Electrochemical Corrosion Behavior of Laser Welded 2205 Duplex Stainless-Steel in Artificial Seawater Environment under Different Acidity and Alkalinity Conditions
Abstract
:1. Introduction
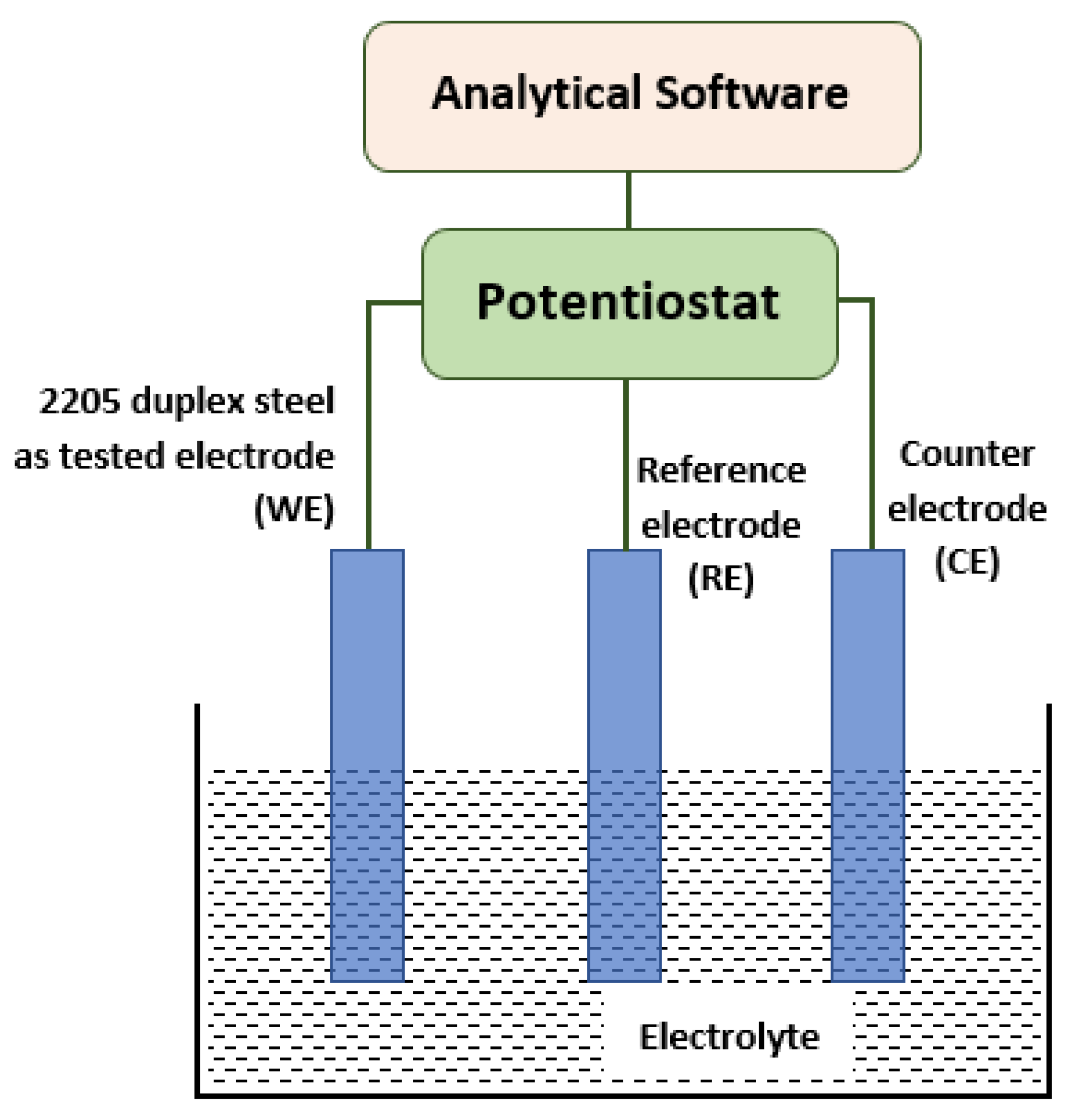
2. Materials and Methods
3. Results and Discussions
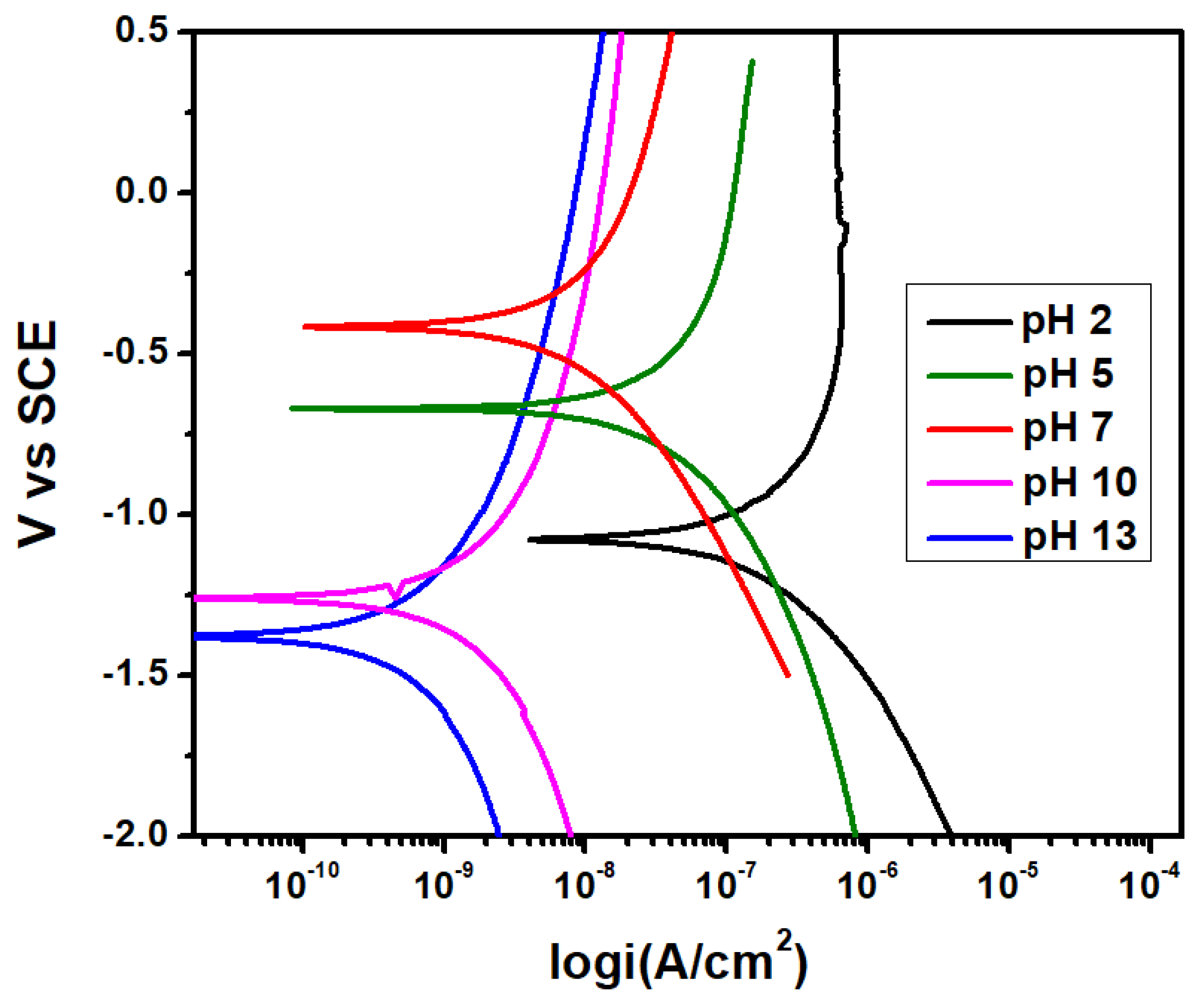
3.1. Potentiodynamic Polarization Studies
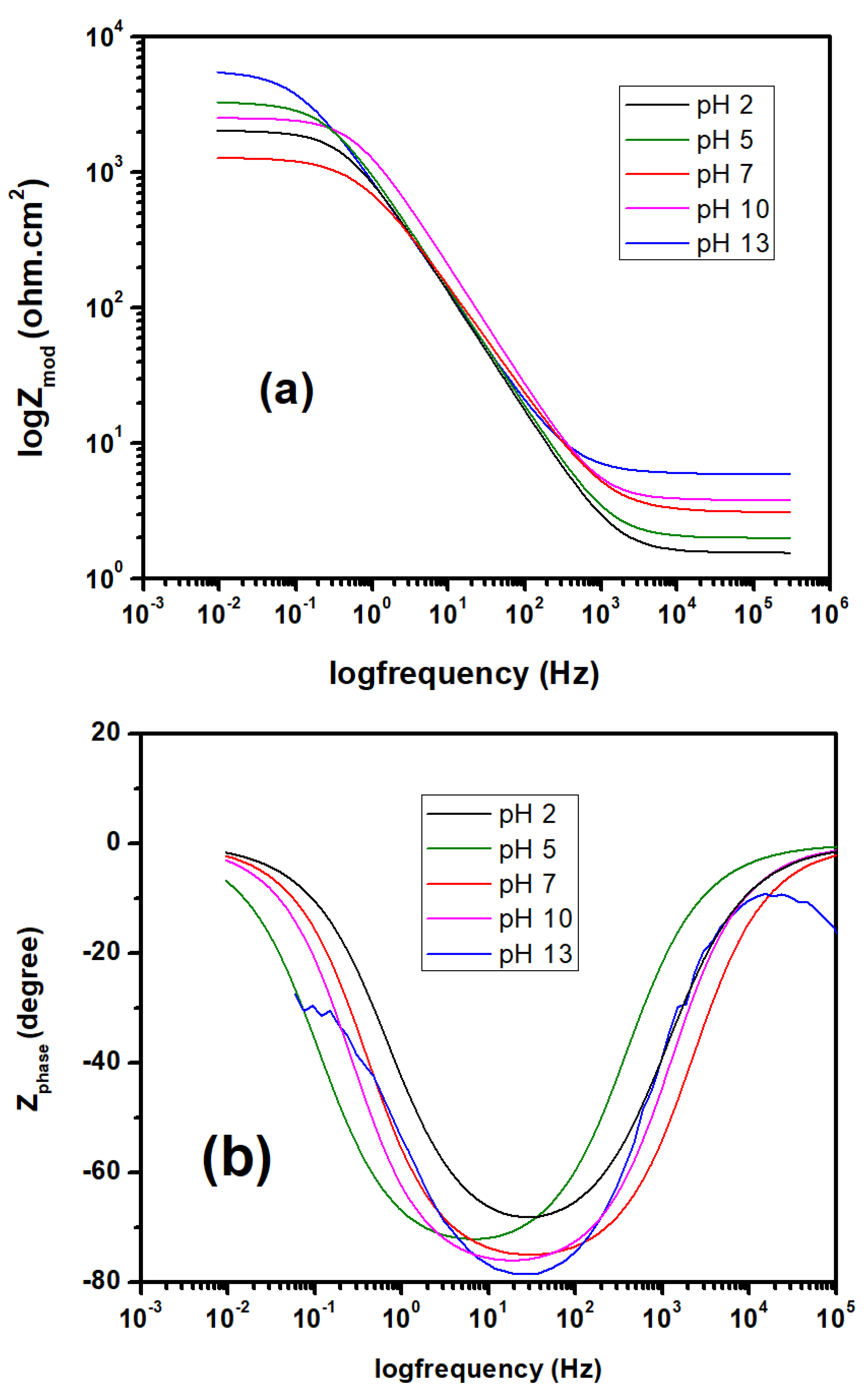
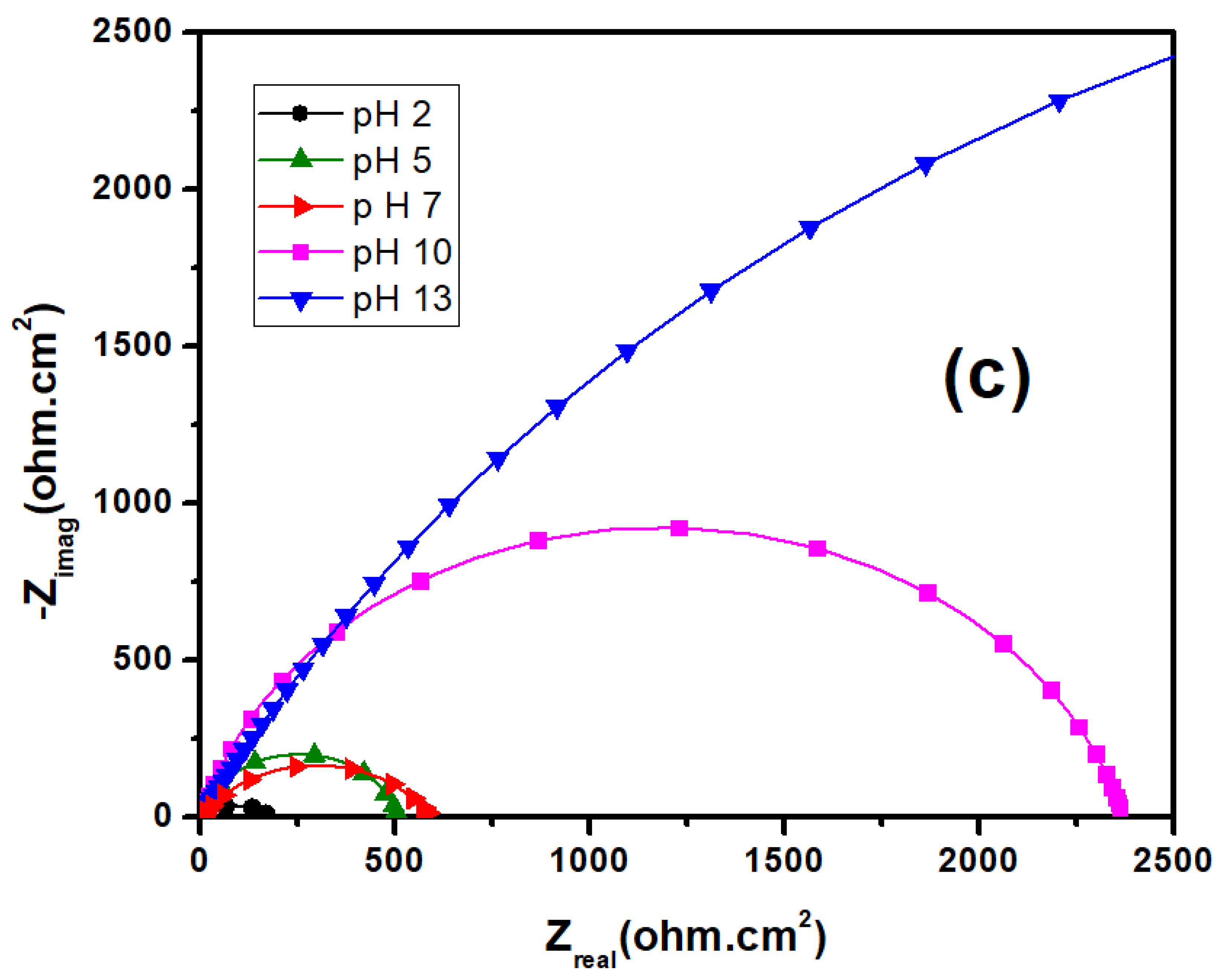
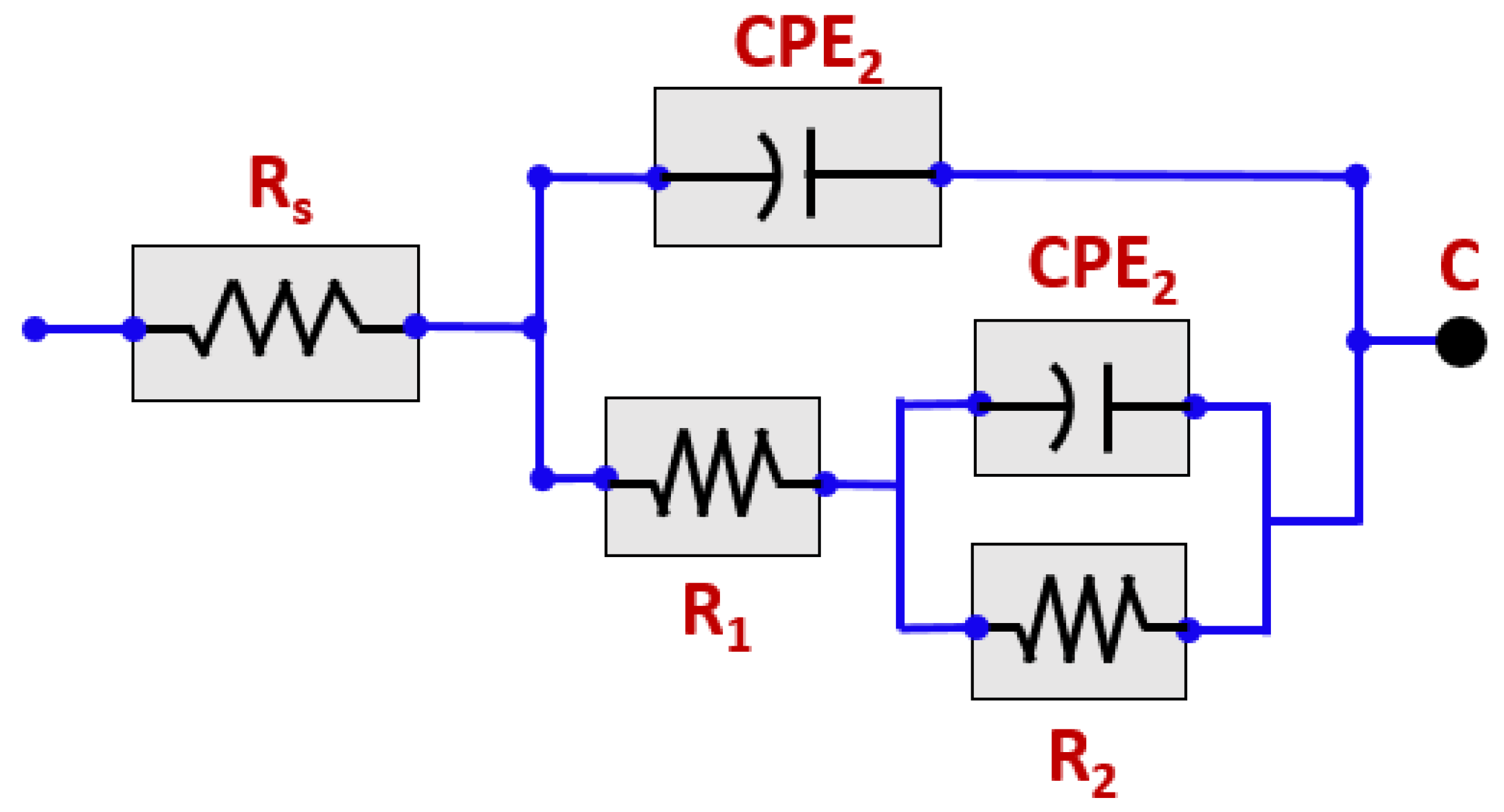
3.2. EIS Studies
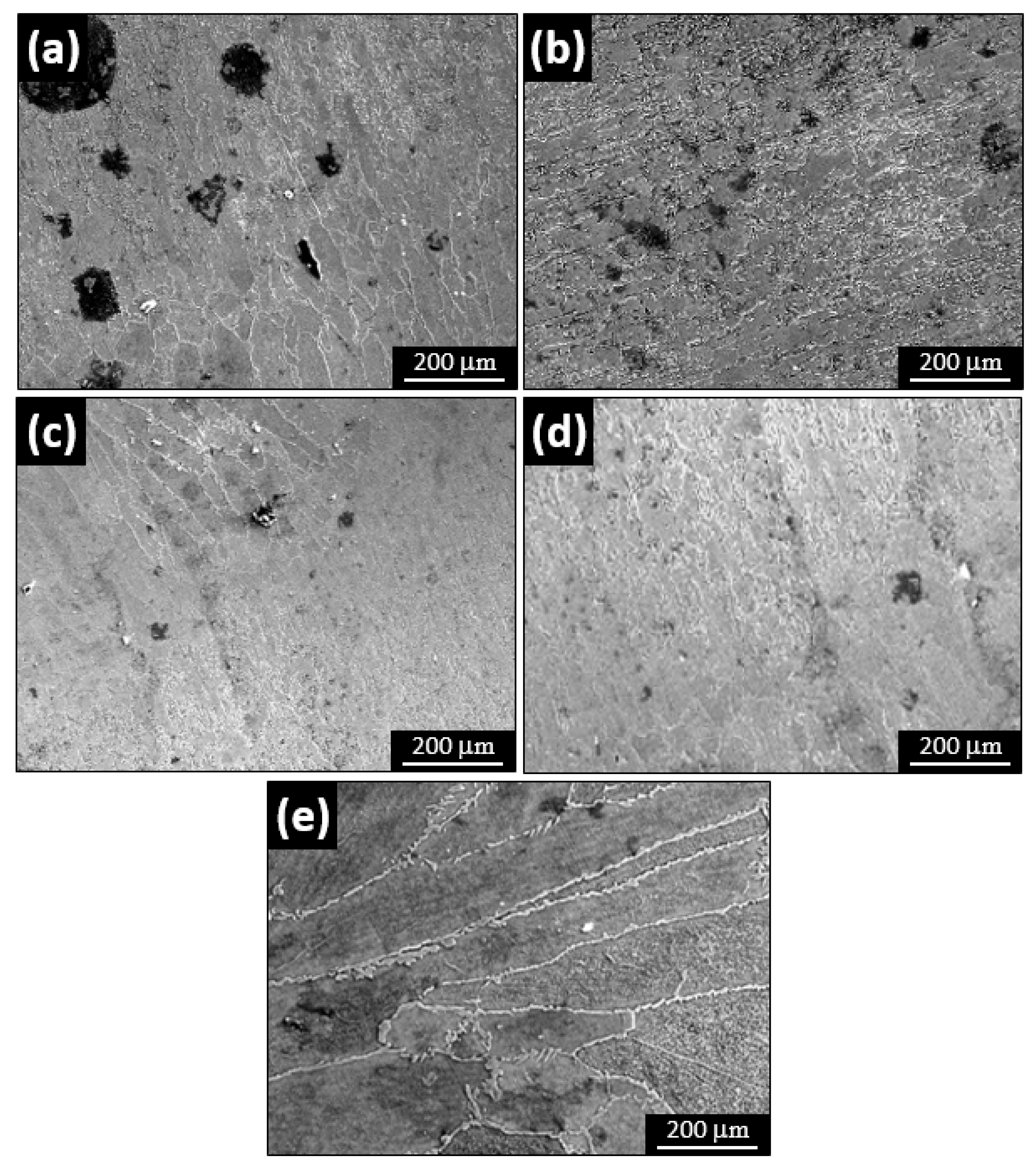
3.3. SEM Analysis
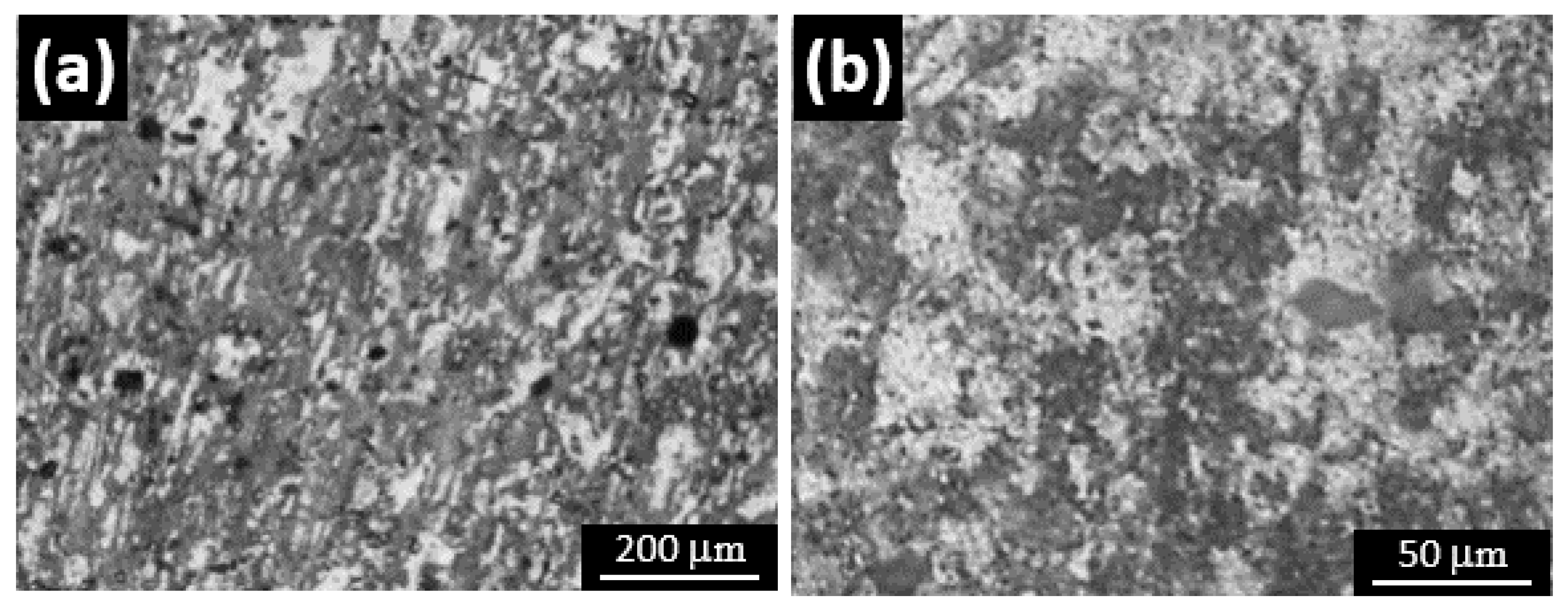
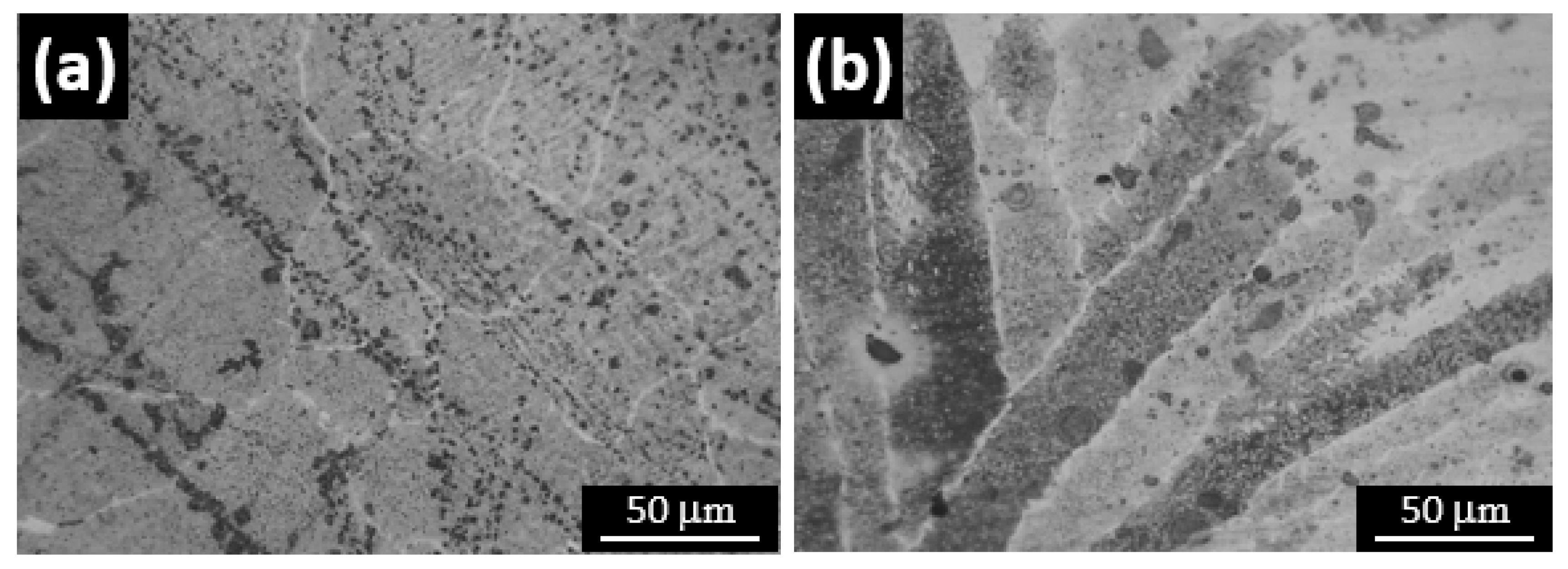
3.4. Microstructure Analysis
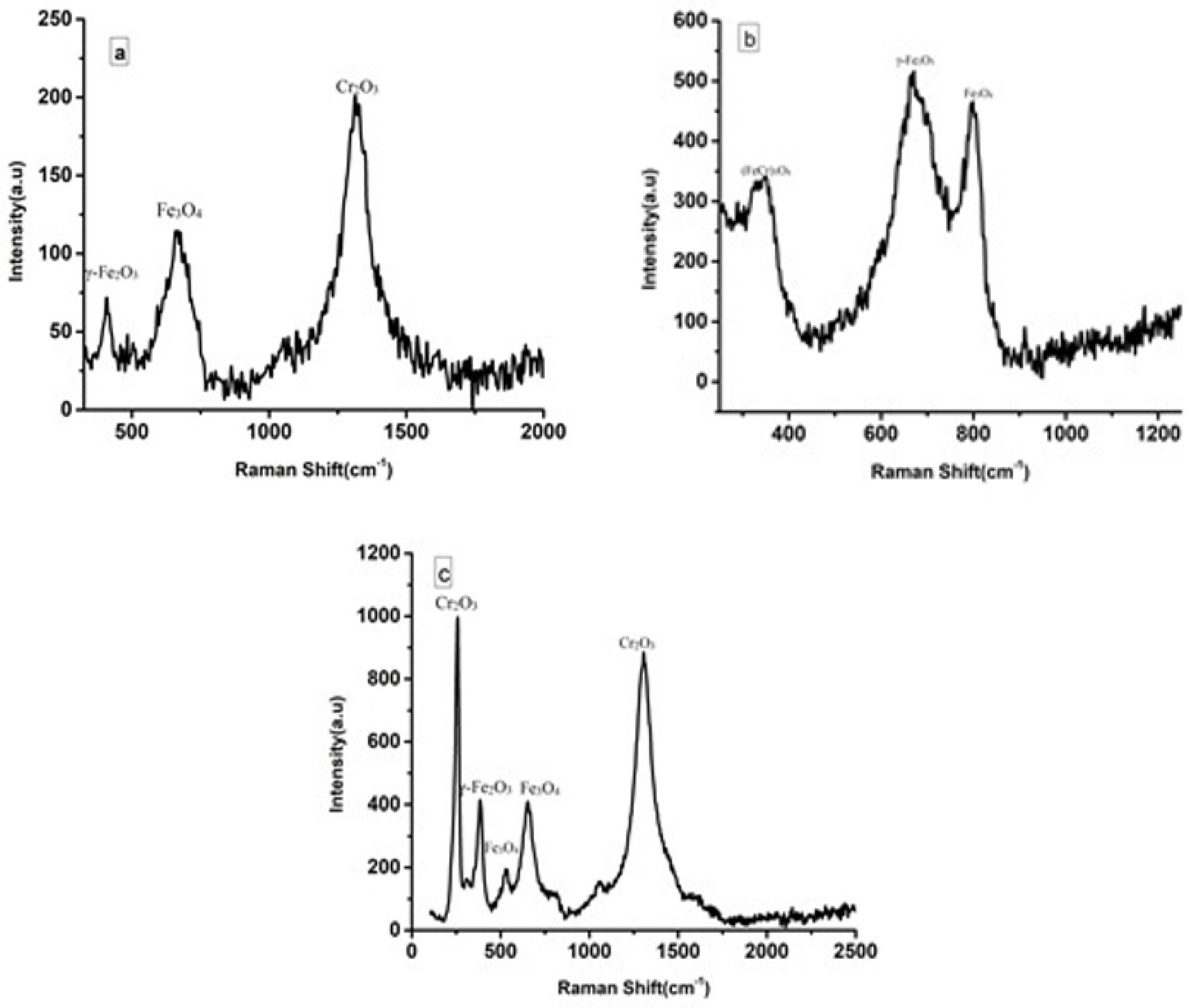
3.5. Raman Measurements
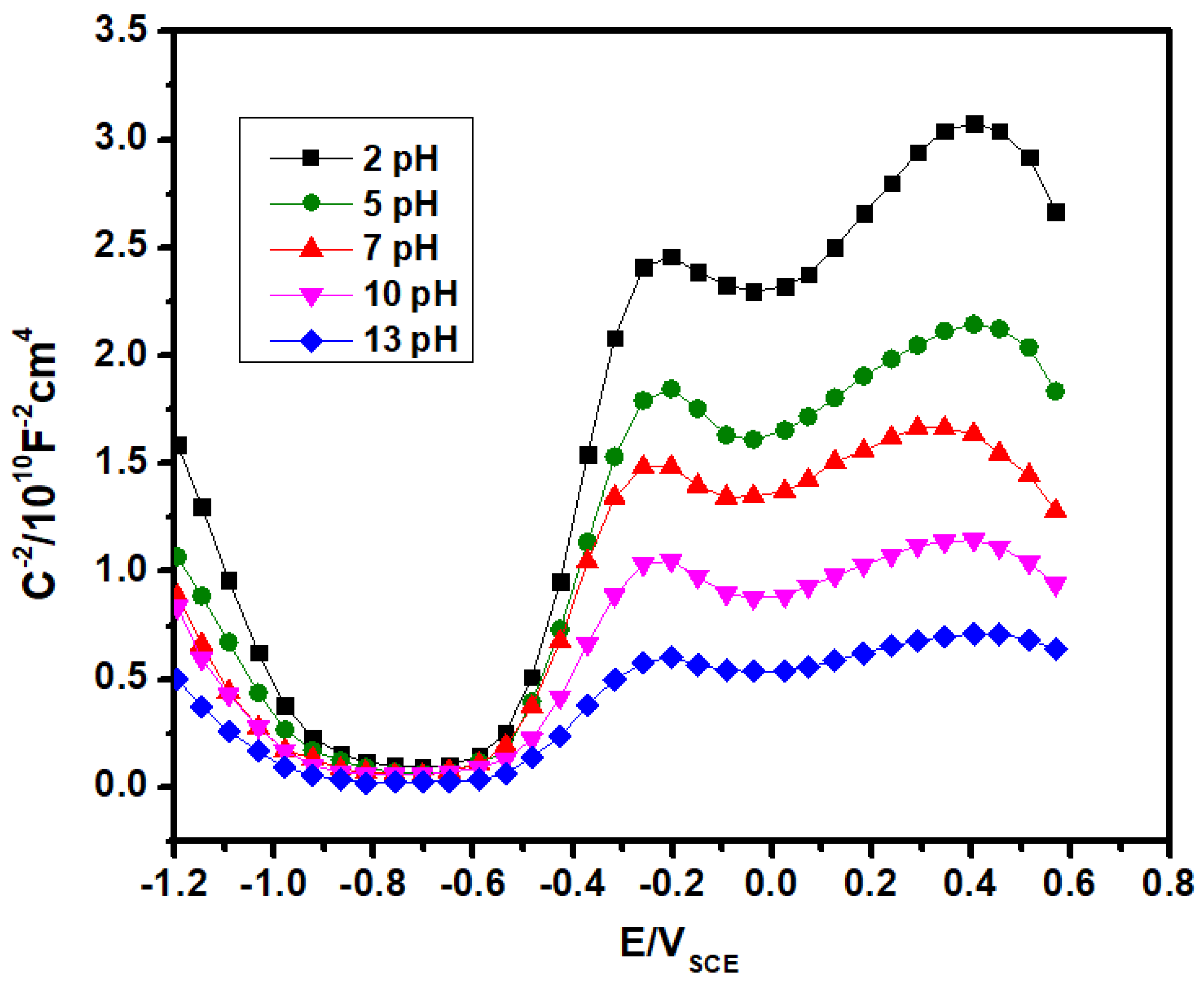
3.6. Mott–Schottky Analysis
4. Conclusions
- 2205 DSS exhibited high corrosion resistance with increasing solution pH. At pH 13 it shows highest icorr value compared to other samples.
- Overall impedance values are increased with the higher pH values, which improving the corrosion resistance, and same trend was confirmed by potentiodynamic polarization curves, too.
- FE-SEM micrographs reveals that the number and size of the pits due to pitting corrosion are increasing in the more acidic solution (low pH vales). It is also observed that the most of the pit found in the base metal compared to fusion zone. So as the variation of solution pH mainly create pitting corrosion, the fusion zone and HAZ were not much affected by corrosion attack compared to base metal.
- Raman measurement confirms the presence of austenite and ferrite phase on the passivation layer created on the surface of 2205 duplex stainless-steel.
- At different applied potential ranges, capacitance studies reveal that the passive films can simulate n-type and p-type semiconducting characteristics. In case of 2205 duplex stainless-steel, these passive films can be represented by two oxide layer structure. The inner layer is composed from chromium oxides and the outer layer is formed from iron oxides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shome, M. Effect of heat-input on austenite grain size in the heat-affected zone of HSLA-100 steel. Mater. Sci. Eng. A 2007, 446, 454–460. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Uzzaman, T. Ameliorative Corrosion Resistance and Microstructure Characterization of 2205 Duplex Stainless Steel by Regulating the Parameters of Pulsed Nd:YAG Laser Beam Welding. Metals 2021, 11, 1206. [Google Scholar] [CrossRef]
- Munoz, A.I.; Anton, J.G.; Guinon, J.L.; Herranz, V.P. Inhibition effect of chromate on the passivation and pitting corrosion of a duplex stainless steel in LiBr solutions using electrochemical techniques. Corros. Sci. 2007, 49, 3200–3225. [Google Scholar] [CrossRef]
- Chen, T.H.; Weng, K.L.; Yang, J.R. The effect of high-temperature exposure on the microstructural stability and toughness property in a 2205 duplex stainless steel. Mater. Sci. Eng. A 2002, 338, 259–270. [Google Scholar] [CrossRef]
- Dhooge, A.; Deleu, E. Low temperature fracture toughness of thick duplex and superduplex stainless steel weldments. Weld. World 1997, 39, 47–52. [Google Scholar]
- Kan, B.; Wu, W.; Yang, Z.; Zhang, X.; Li, J. Effects of hydrostatic pressure and pH on the corrosion behavior of 2205 duplex stainless steel. J. Electroanal. Chem. 2021, 886, 115134. [Google Scholar] [CrossRef]
- Baeslack, W.A., III; Lippold, J.C. Phase transformation behaviour in duplex stainless steel weldments. Met. Constr. 1988, 20, 26R–31R. [Google Scholar]
- Zeng, H.; Yang, Y.; Zeng, M.; Li, M. Effect of dissolved oxygen on electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater. J. Mater. Sci. Technol. 2021, 66, 177–185. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Karlsson, L.; Andersson, J.O. Secondary austenite formation and its relation to pitting corrosion in duplex stainless steel weld metal. Mater. Sci. Technol. 1995, 11, 276–283. [Google Scholar] [CrossRef]
- Hertzman, S.; Huhtala, T.; Karlsoon, L.; Nilsson, J.O.; Nilsson, M.; Pettersson, R.J.; Wilson, A. Microstructure–property relations of Mo- and Walloyed super duplex stainless weld metals. Mater. Sci. Technol. 1997, 13, 604–613. [Google Scholar] [CrossRef]
- Hsieh, R.I.; Liou, H.Y.; Pan, Y.T. Effects of cooling time and alloying elements on the microstructure of the Gleeble-simulated heat-affected zone of 22% Cr duplex stainless steels. J. Mater. Eng. Perform. 2001, 10, 526–536. [Google Scholar] [CrossRef]
- Liou, H.Y.; Hsieh, R.I.; Tsai, W.T. Microstructure and pitting corrosion in simulated heat-affected zones of duplex stainless steels. Mater. Chem. Phys. 2002, 74, 33–42. [Google Scholar] [CrossRef]
- Prakash, S. Development of advanced alloys with improved resistance to corrosion and stress corrosion cracking (SCC) in power plants. In Structural Alloys for Power Plants; Woodhead Publishing: Cambridge, UK, 2014; pp. 294–318. [Google Scholar]
- Łabanowski, J.; Prokop-Strzelczyńska, K.; Rogalski, G.; Fydrych, D. The effect of wet underwater welding on cold cracking susceptibility of duplex stainless steel. Adv. Mater. Sci. 2016, 16, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Świerczyńska, A.; Łabanowski, J.; Michalska, J.; Fydrych, D. Corrosion behavior of hydrogen charged super duplex stainless steel welded joints. Mater. Corros. 2017, 68, 1037–1045. [Google Scholar] [CrossRef]
- Qi, K.; Li, R.; Wang, G.; Li, G.; Liu, B.; Wu, M. Microstructure and Corrosion Properties of Laser-Welded SAF 2507 Super Duplex Stainless Steel Joints. J. Mater. Eng. Perform. 2019, 28, 287–295. [Google Scholar] [CrossRef]
- Calliari, I.; Gennari, C.; Hurtado Delgado, E.; Miranda Perez, A.F.; Rodriguez Vargas, B.R. Laser welding of plastically deformed lean duplex stainless steel. Metall. Ital. 2018, 1, 5–10. [Google Scholar]
- Hu, S.; Zheng, D.; Zhao, G.; Li, G.; Tang, H. The effect of welded joint properties on the surface characteristics of laser-welded 2205 duplex stainless steel. Adv. Mech. Eng. 2018, 10, 1687814018797449. [Google Scholar] [CrossRef] [Green Version]
- Tuz, L. Evaluation of Microstructure and Selected Mechanical Properties of Laser Beam Welded S690QL High-Strength Steel. Adv. Mater. Sci. 2018, 18, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Lisiecki, A.; Kurc-Lisiecka, A. Automated Laser Welding of AISI 304 Stainless Steel by Disk Laser. Arch. Metall. Mater. 2018, 4, 1663–1672. [Google Scholar]
- Abdo, H.S.; Sarkar, A.; Gupta, M.; Sahoo, S.; Mohammed, J.A.; Ragab, S.A.; Seikh, A.H. Low-Cost High-Performance SnO2–Cu Electrodes for Use in Direct Ethanol Fuel Cells. Crystals 2021, 11, 55. [Google Scholar] [CrossRef]
- Tęczar, P.; Majkowska-Marzec, B.; Bartmański, M. The influence of laser alloying of Ti13Nb13Zr on surface topography and properties. Adv. Mater. Sci. 2019, 19, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Janicki, D.; Górka, J.; Kwaśny, W.; Gołombek, K.; Kondracki, M.; Żuk, M. Diode laser surface alloying of armor steel with tungsten carbide. Arch. Metall. Mater. 2017, 62, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Kik, T.; Górka, J. Numerical Simulations of Laser and Hybrid S700MC T-Joint Welding. Materials 2019, 12, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintino, L.; Costa, A.; Miranda, R.; Yapp, D.; Kumar, V.; Kong, C.J. Welding with high power fiber lasers–A preliminary study. Mater. Des. 2007, 28, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Abdo, H.S.; Seikh, A.H.; Mandal, B.B.; Mohammed, J.A.; Ragab, S.A.; Abdo, M.S. Microstructural Characterization and Corrosion-Resistance Behavior of Dual-Phase Steels Compared to Conventional Rebar. Crystals 2020, 10, 1068. [Google Scholar] [CrossRef]
- Blanco, G.; Bautista, A.; Takenouti, H. Influence ofthe forming process ofcorrugated stainless steels on their corrosion behaviourin simulated pore solutions. Cem. Concr. Compos. 2006, 28, 212. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Luqman, M.; Ragab, S.A.; Almotairy, S.M. Influence of Chloride Ions on Electrochemical Corrosion Behavior of Dual-Phase Steel over Conventional Rebar in Pore Solution. Appl. Sci. 2020, 10, 4568. [Google Scholar] [CrossRef]
- Freire, L.; Carmezima, M.J.; Ferreira, M.G.S. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2011, 56, 5280. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Fouly, A.; Hashmi, F.H. Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique. Processes 2021, 9, 1469. [Google Scholar] [CrossRef]
- Malik, A.U.; Kutty, P.C.M.; Siddiqi, N.A.; Andijani, N.; Ahmed, S. The influence of pH and chloride concentration on the corrosion behaviour of AISI 316L steel in aqueous solutions. Corros. Sci. 1992, 33, 1809. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Li, X.G.; Xiao, K. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride. Electrochim. Acta 2012, 64, 211–220. [Google Scholar] [CrossRef]
- Li, L.F.; Jiang, Z.H.; Riquier, Y. High-temperature oxidation of duplex stainless steels in air mixed gas of air and CH4. Corros. Sci. 2005, 47, 57–68. [Google Scholar] [CrossRef]
- Thiberau, R.J.; Brown, C.W.; Heidersbach, R.H. Raman spectra of possible corrosion products on iron at 100 to 150 °C in air. Appl. Spectrosc. 1978, 32, 532–535. [Google Scholar] [CrossRef]
- Thierry, D.; Persson, D.; Leygraf, C.; Boucherit, N.; Hugot-Le Goff, A. Raman spectroscopy and XPS investigations of anodic corrosion films formed on Fe-mo alloys in alcaline solutions. Corros. Sci. 1991, 32, 273–284. [Google Scholar] [CrossRef]
- Thierry, D.; Persson, D.; Leygraf, C.; Delichere, D.; Joiret, S.; Pallota, C. In-situ Raman spectroscopy combined with X-ray photoelectron spectroscopy and nuclear microanalyisis for studies of anodic corrosion film formation on Fe-Cr single crystals. J. Electrochem. Soc. 1988, 135, 305–310. [Google Scholar] [CrossRef]
- Verble, J.R. Temperature-dependent light scattering studies of the Verwley transition and electronic disorder in magnetite. Phys. Rev. B 1974, 9, 5236–5248. [Google Scholar] [CrossRef]
- da Cunha Belo, M.; Walls, M.; Hakiki, N.E.; Corset, J.; Picquenard, E.; Sagon, G. Composition structure and properties of the oxide films formed on the stainless steels 316 L in a primary type PWR environment. Corros. Sci. 1998, 40, 447–463. [Google Scholar] [CrossRef]
- Beattie, I.R.; Gilson, T.R. The single-crystal Raman spectra of nearly opaque materials. J. Chem. Soc. A 1970, 980–986. [Google Scholar] [CrossRef]
- Fabis, P.; Heindersbach, R.; Brown, C.; Rockett, T. Oxide scale formation on iron chromium alloys in elevated temperature air environments. Corrosion 1981, 37, 700–711. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Azevedo, C.; Bezerra, P.S.A.; Esteves, F.; Joia, C.J.B.M.; Mattos, O.R. Hydrogen permeation studied by electrochemical techniques. Electrochim. Acta 1999, 44, 4431–4442. [Google Scholar] [CrossRef]
- Gomes, W.P.; Vanmackelbergh, D. Influence of formation conditions on impedance properties of nickel passive layers formed in 1 M KOH. Electrochim. Acta 1996, 41, 967. [Google Scholar] [CrossRef]
- Dean, M.H.; Stimming, U. The electronic properties of disordered passive films. Corros. Sci. 1989, 29, 199. [Google Scholar] [CrossRef]
- Simoes, A.M.P.; Ferreira, M.G.S.; Rondot, B.; da Cunha Belo, M. Study of Passive Formed on AISI 304 Stainless Steel by Impedance Measurements and Photoelectrochemistry. J. Electrochem. Soc. 1990, 137, 82. [Google Scholar] [CrossRef]
- Yang, M.Z.; Luo, J.L.; Patchet, B.M. Correlation of Hydrogen-Facilitated Pitting of AISI 304 Stainless Steel to Semiconductivity of Passive Films. Thin Solid Film. 1999, 354, 142. [Google Scholar] [CrossRef]

| C | Mn | Si | S | P | B | Al |
|---|---|---|---|---|---|---|
| 0.03 | 1.34 | 0.39 | <0.003 | 0.03 | 0.0035 | 0.0039 |
| Cr | Mo | Cu | Nb | Ni | Ti | Co |
| 22.5 | 3.03 | 0.24 | 0.016 | 5.6 | <0.005 | 0.128 |
| Laser Power | Welding Speed | Heat Input | Argon Flow Rate |
|---|---|---|---|
| 1500 W | 30 mm/s | 65 J/mm | 15 L/min |
| Specimen | Solution | pH |
|---|---|---|
| 1 | 3.5% NaCl + 5%HCl | 2 |
| 2 | 3.5% NaCl + 5%HCl | 5 |
| 3 | 3.5% NaCl | 7 |
| 4 | 3.5% NaCl + 0.1 M NaOH + 0.1 M KOH | 10 |
| 5 | 3.5% NaCl + 0.1 M NaOH + 0.1 M KOH | 13 |
| Solution | Ecorr (V vs. SCE) | icorr (A/cm2) |
|---|---|---|
| pH 13 | 0.3755 | 8 × 10−09 |
| pH 10 | 0.3518 | 3 × 10−08 |
| pH 7 | 0.1831 | 1 × 10−07 |
| pH 5 | 0.2339 | 6 × 10−07 |
| pH 2 | 0.3157 | 4 × 10−06 |
| Solution | Rs (ohm-cm2) | CPE1 (ohm−1 cm−2 s−n) | R1 (ohm-cm2) | CPE2 (ohm−1 cm−2 s−n) | R2 (kohm-cm2) |
|---|---|---|---|---|---|
| pH 2 | 9.67 | 71.71 | 11.04 | 278.70 | 109.7 |
| pH 5 | 7.94 | 62.81 | 13.12 | 4.43 | 155.90 |
| pH 7 | 6.97 | 66.58 | 22.86 | 374.6 | 211.50 |
| pH 10 | 5.82 | 78.89 | 33.38 | 239.1 | 267.48 |
| pH 13 | 7.35 | 60.21 | 56.78 | 1103.3 | 310.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, H.S.; Seikh, A.H.; Abdus Samad, U.; Fouly, A.; Mohammed, J.A. Electrochemical Corrosion Behavior of Laser Welded 2205 Duplex Stainless-Steel in Artificial Seawater Environment under Different Acidity and Alkalinity Conditions. Crystals 2021, 11, 1025. https://doi.org/10.3390/cryst11091025
Abdo HS, Seikh AH, Abdus Samad U, Fouly A, Mohammed JA. Electrochemical Corrosion Behavior of Laser Welded 2205 Duplex Stainless-Steel in Artificial Seawater Environment under Different Acidity and Alkalinity Conditions. Crystals. 2021; 11(9):1025. https://doi.org/10.3390/cryst11091025
Chicago/Turabian StyleAbdo, Hany S., Asiful H. Seikh, Ubair Abdus Samad, Ahmed Fouly, and Jabair Ali Mohammed. 2021. "Electrochemical Corrosion Behavior of Laser Welded 2205 Duplex Stainless-Steel in Artificial Seawater Environment under Different Acidity and Alkalinity Conditions" Crystals 11, no. 9: 1025. https://doi.org/10.3390/cryst11091025
APA StyleAbdo, H. S., Seikh, A. H., Abdus Samad, U., Fouly, A., & Mohammed, J. A. (2021). Electrochemical Corrosion Behavior of Laser Welded 2205 Duplex Stainless-Steel in Artificial Seawater Environment under Different Acidity and Alkalinity Conditions. Crystals, 11(9), 1025. https://doi.org/10.3390/cryst11091025








