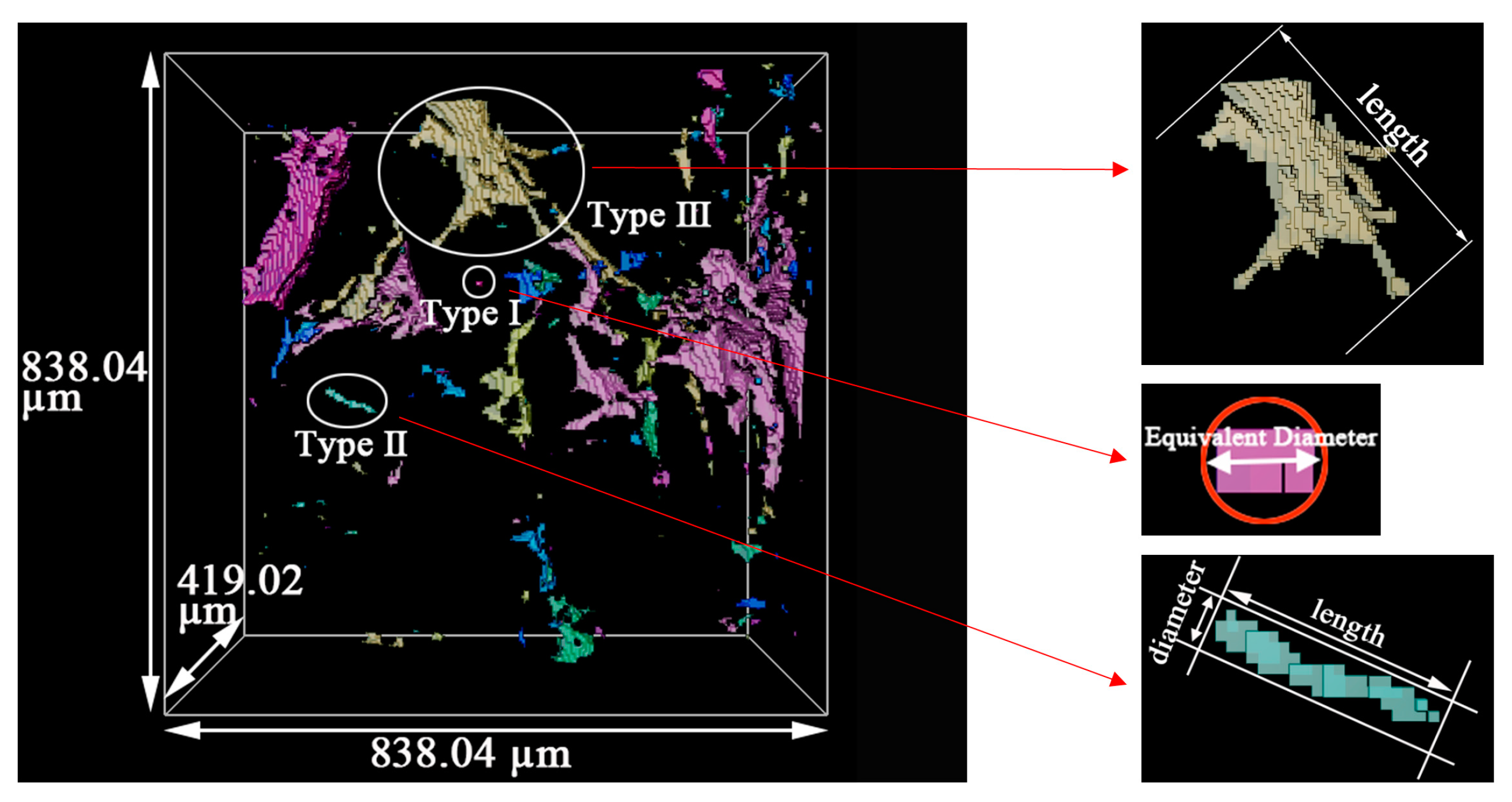
- The positions of pores in LiH did not change. The sizes of pores rose in the range of 25–200 °C and fell from 200 °C to 500 °C. Some small round-shaped (Type I) pores with an Equivalent Diameter under 9 μm appeared at 200 °C and disappeared at elevated temperatures. Some adjacent pores of all types connected at 200 °C, and some branched crack-like (Type III) pores gradually disconnected with an increasing temperature.
- (3)
- The reason for the most significant change of pores at 200 °C might be the expansion of pores caused by the release of residual hydrogen. After 200 °C, the migration and diffusion of some hydrogen in pores, resulting in the contraction of pores, might be the reason for the change in the porosity, amount of pores, total volume of pores and pore size with an increasing temperature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golubev, V.K.; Rabinovich, K.G.; Polyakov, L.V. The effect of pressing conditions on the strength oflithium hydride upon compre. J. Appl. Mech. Tech. Phys. 1997, 38, 961–962. [Google Scholar] [CrossRef]
- Rabinovich, K.G.; Golubev, V.K. Strength and Fracture of Lithium Hydride Crystals underUniaxial Compression. Strength Mater. 1999, 31, 476–478. [Google Scholar] [CrossRef]
- Golubev, V.K.; Rabinovich, K.G. Influence of the initial state on the strength of pressed lithium hydride. Strength Mater. 1999, 31, 625–628. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Li, Q.; Yang, X.; Le, G. A review of irradiation stability of Lithium hydride neutron shielding material. Mater. Sci. Technol. 2016, 32, 434–437. [Google Scholar] [CrossRef]
- Johnson, G.A. Shield design for lunar surface applications. In Proceedings of the AIP Conference Proceedings; pp. 701–706. Available online: https://aip.scitation.org/doi/abs/10.1063/1.2169251 (accessed on 31 August 2021). [CrossRef]
- Messer, C.E. A Survey Report on Lithium Hydride, Medium ed.; United States Atomic Energy Commission, Technical Information Service: Washington, DC, USA, 1960; p. 64. [Google Scholar]
- Qiu, Z.C.; Huang, H.; Shuai, M.B. Mechanical properties of lithium hydride under different environmental conditions. Ordnance Mater. Sci. Eng. 2006, 29, 43–46. [Google Scholar] [CrossRef]
- Qiu, Z.C.; Liu, K.Z.; Wang, X.S. Tensile properties of lithium hydride at high temperature. Rare Met. Mater. Eng. 2006, 35, 1560–1563. [Google Scholar]
- Gogotsi, G.A.; Negovskii, A.N. Use of nondestructive testing methods in evaluation of thermal damage for ceramics under conditions of nonstationary thermal effects. Strength Mater. 1985, 17, 52–56. [Google Scholar] [CrossRef]
- Gogotsi, G.A.; Negovskii, A.N.; Zubov, V.I.; Dezhkunov, N.V. Controlling surface defects in assessing thermal damage to porous ceramics using the luminescent capillary method. Refractories 1984, 25, 274–277. [Google Scholar] [CrossRef]
- Gogotsi, G.A.; Negovskii, A.N.; Zubov, V.I.; Vityuk, P.S.; Glukhovskii, V.P.; Rapoport, Y.M. Using the ultrasonic spectral method for assessing thermal damage to refractory ceramics. Refractories 1984, 25, 219–222. [Google Scholar] [CrossRef]
- Konsztowicz, K.J. Crack Growth and Acoustic Emission in Ceramics During Thermal Shock. J. Am. Ceram. Soc. 1990, 73, 502–508. [Google Scholar] [CrossRef]
- Sitnikov, A.I.; Tel’Nova, G.B.; Shvorneva, L.I.; Fedotov, A.Y.; Solntsev, K.A. Study of the thermomechanical properties of a ceramic based on sodium polyaluminate with the structure of Na-β-alumina. Refract. Ind. Ceram. 2013, 53, 304–309. [Google Scholar] [CrossRef]
- Singh, D.; Singh, J.P.; Wheeler, M.J. Mechanical behavior of SiC(f)/SiC composites and correlation to in situ fiber strength at room and elevated temperatures. J. Am. Ceram. Soc. 1996, 79, 591–596. [Google Scholar] [CrossRef]
- Cao, X.; Wang, B.; Ma, X.; Feng, L.; Shen, X.; Wang, C. Oxidation behavior of melt-infiltrated SiC–TiB2 ceramic composites at 500–1300 °C in air. Ceram. Int. 2021, 47, 9881–9887. [Google Scholar] [CrossRef]
- Cheng, X.; He, R.; Qu, Z.; Ai, S.; Fang, D. High temperature flexural strength of B4C–ZrB2 ceramic at 1000–1600 °C in air. Ceram. Int. 2015, 41, 14574–14578. [Google Scholar] [CrossRef]
- Kong, W.; Wei, Y.; Wang, S.; Chen, J.; Wang, Y. Research progress on cement-based materials by X-ray computed tomography. Int. J. Pavement Res. Technol. 2020, 13, 366–375. [Google Scholar] [CrossRef]
- Xu, F.; Hu, X.F.; Niu, Y.; Zhao, J.H.; Yuan, Q.X. In situ observation of grain evolution in ceramic sintering by SR-CT technique. Trans. Nonferrous Met. Soc. China 2009, 19, s684–s688. [Google Scholar] [CrossRef]
- Xu, F.; Hu, X.F.; Lu, B.; Zhao, J.H.; Wu, X.P.; Yuan, Q.X. Microstructures-evolution observation of boron carbide ceramic during sintering process by synchrotron radiation X-ray computed tomography. Wuji Cailiao Xuebao/J. Inorg. Mater. 2009, 24, 175–181. [Google Scholar] [CrossRef]
- Xu, F.; Hu, X.; Zhao, J.; Yuan, Q. In situ observation of the microstructure-evolution in silicon nitride ceramics sintering by synchrotron radiation X-ray computed tomography. Acta Chim. Sin. 2009, 67, 1205–1210. [Google Scholar]
- Xu, F.; Hu, X.F.; Miao, H.; Zhao, J.H. In situ investigation of ceramic sintering by synchrotron radiation X-ray computed tomography. Opt. Lasers Eng. 2010, 48, 1082–1088. [Google Scholar] [CrossRef]
- Li, Y.C.; Xu, F.; Hu, X.F.; Qu, H.Y.; Miao, H.; Zhang, Z.; Xiao, T.Q. In situ investigation of SiC powder’s microwave sintering by SR-CT technique. Sci. China Technol. Sci. 2011, 54, 1382–1388. [Google Scholar] [CrossRef]
- Okuma, G.; Watanabe, S.; Shinobe, K.; Nishiyama, N.; Takeuchi, A.; Uesugi, K.; Tanaka, S.; Wakai, F. 3D multiscale-imaging of processing-induced defects formed during sintering of hierarchical powder packings. Sci. Rep. 2019, 9, 11595. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Lannutti, J.J.; Klobes, P.; Meyer, K. X-ray computed tomography and mercury porosimetry for evaluation of density evolution and porosity distribution. J. Am. Ceram. Soc. 2000, 83, 518–522. [Google Scholar] [CrossRef]
- Rane, K.; Petrò, S.; Strano, M. Evolution of porosity and geometrical quality through the ceramic extrusion additive manufacturing process stages. Addit. Manuf. 2020, 32, 101038. [Google Scholar] [CrossRef]
- Hondo, T.; Kato, Z.; Yasuda, K.; Wakai, F.; Tanaka, S. Coarse pore evolution in dry-pressed alumina ceramics during sintering. Adv. Powder Technol. 2016, 27, 1006–1012. [Google Scholar] [CrossRef]
- Hondo, T.; Yasuda, K.; Wakai, F.; Tanaka, S. Influence of binder layer of spray-dried granules on occurrence and evolution of coarse defects in alumina ceramics during sintering. J. Eur. Ceram. Soc. 2018, 38, 1846–1852. [Google Scholar] [CrossRef]
- Jia, R.; Wang, C.; Yu, S. Test of apparent porosity and closed porosity of carbon materials—Effective density method. Carbon Tech. 2009, 28, 50–52. [Google Scholar]
- Bo, L.; Kaihui, H.; Dongwei, S.; Weicai, Y.; Yonggang, C.; Mei, L.; Jun, S. Research on measurement of residual stresses of hemispherical lithium hydride by blind-hole method. Fusion Eng. Des. 2014, 89, 365–369. [Google Scholar] [CrossRef]
- Grigoriev, M.V.; Dyachkova, I.G.; Buzmakov, A.V.; Povolotsky, M.A.; Kokhan, V.V.; Chukalina, M.V.; Uvarov, V.I. μCT Analysis of Porous Cermet Membranes with the Use of Enhanced Filtration and Binarization Algorithms. J. Surf. Investig. 2020, 14, 1294–1303. [Google Scholar] [CrossRef]
- Shou, Y.D.; Zhao, Z.; Zhou, X.P. Sensitivity analysis of segmentation techniques and voxel resolution on rock physical properties by X-ray imaging. J. Struct. Geol. 2020, 133, 103978. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Dias Velasco, F.R. Thresholding Using the ISODATA Clustering Algorithm. IEEE Trans. Syst. Man Cybern. 1980, 10, 771–774. [Google Scholar]
- Weickert, J.; Romeny, B.M.H.; Viergever, M.A. Efficient and Reliable Schemes for Nonlinear Diffusion Filtering. IEEE Trans. Image Process. 1998, 7, 398–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, A.; Naito, S. Facile Hydrogen Release on the Composites of Lithium Hydride with Carbonaceous and Polymer Materials. J. Jpn. Pet. Inst. 2019, 62, 87–96. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Peng, L.; Zhang, W.; Li, Q.; Li, Q.; Ye, L. In Situ Evolution of Pores in Lithium Hydride at Elevated Temperatures Characterized by X-ray Computed Tomography. Crystals 2021, 11, 1093. https://doi.org/10.3390/cryst11091093
Shi Y, Peng L, Zhang W, Li Q, Li Q, Ye L. In Situ Evolution of Pores in Lithium Hydride at Elevated Temperatures Characterized by X-ray Computed Tomography. Crystals. 2021; 11(9):1093. https://doi.org/10.3390/cryst11091093
Chicago/Turabian StyleShi, Yifan, Lei Peng, Wangzi Zhang, Qiang Li, Qishou Li, and Linsen Ye. 2021. "In Situ Evolution of Pores in Lithium Hydride at Elevated Temperatures Characterized by X-ray Computed Tomography" Crystals 11, no. 9: 1093. https://doi.org/10.3390/cryst11091093
APA StyleShi, Y., Peng, L., Zhang, W., Li, Q., Li, Q., & Ye, L. (2021). In Situ Evolution of Pores in Lithium Hydride at Elevated Temperatures Characterized by X-ray Computed Tomography. Crystals, 11(9), 1093. https://doi.org/10.3390/cryst11091093





