Tailoring α/β Ratio of Pollen-Like Anhydrous Lactose as Ingredient Carriers for Controlled Dissolution Rate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Pollen-Like Anhydrous Lactose with Tailored α/β Ratio
2.3. Ingredient Loading, Tableting and Release
2.4. Instrumental Analysis
3. Results and Discussion
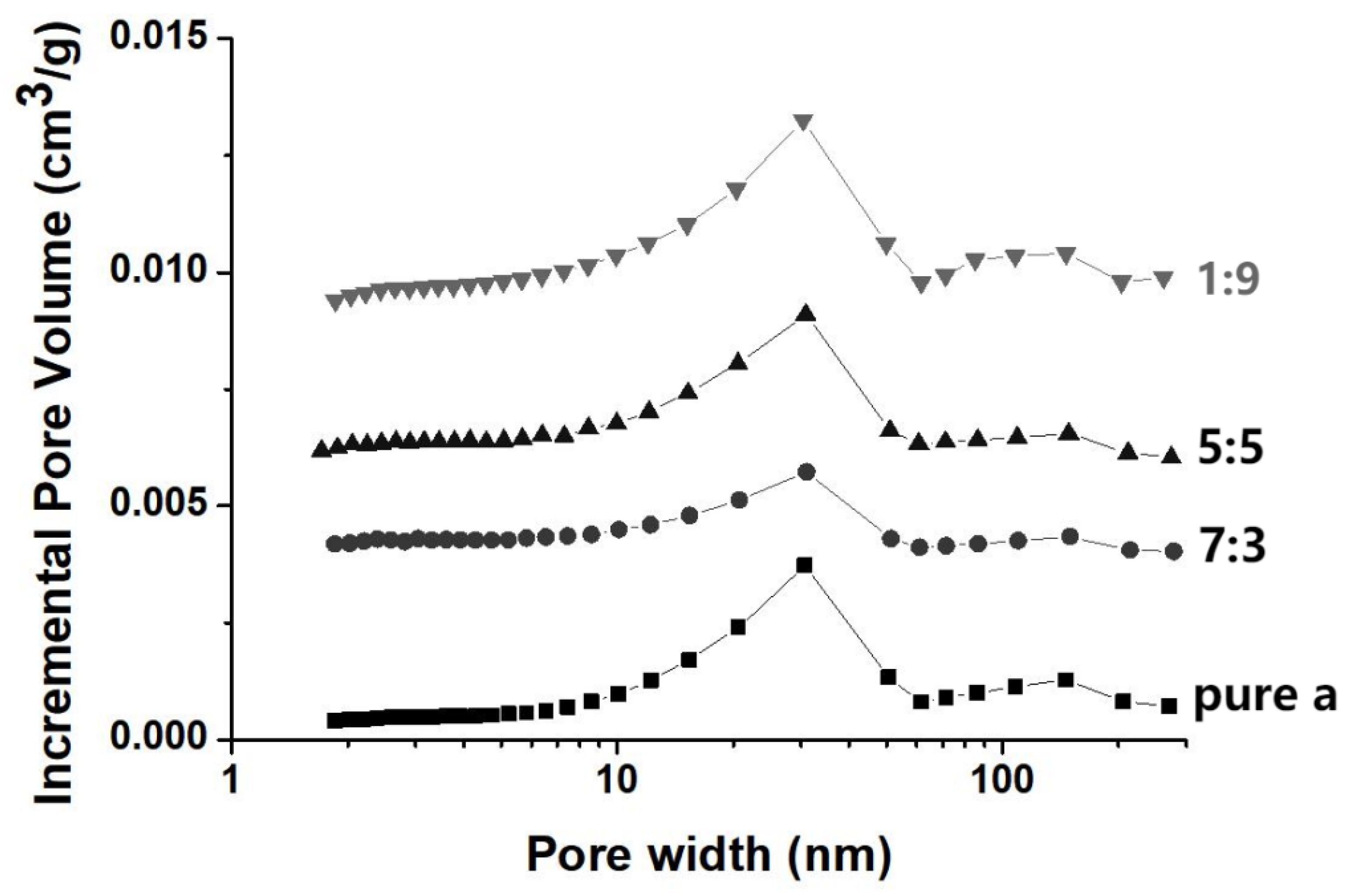
3.1. Pollen-Like Anhydrous Lactose for Ingredient Loading
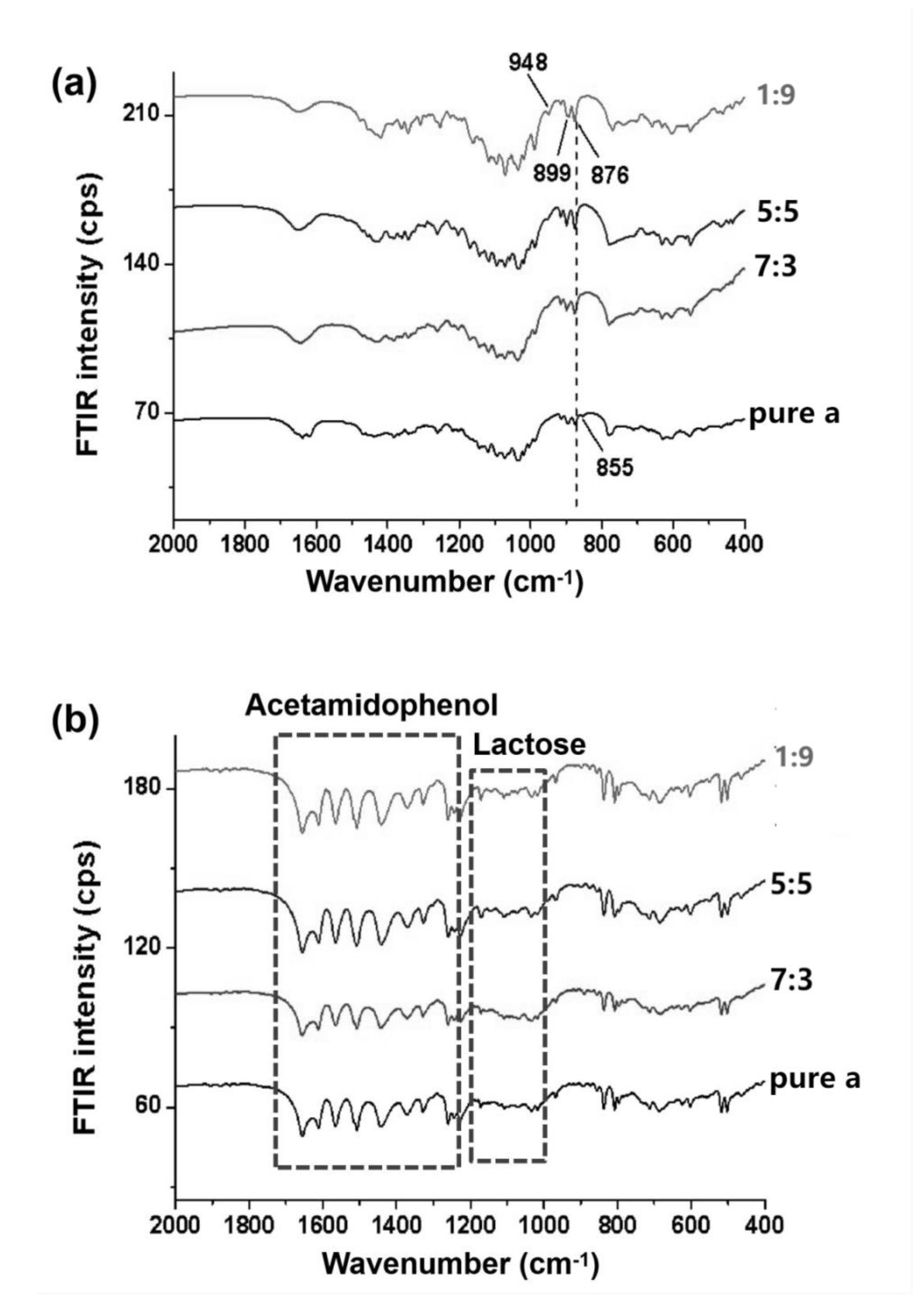
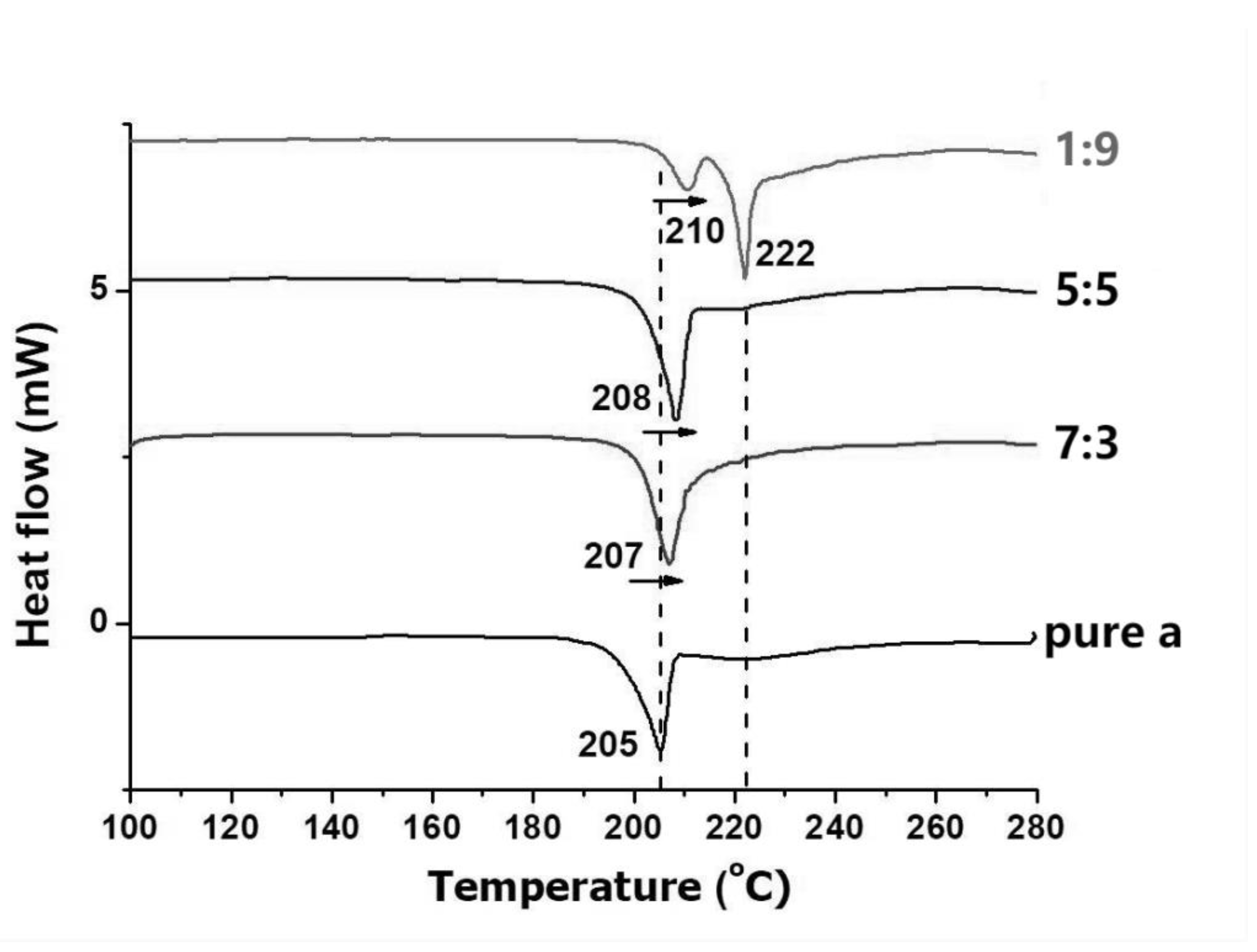
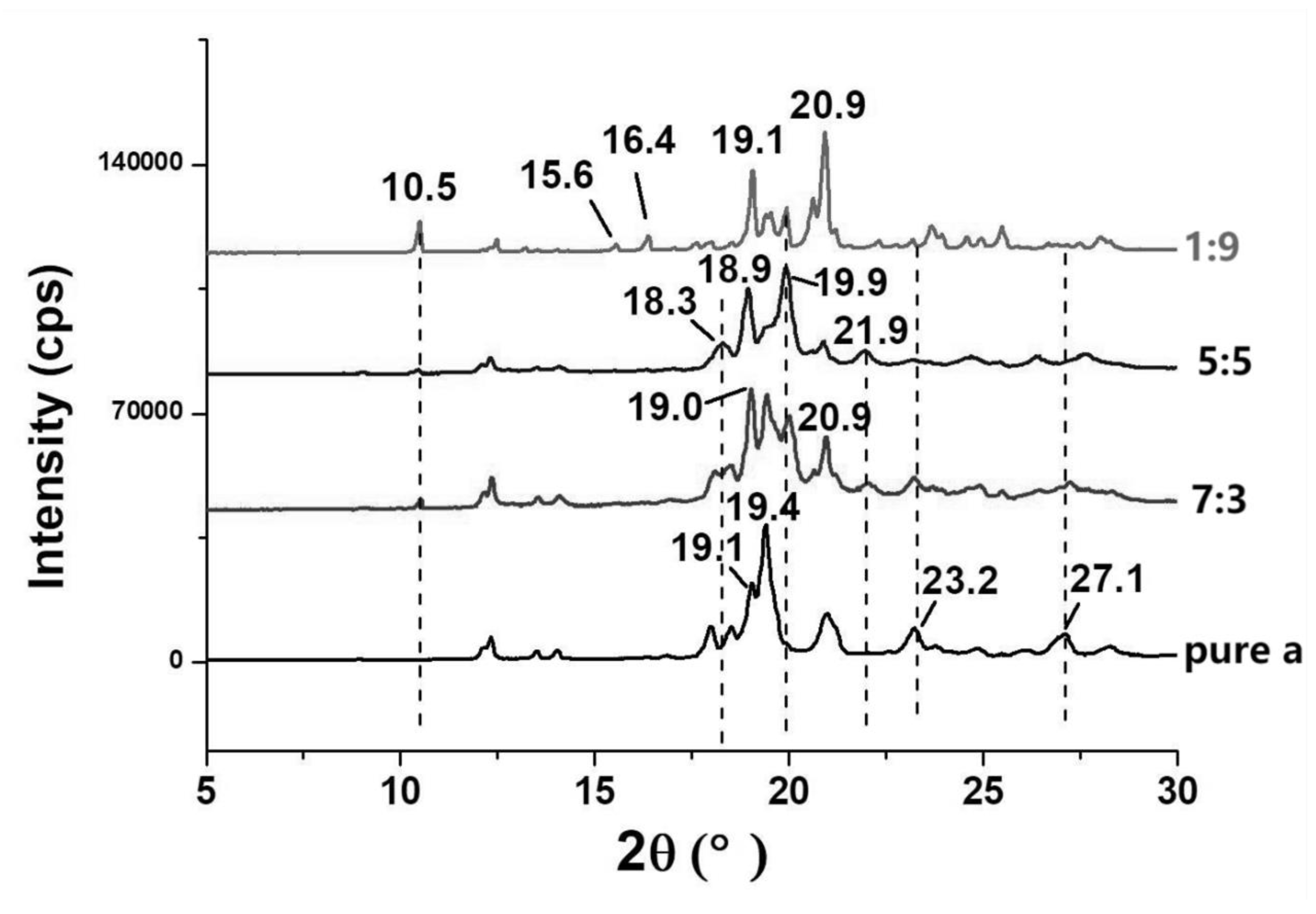
3.2. Crystalline Features of α/β-Lactose co-Crystals
3.3. Loading Efficiency
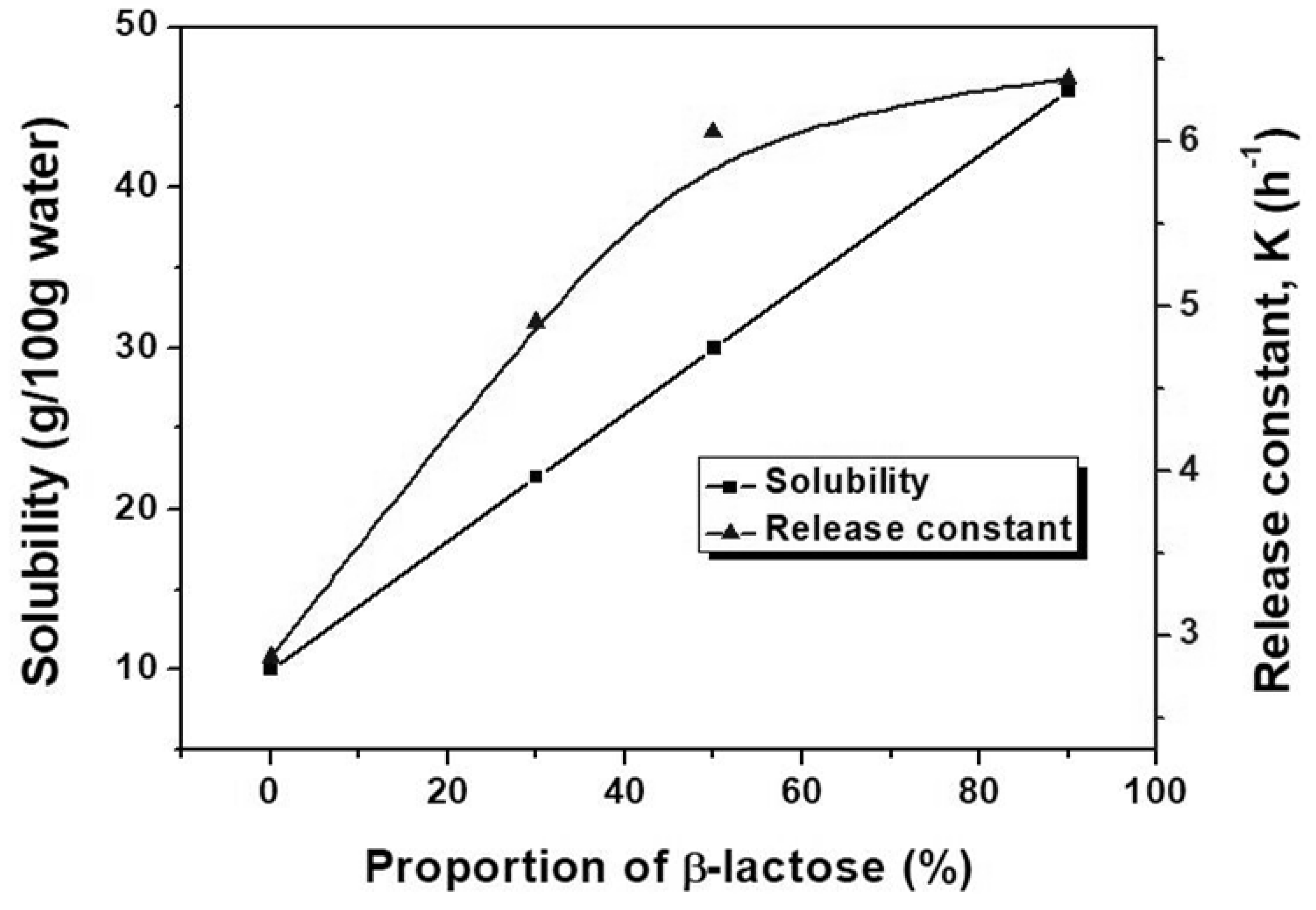
3.4. Tailored α/β Ratio for Controlled Release
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Young, C.; Hillers, J.; Freeman, A. Production, consumption, and pricing of milk and its components. J. Dairy Sci. 1986, 69, 272–281. [Google Scholar] [CrossRef]
- Linn, J. Factors affecting the composition of milk from dairy cows. In Designing Foods: Animal Product Options in the Market Place; National Academy Press: Washington, DC, USA, 1988; p. 224. [Google Scholar]
- Heck, J.M.L.; van Valenberg, H.; Dijkstra, J.; van Hooijdonk, A.C.M. Seasonal variation in the Dutch bovine raw milk composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef]
- Costa, A.; Lopez-Villalobos, N.; Sneddon, N.; Shalloo, L.; Franzoi, M.; de Marchi, M.; Penasa, M. Invited review: Milk lactose—Current status and future challenges in dairy cattle. J. Dairy Sci. 2019, 102, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline cellulose, lactose and lignin blends: Process mapping of dry granulation via roll compaction. Powder Technol. 2018, 341, 38–50. [Google Scholar] [CrossRef]
- Illanes, A.; Guerrero, C.; Vera, C.; Wilson, L.; Conejeros, R.; Scott, F. Lactose-Derived Prebiotics: A Process Perspective; Academic Press: Cambridge, MA, USA, 2016; pp. 1–31. [Google Scholar]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009; pp. 359–377. [Google Scholar]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Tan, S.; Ebrahimi, A.; Liu, X.; Langrish, T. Role of templating agents in the spray drying and postcrystallization of lactose for the production of highly porous powders. Dry. Technol. 2018, 36, 1882–1891. [Google Scholar] [CrossRef]
- Huppertz, T.; Gazi, I. Lactose in dairy ingredients: Effect on processing and storage stability. J. Dairy Sci. 2016, 99, 6842–6851. [Google Scholar] [CrossRef]
- Altamimi, M.J.; Wolff, K.; Nokhodch, A.; Martin, G.P.; Royall, P.G. Variability in the α and β anomer content of commercially available lactose. Int. J. Pharm. 2019, 555, 237–249. [Google Scholar] [CrossRef]
- Murphy, B.M.; Prescott, S.W.; Larson, I. Measurement of lactose crystallinity using Raman spectroscopy. J. Pharm. Biomed. Anal. 2005, 38, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.C.; Grush, E.; Mauer, L.J. RH-temperature stability diagram of α- and β-anhydrous and monohydrate lactose crystalline forms. Food Res. Int. 2019, 127, 108717. [Google Scholar] [CrossRef]
- Agrawal, S.; Paterson, T.; Jones, J.; McLeod, J.; Bronlund, J. A mathematical model based parametric sensitivity analysis of an evaporative crystallizer for lactose monohydrate. Food Bioprod. Process. 2015, 97, 1–11. [Google Scholar] [CrossRef]
- Ramesh, P.; Kritikos, A.; Tsilomelekis, G. Effect of metal chlorides on glucose mutarotation and possible implications on humin formation. React. Chem. Eng. 2018, 4, 273–277. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H. Lactose. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 21–32. [Google Scholar]
- Lara-Mota, E.E.; Nicolás–Vázquez, M.I.; López-Martínez, L.A.; Espinosa-Solis, V.; Cruz-Alcantar, P.; Toxqui-Teran, A.; Saavedra-Leos, M.Z. Phenomenological study of the synthesis of pure anhydrous β-lactose in alcoholic solution. Food Chem. 2020, 340, 128054. [Google Scholar] [CrossRef] [PubMed]
- Barham, A.S.; Hodnett, B.K. In situ x-ray diffraction study of the crystallization of spray-dried lactose. Cryst. Growth Des. 2005, 5, 1965–1970. [Google Scholar] [CrossRef]
- Haque, M.K.; Roos, Y.H. Crystallization and X-ray diffraction of spray-dried and freeze-dried amorphous lactose. Carbohydr. Res. 2005, 340, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Chin, G.Q.; Ching, T.W.; Woo, M.W.; Fu, N.; Chen, X.D. Precipitating smooth amorphous or pollen structured lactose microparticles. Chem. Eng. J. 2013, 226, 312–318. [Google Scholar] [CrossRef]
- Woo, M.W.; Mansouri, S.; Chen, X.D. Antisolvent vapor precipitation: The future of pulmonary drug delivery particle production? Expert Opin. Drug Deliv. 2014, 11, 307–311. [Google Scholar] [CrossRef][Green Version]
- Hibbins, A.R.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; du Toit, L.C.; Pillay, V. Design of a versatile pH-responsive hydrogel for potential oral delivery of gas-tric-sensitive bioactives. Polymers 2017, 9, 74. [Google Scholar] [CrossRef]
- Parimaladevi, P.; Srinivasan, K. Influence of supersaturation level on the morphology of α-lactose monohydrate crystals. Int. Dairy J. 2014, 39, 301–311. [Google Scholar] [CrossRef]
- Parimaladevi, P.; Srinivasan, K. Achievement of favorable uniform crystal size distribution of alpha-lactose monohydrate (α-LM) through swift cooling process. J. Food Eng. 2015, 151, 1–6. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Saffari, M.; Dehghani, F.; Langrish, T. Incorporation of acetaminophen as an active pharmaceutical ingredient into porous lactose. Int. J. Pharm. 2016, 499, 217–227. [Google Scholar] [CrossRef]
- López-Pablos, A.L.; Leyva-Porras, C.C.; Silva-Cázares, M.B.; Longoria-Rodríguez, F.E.; Pérez-García, S.A.; Vértiz-Hernández, A.; Saavedra-Leos, M.Z. Preparation and characterization of high purity anhydrous β-lactose from α-lactose monohydrate at mild temperature. Int. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Tan, S.; Ebrahimi, A.; Langrish, T. Template-directed flower-like lactose with micro-meso-macroporous structure. Mater. Des. 2017, 117, 178–184. [Google Scholar] [CrossRef]
- Tan, S.; Zhong, C.; Langrish, T. Pre-gelation assisted spray drying of whey protein isolates (WPI) for microencapsulation and controlled release. LWT 2020, 117, 108625. [Google Scholar] [CrossRef]
- Smith, G.; Hussain, A.; Bukhari, N.I.; Ermolina, I. Quantification of residual crystallinity of ball-milled, commercially available, anhydrous β-lactose by differential scanning calorimetry and terahertz spectroscopy. J. Therm. Anal. Calorim. 2015, 121, 327–333. [Google Scholar] [CrossRef]
- Gombás, A.; Szabó-Révész, P.; Kata, M.; Regdon, G., Jr.; Erős, I. Quantitative determination of crystallinity of α-lactose monohydrate by DSC. J. Therm. Anal. Calorim. 2002, 68, 503–510. [Google Scholar] [CrossRef]
- Segun, P.; Ajala, O.T.; Aremu, O.I. The effect of formulation variables on the release kinetics of paracetamol tablet formulations. J. Pharm. Health Sci. 2018, 6, 103–111. [Google Scholar]


| α/β Ratio | BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) | Loading Efficiency (%) |
|---|---|---|---|
| Pure α | 13.0 | 0.0271 | 32.7 |
| 7:3 | 13.6 | 0.0209 | 30.6 |
| 5:5 | 16.3 | 0.0316 | 31.8 |
| 1:9 | 15.4 | 0.0380 | 33.4 |
| α/β Ratio | First-Order Release Constant, k (h−1) | R-Squared |
|---|---|---|
| Pure α | 2.87 | 0.688 |
| 7:3 | 4.91 | 0.844 |
| 5:5 | 6.06 | 0.936 |
| 1:9 | 6.39 | 0.925 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Wang, B.; Fu, L.; Chen, C.; Liu, W.; Tan, S. Tailoring α/β Ratio of Pollen-Like Anhydrous Lactose as Ingredient Carriers for Controlled Dissolution Rate. Crystals 2021, 11, 1049. https://doi.org/10.3390/cryst11091049
Xiang J, Wang B, Fu L, Chen C, Liu W, Tan S. Tailoring α/β Ratio of Pollen-Like Anhydrous Lactose as Ingredient Carriers for Controlled Dissolution Rate. Crystals. 2021; 11(9):1049. https://doi.org/10.3390/cryst11091049
Chicago/Turabian StyleXiang, Jia, Bo Wang, Le Fu, Chuanpin Chen, Wenjie Liu, and Songwen Tan. 2021. "Tailoring α/β Ratio of Pollen-Like Anhydrous Lactose as Ingredient Carriers for Controlled Dissolution Rate" Crystals 11, no. 9: 1049. https://doi.org/10.3390/cryst11091049
APA StyleXiang, J., Wang, B., Fu, L., Chen, C., Liu, W., & Tan, S. (2021). Tailoring α/β Ratio of Pollen-Like Anhydrous Lactose as Ingredient Carriers for Controlled Dissolution Rate. Crystals, 11(9), 1049. https://doi.org/10.3390/cryst11091049








