Molecular and Supramolecular Structures of Cd(II) Complexes with Hydralazine-Based Ligands; A New Example for Cyclization of Hydrazonophthalazine to Triazolophthalazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis
2.2.1. Synthesis of HL and HLOH
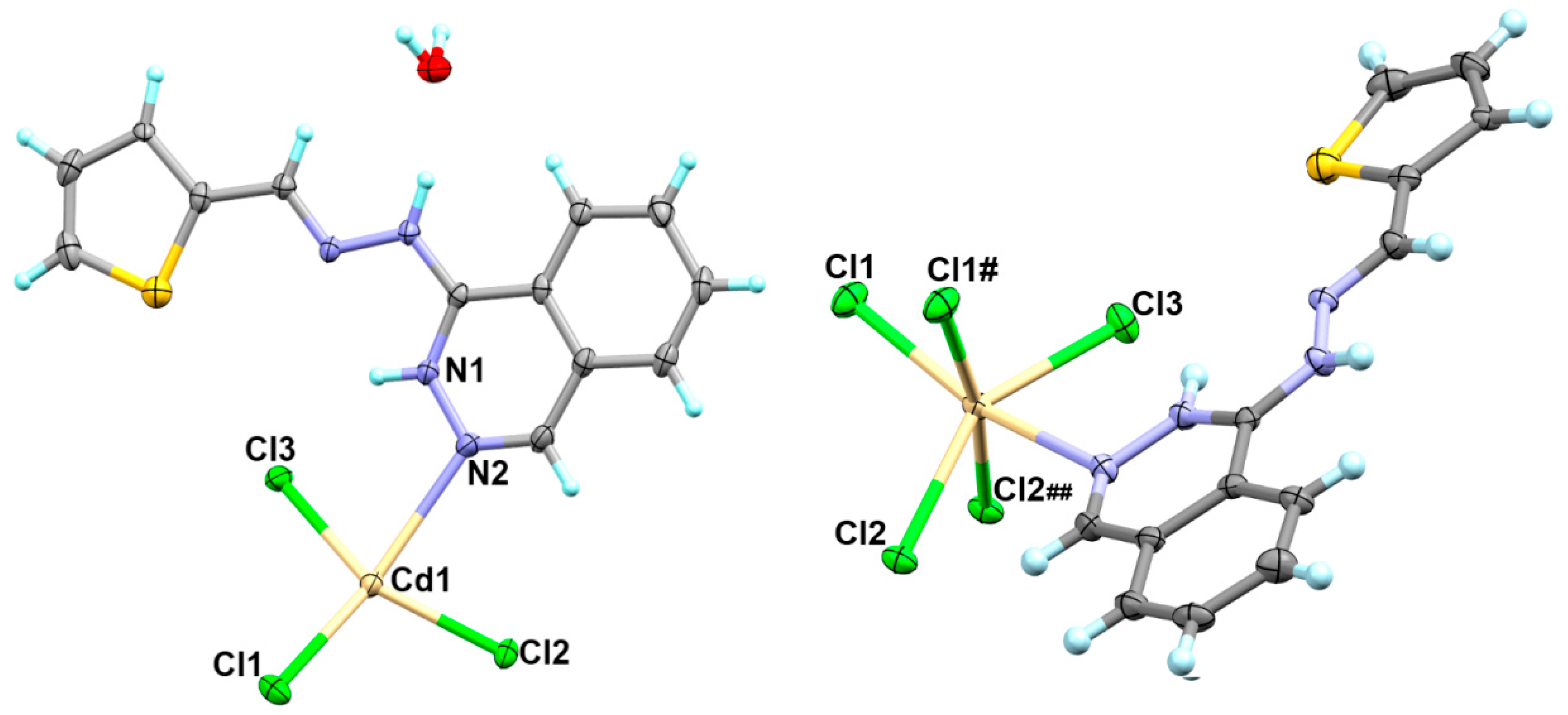
2.2.2. Synthesis of [Cd(H2L)Cl3]n*H2O; 1
2.2.3. Synthesis of [Cd(TPT)(SCN)2]n*H2O; 2
2.2.4. Synthesis of [Cd3(Hydralazine)2(H2O)2Cl6]; 3
2.3. Methods and Calculations
2.4. Crystal Structure Determination
3. Results
4. Discussion
4.1. X-ray Crystal Structure Description
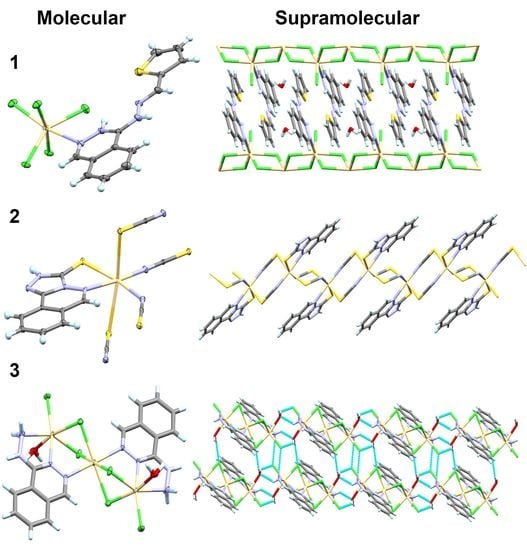
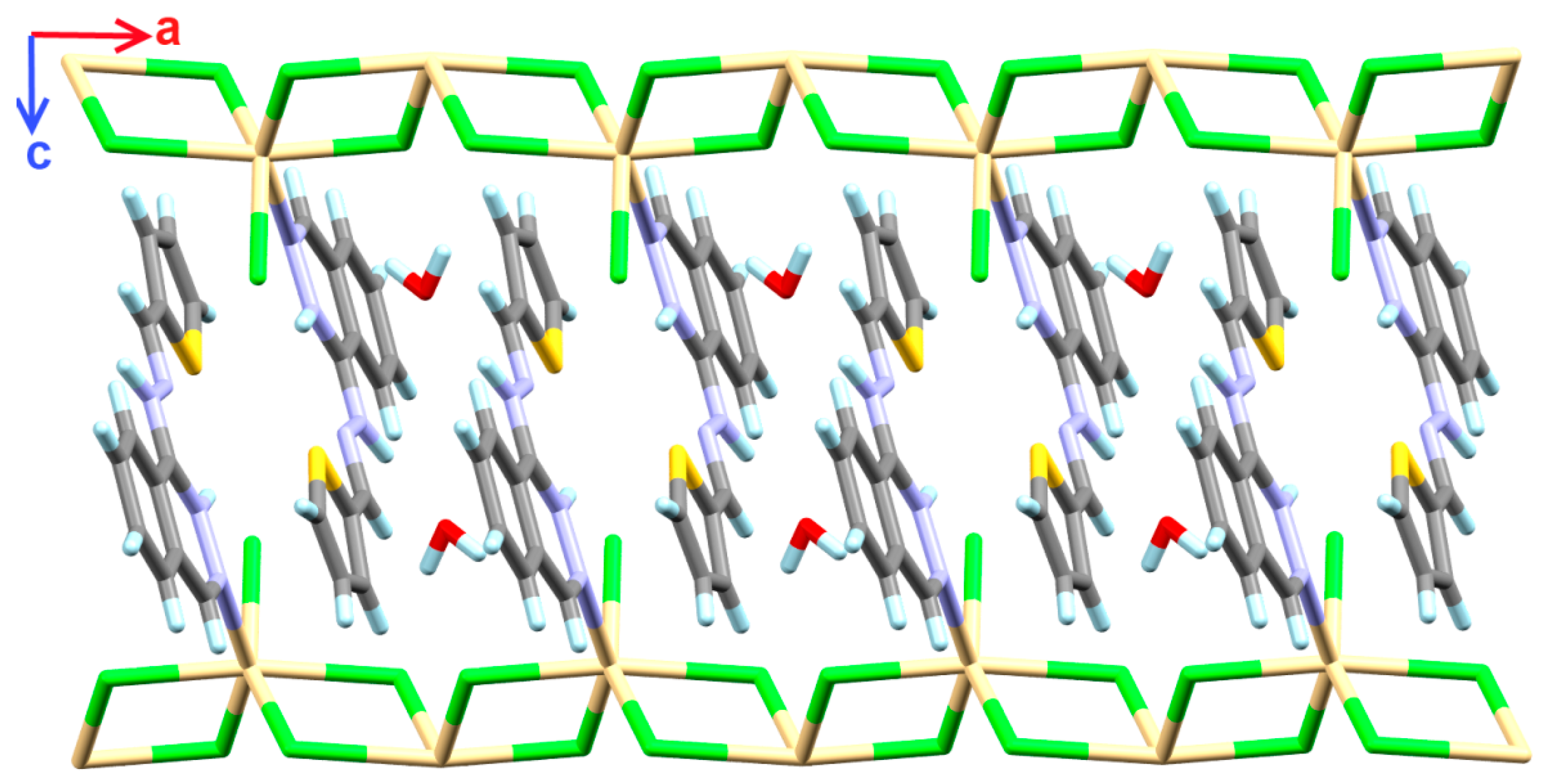
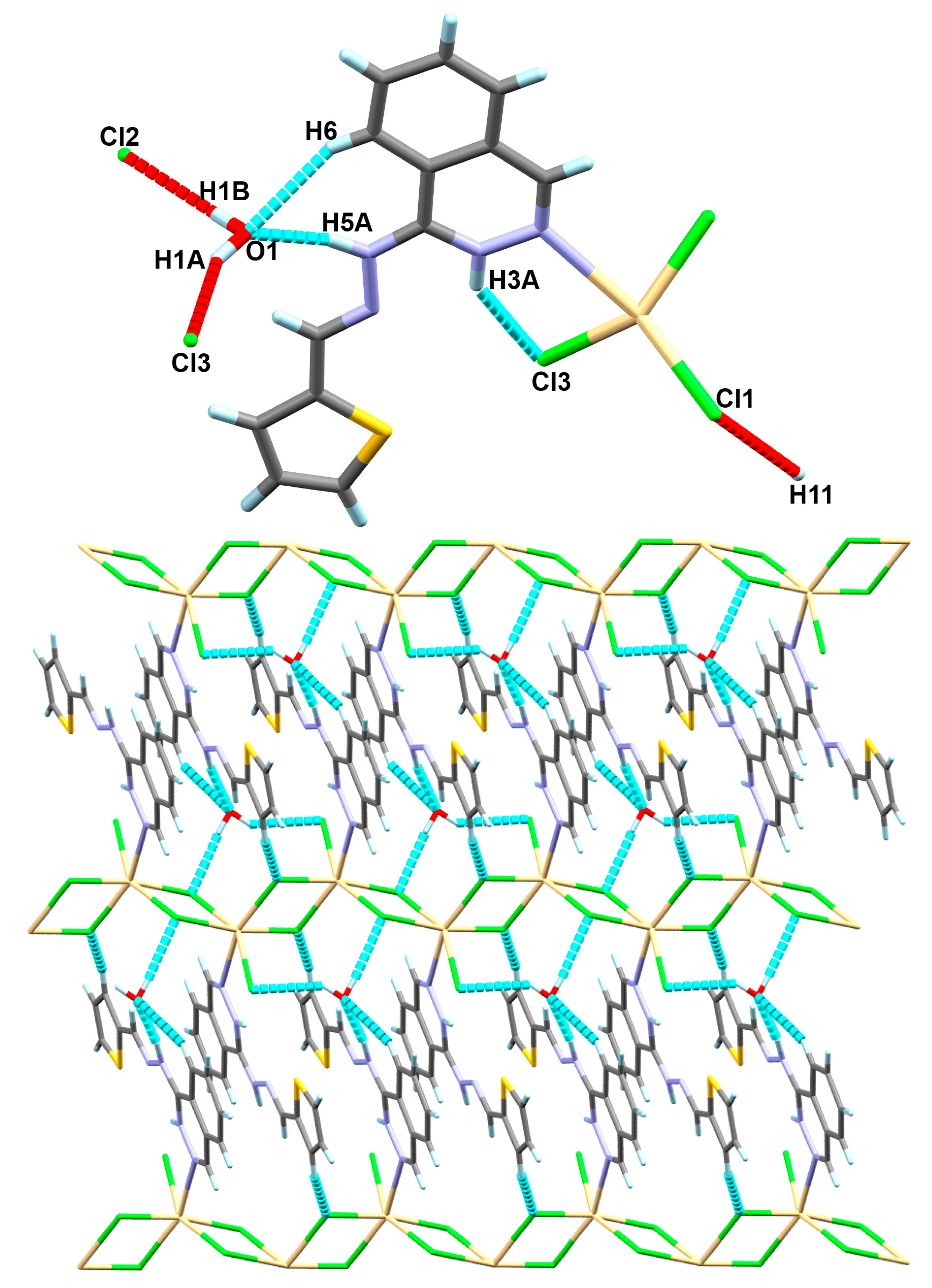
4.1.1. [Cd(H2L)Cl3]n*H2O Complex; (1)
4.1.2. [Cd(TPT)(SCN)2]n*H2O Complex; (2)
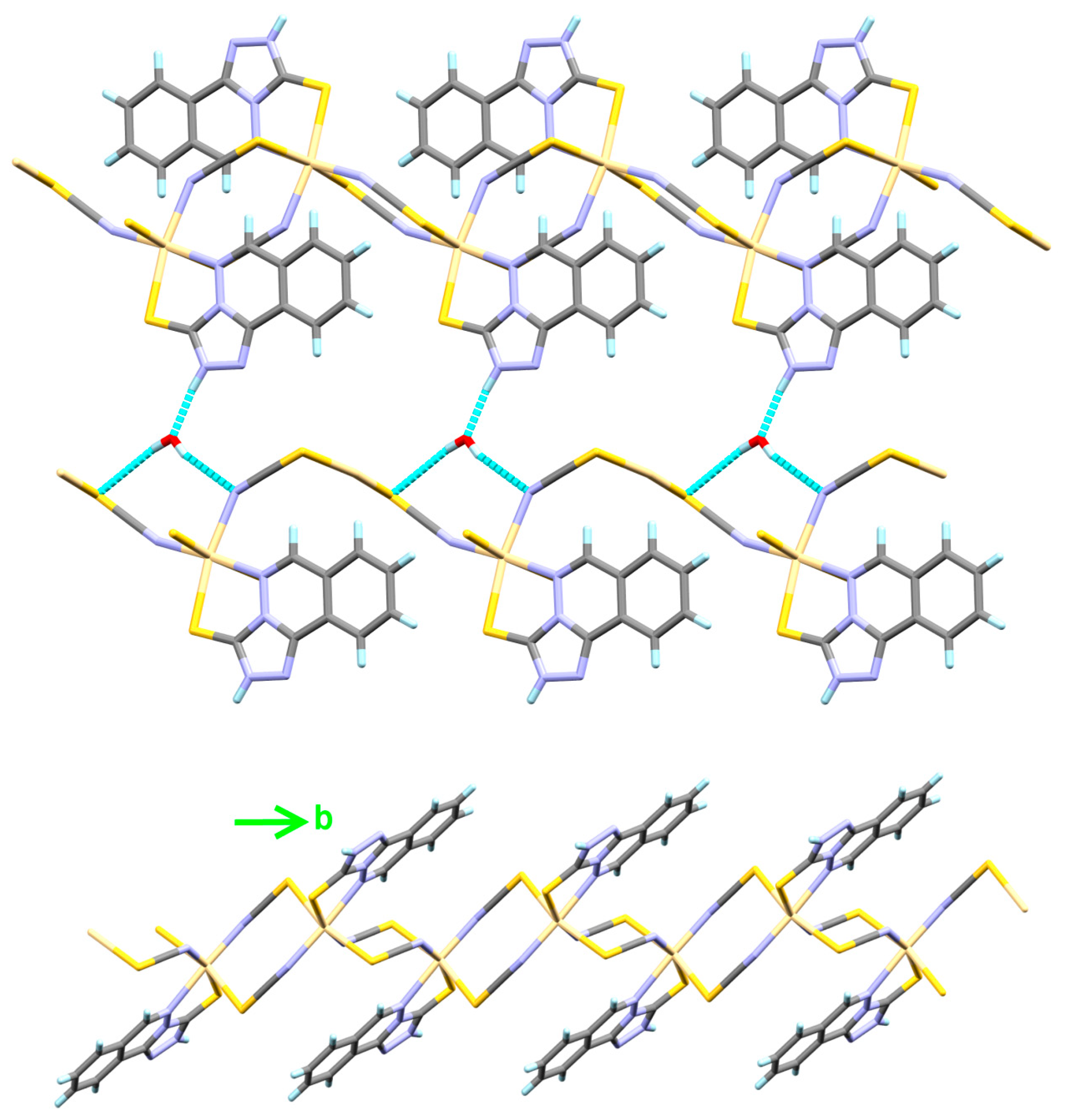
4.1.3. [Cd3(Hydralazine)2(H2O)2Cl6] Complex; (3)
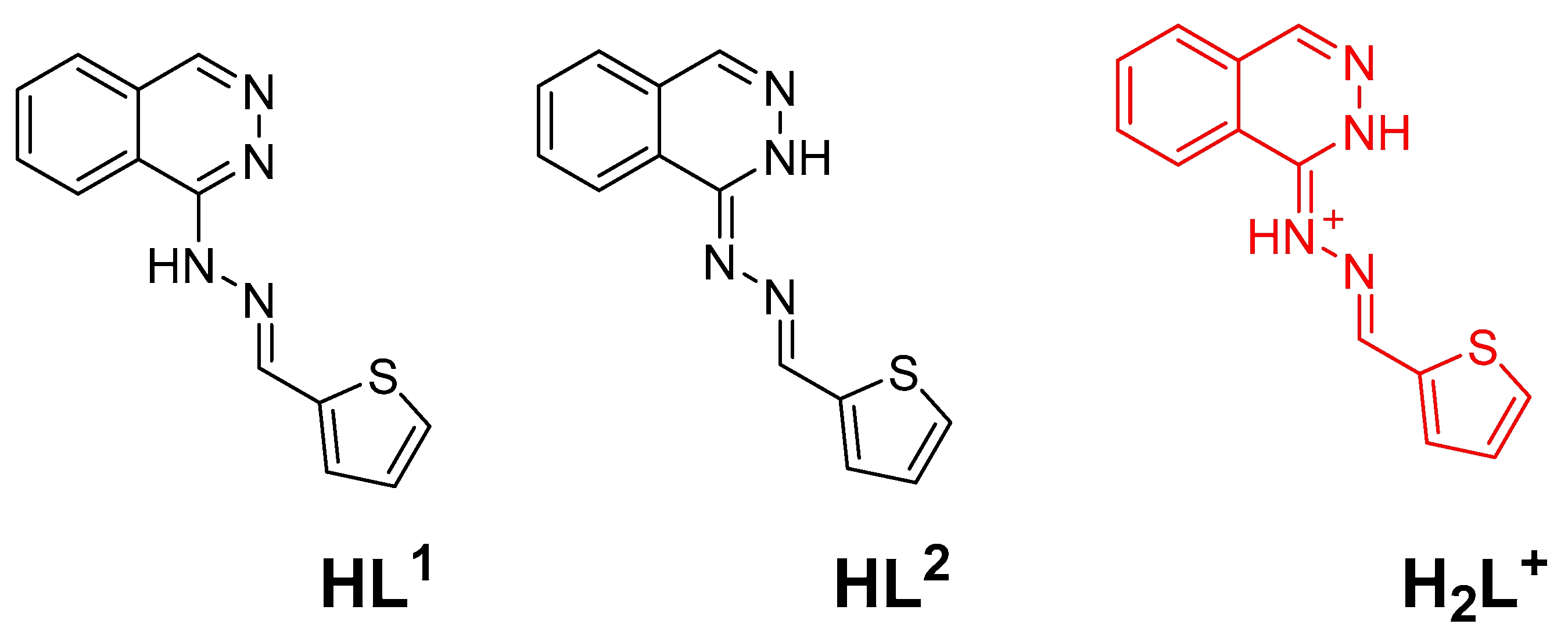
4.2. Tautomerism and Proton Affinity of HL
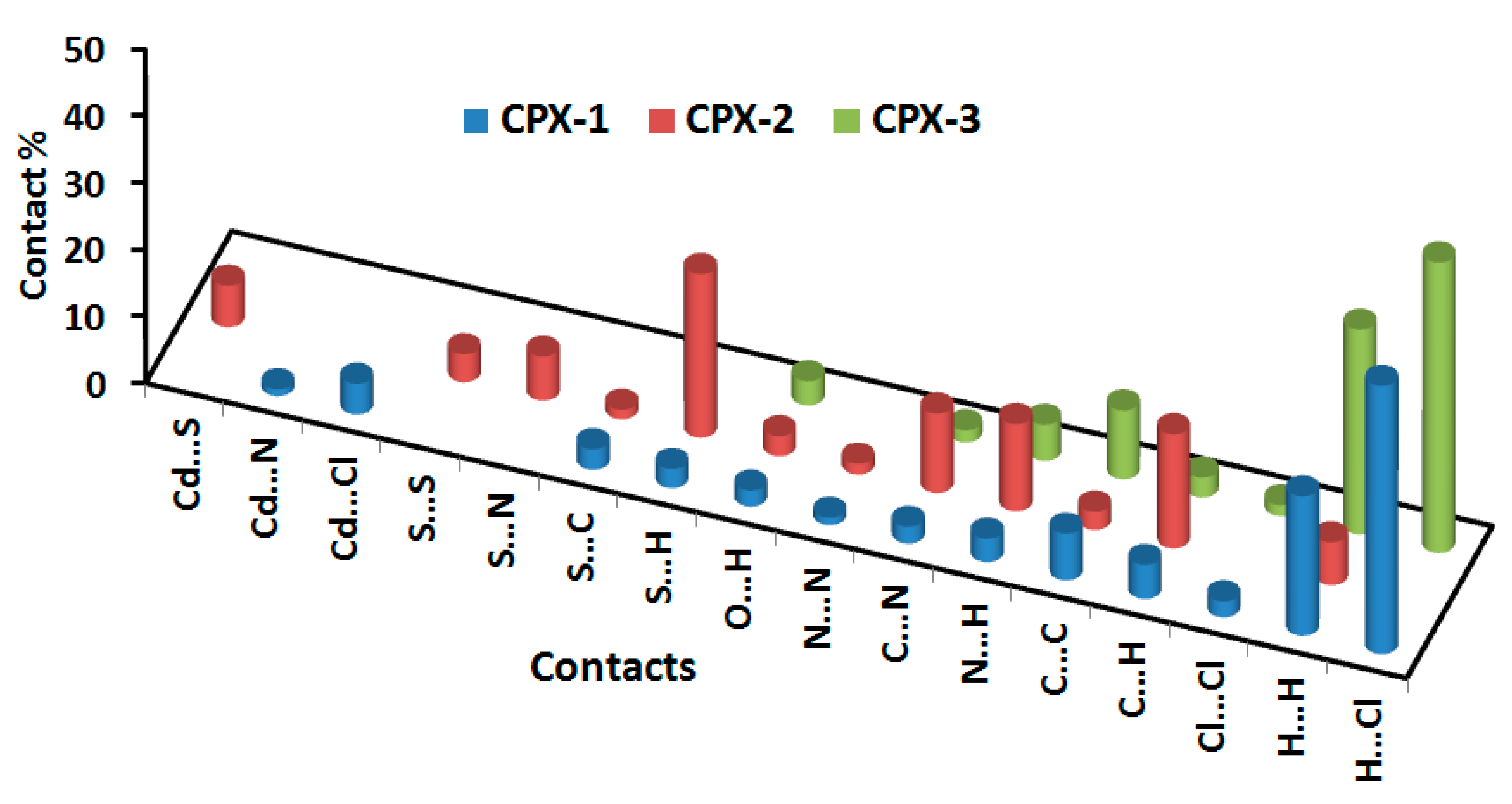
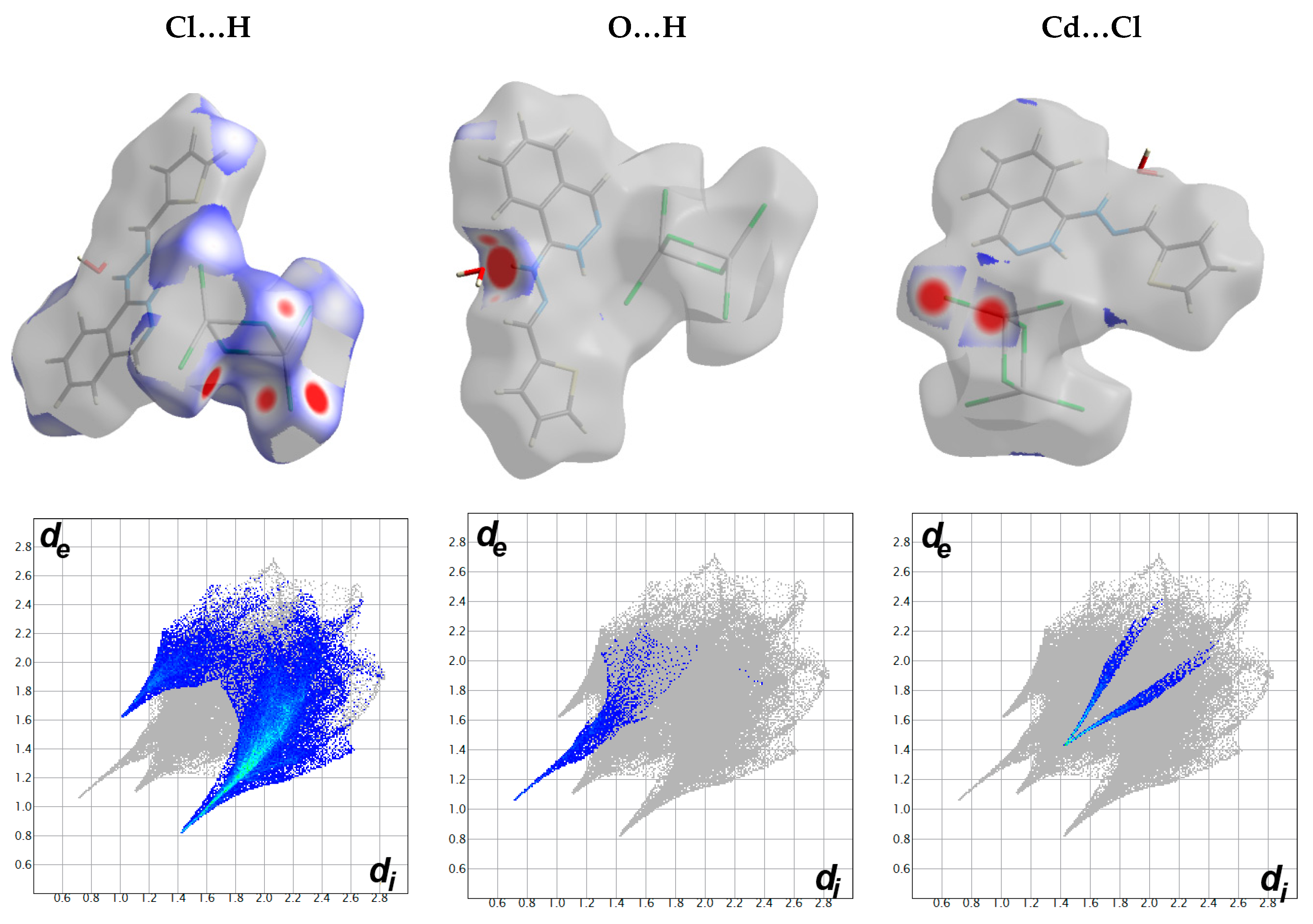
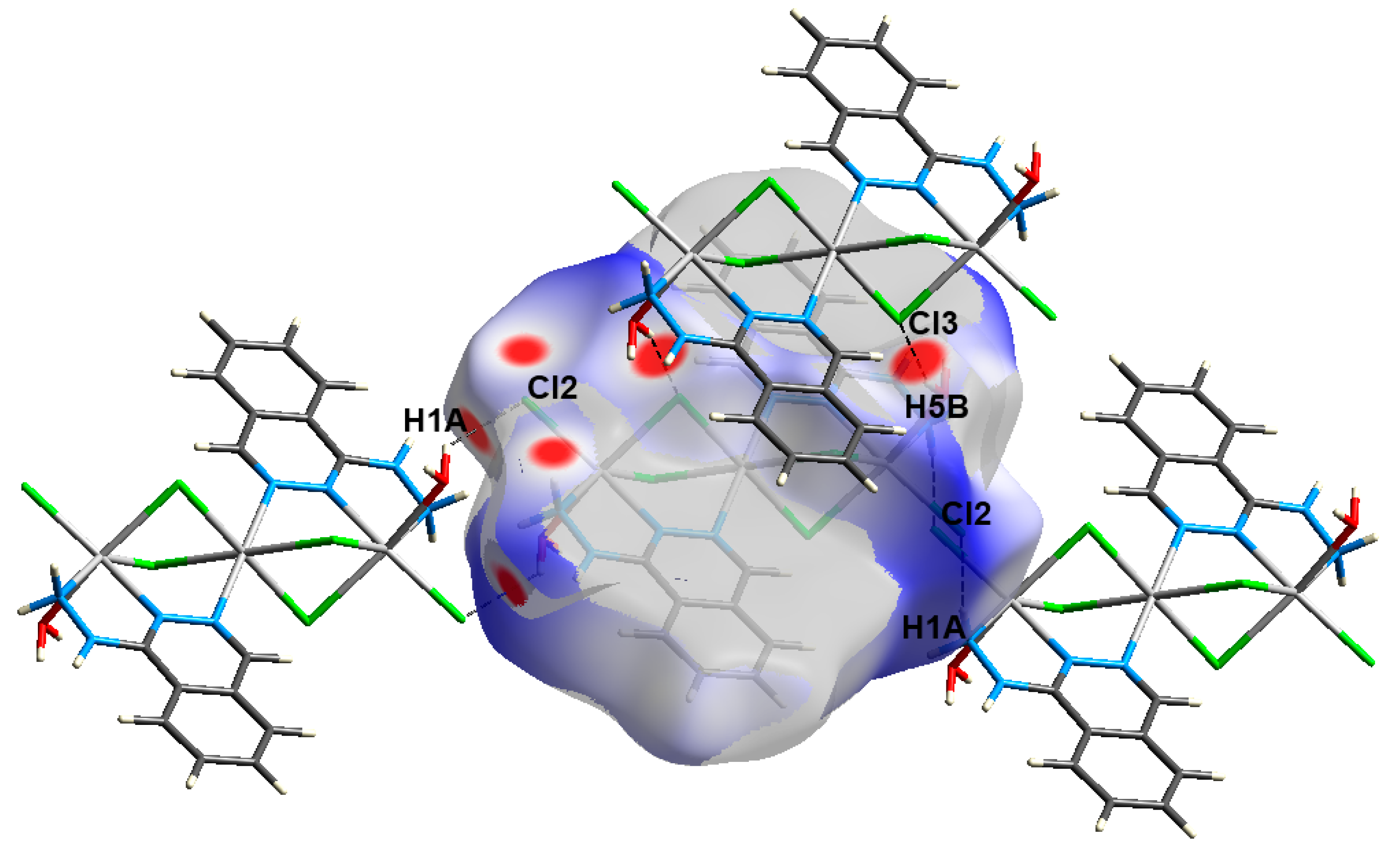
4.3. Analysis of Molecular Packing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Gong, G.; Wei, C.; Jin, X.; Quan, Z. Synthesis and anti-inflammatory activity evaluation of a novel series of 6-phenoxy-[1,2,4]triazolo[3,4-a]phthalazine-3-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 1576–1579. [Google Scholar] [CrossRef]
- El-Shamy, I.E.; Abdel-Mohsen, A.M.; Alsheikh, A.A.; Fouda, M.M.G.; Al-Deyab, S.S.; El-Hashash, M.A.; Jancar, J. Synthesis, biological, anti-inflammatory activities and quantum chemical calculation of some [4-(2,4,6-trimethylphenyl)-1(2H)-oxo-phthalazin-2yl] acetic acid hydrazide derivatives. Dye. Pigment. 2015, 113, 357–371. [Google Scholar] [CrossRef]
- Behalo, M.S. An efficient one-pot catalyzed synthesis of 2,5-disubstituted-1,3,4-oxadiazoles and evaluation of their antimicrobial activities. RSC Adv. 2016, 6, 103132–103136. [Google Scholar] [CrossRef]
- Hollo, B.; Magyari, J.; Radovanovic, V. Ž.; Vučkovic, G.; Tomic, Z.D.; Szilágyi, I.M.; Pokol, G.; Szécsény, K.M. Synthesis, characterisation and antimicrobial activity of bis(phthalazine-1-hydrazone)-2,6-diacetylpyridine and its complexes with CoIII, NiII, CuII and ZnII. Polyhedron 2014, 80, 142–150. [Google Scholar] [CrossRef]
- Issac, Y.A.; El-Karim, I.G.; Donia, S.G.; Behalo, M.S. Novel synthesis of phthalazine derivatives as antimicrobial agents. Sulfur Lett. 2002, 25, 183–190. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Xue, D.Q.; He, P.; Shao, K.P.; Chen, P.J.; Gu, Y.F.; Ren, J.L.; Shan, L.H.; Liu, H.M. Synthesis and antimicrobial activities of novel 1,2,4-triazolo [3,4-a] phthalazine derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Glišić, B.Đ.; Senerovic, L.; Comba, P.; Wadepohl, H.; Veselinovic, A.; Milivojevic, D.R.; Djuran, M.I.; Runic, J.N. Silver(I) complexes with phthalazine and quinazoline as effective agents against pathogenic Pseudomonas aeruginosa strains. J. Inorg. Biochem. 2016, 155, 115–128. [Google Scholar] [CrossRef]
- El-hashash, M.A.; Soliman, A.Y.; EL-Sham, I.E. Synthesis and antimicrobial evaluation of some annelated phthalazine derivatives and acyclo C-nucleosides from 1-chloro-4-(2,4,6-trimethylphenyl) phthalazine precursor. Turk. J. Chem. 2012, 36, 347–366. [Google Scholar]
- Sagar, P.; Kumar, V.; Suresh, L.; Chandramouli, G.V.P. Ionic liquid catalysed multicomponent synthesis, antifungal activity, docking studies and in silico ADMET properties of novel fused Chromeno-Pyrazolo-Phthalazine derivatives. J. Saudi Chem. Soc. 2017, 21, 306–314. [Google Scholar] [CrossRef]
- Savinia, L.; Chiasserinia, L.; Travaglia, V.; Pelleranoa, C.; Novellinob, E.; Cosentinoc, S.; Pisano, M.B. New α-(N)-heterocyclichydrazones: Evaluation of anticancer, anti-HIV and antimicrobial activity. Eur. J. Med. Chem. 2004, 39, 113–122. [Google Scholar] [CrossRef]
- El-Helby, A.A.; Ayyad, R.R.; Sakr, H.M.; Abdelrahim, A.S.; El-Adl, K.; Sherbiny, F.S.; Eissa, I.H.; Khalifa, M.M. Design, synthesis, molecular modeling and biological evaluation of novel 2,3-dihydrophthalazine-1,4-dione derivatives as potential anticonvulsant agents. J. Mol. Struct. 2017, 1130, 333–351. [Google Scholar] [CrossRef]
- Wasfy, A.F.; Behalo, M.S.; Aly, A.A.; Sobhi, N.M. An efficient synthesis of some new 1, 4-disubstituted phthalazine derivatives and their anticancer activity. Der PharmaChemica 2013, 5, 82–96. [Google Scholar]
- Amin, K.M.; Barsoum, F.F.; Awadallah, F.M.; Mohamed, N.E. Identification of new potent phthalazine derivatives with VEGFR-2 and EGFR kinase inhibitory activity. Eur. J. Med. Chem. 2016, 123, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Abou-Seri, S.M.; El Kerdawy, A.M.; Ayyad, R.R.; Hamdy, A.M.; Ghabbour, H.A.; Ali, M.M.; Abou El Ella, D.A. Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl)phthalazine derivatives. Eur. J. Med. Chem. 2016, 113, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Albering, J.H.; Farooq, M.; Wadaan, M.A.M.; El-Faham, A. Synthesis, structural and biological studies of two new Co (III) complexes with tridentate hydrazone ligand derived from the antihypertensive drug hydralazine. Inorg. Chim. Acta 2017, 466, 16–29. [Google Scholar] [CrossRef]
- Sousa, C.; Freire, C.; de Castro, B. Synthesis and Characterization of Benzo-15-Crown-5 Ethers with Appended N2O Schiff Bases. Molecules 2003, 8, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Shahsavani, M.B.; Ahmadi, S.; Aseman, M.D.; Nabavizadeh, S.M.; Rashidi, M.; Asadi, Z.; Erfani, N.; Ghasemi, A.; Saboury, A.A.; Niazi, A.; et al. Anticancer activity assessment of two novel binuclear platinum (II) complexes. J. Photochem. Photobiol. B Biol. 2016, 161, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Tomek, P.; Palmer, B.D.; Flanagan, J.U.; Sun, C.; Raven, E.L.; Ching, L. Discovery and evaluation of inhibitors to the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1 (IDO1): Probing the active site-inhibitor interactions. Eur. J. Med. Chem. 2017, 126, 983–996. [Google Scholar] [CrossRef]
- Xue, D.Q.; Zhang, X.Y.; Wang, C.J.; Ma, L.Y.; Shao, K.P.; Chen, P.J.; Gu, Y.F.; Zhang, X.S.; Wang, C.F.; Ji, C.H.; et al. Synthesis and anticancer activities of novel 1,2,4-triazolo[3,4-a]phthalazine derivatives. Eur. J. Med. Chem. 2014, 85, 235–244. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Eldehna, W.M.; Ali, M.M.; Abou El Ella, D.A. 1-Piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: Synthesis and in vitro biological evaluation. Eur. J. Med. Chem. 2016, 107, 165–179. [Google Scholar] [CrossRef]
- Subramanian, G.; Babu Rajeev, C.P.; Mohan, C.D.; Sinha, A.; Chu, T.T.; Anusha, S.; Ximei, H.; Fuchs, J.E.; Bender, A.; Rangappa, K.S.; et al. Synthesis and in vitro evaluation of hydrazinyl phthalazines against malaria parasite, Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2016, 15, 3300–3306. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Palenik, G.J. Binuclear Complexes. Synthesis and Characterization of the Binuclear Ligand 1,4-Dihydrazinophthalazine Bis(2’-pyridinecarboxaldimine) and the Nickel Complex p-Chloro-tetraaqua[1,4-dihydrazinophthalazine bis( 2’-pyridinecarboxaldimine)]dinickel(II) Chloride Dihydrate. Inorg. Chem. 1977, 16, 1127–1133. [Google Scholar]
- Lee, J.J.; Choi, Y.W.; You, G.R.; Lee, S.Y.; Kim, C. A phthalazine-based two-in-one chromogenic receptor for detecting Co2+ and Cu2+ in an aqueous environment. Dalton Trans. 2015, 44, 13305–13314. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Tárraga, A.; Otón, F. Imidazole derivatives: A comprehensive survey of their recognition properties. Org. Biomol. Chem. 2012, 10, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Mitsumori, K.; Niho, N.; Satoh, S.; Hiratsuka, H.; Satoh, M.; Sumiyoshi, M.; Nishijima, M.; Katsuki, Y.; Suzuki, J.; et al. Assessment of renal toxicity by analysis of regeneration of tubular epithelium in rats given low-dose cadmium chloride or cadmium-polluted rice for 22 months. Arch. Toxicol. 2000, 74, 571–577. [Google Scholar] [CrossRef]
- Chen, L.; Lei, L.; Jin, T.; Nordberg, M.; Nordberg, G.F. Plasma Metallothionein Antibody, Urinary Cadmium, and Renal Dysfunction in a Chinese Type 2 Diabetic Population. Diabetes Care 2006, 29, 2682–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonio, M.T.; Corredor, L.; Leret, M.L. Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol. Lett. 2003, 143, 331–340. [Google Scholar] [CrossRef]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Lane, T.W.; Morel, F.M.M. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.-Q.; Fan, J.; Wang, R.-H.; Zhou, Y.-F.; Yuan, D.-Q.; Hong, M.-C. Syntheses, crystal structures and photoluminescence of two Cd(II) coordination polymers derived from a flexible bipyridyl ligand. J. Mol. Struct. 2004, 705, 29–34. [Google Scholar] [CrossRef]
- Li, W.; Evans, O.-R.; Xiong, R.-G.; Wang, Z. Supramolecular Engineering of Chiral and Acentric 2D Networks. Synthesis, Structures, and Second-Order Nonlinear Optical Properties of Bis(nicotinato)zinc and Bis{3-[2-(4-pyridyl)ethenyl]benzoato}cadmium. J. Am. Chem. Soc. 1998, 120, 13272–13273. [Google Scholar]
- Lin, W.; Wang, Z.; Ma, L. A Novel Octupolar Metal−Organic NLO Material Based on a Chiral 2D Coordination Network. J. Am. Chem. Soc. 1999, 121, 11249–11250. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Zhang, J.; Cheng, J.-K.; Kang, Y.; Li, Z.-J.; Yao, Y.-G. 1D chain structure, NLO and luminescence properties of [Zn2 (bpp) (pht)2]. Inorg. Chem.Commun. 2004, 7, 1139–1141. [Google Scholar] [CrossRef]
- Cooke, N.H.C.; Viavattene, R.L.; Eksteen, R.; Wong, W.S.; Davies, G.; Karger, B.L. Use of metal ions for selective separations in high-performance liquid chromatography. J. Chromatogr. 1978, 149, 391–415. [Google Scholar] [CrossRef]
- Le Page, J.N.; Lindner, W.; Davies, G.; Seitz, D.E.; Karger, B.L. Resolution of the optical isomers of dansyl amino acids by reversed phase liquid chromatography with optically active metal chelate additives. Anal. Chem. 1979, 51, 433–435. [Google Scholar] [CrossRef]
- Lindner, W. HPLC-Enantiomerentrennung an gebundenen chiralen Phasen. Nat. Schaften 1980, 67, 354–356. [Google Scholar] [CrossRef]
- Hashemi, L.; Hosseinifard, M.; Amani, V.; Morsali, A.J. Sonochemical Synthesis of Two New Nano-structured Cadmium (II) Supramolecular Complexes. Inorg. Organomet. Polym. 2013, 23, 519–524. [Google Scholar] [CrossRef]
- Mlowe, S.; Lewis, D.J.; Malik, M.A.; Raftery, J.; Mubofu, E.B.; O’Brien, P.; Revaprasadu, N. Bis(piperidinedithiocarbamato)pyridinecadmium(II) as a single-source precursor for the synthesis of CdS nanoparticles and aerosol-assisted chemical vapour deposition (AACVD) of CdS thin films. New J. Chem. 2014, 38, 6073–6080. [Google Scholar] [CrossRef] [Green Version]
- Mirtamizdoust, B.; Hanifehpour, Y.; Behzadfar, E.; Sadeghi-Roodsari, M.; Jung, J.H.; Joo, S.W. A novel nano-structured three-dimensional supramolecular metal-organic framework for cadmium (II): A new precursor for producing nano cadmium oxide. J. Mol. Struct. 2020, 1201, 127191. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Dadashi, J.; Mirtamizdoust, B. Ultrasound-Assisted Synthesis and Crystal Structure of Novel 2D Cd (II) Metal–Organic Coordination Polymer with Nitrite End Stop Ligand as a Precursor for Preparation of CdO Nanoparticles. Crystals 2021, 11, 197. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Bi, C.F.; Fan, Y.H.; Yan, X.C.; Zhang, X.; Zhang, P.F.; Huang, G.M. Synthesis, crystal structures, luminescent properties, theoretical calculation, and DNA interaction of the cadmium (II) and lead (II) complexes with o-aminobenzoic acid and 1, 10-phenanthroline. Russ. J. Coord. Chem. 2015, 41, 274–284. [Google Scholar] [CrossRef]
- Jana, S.K.; Mandal, A.K.; Seth, S.K.; Puschmann, H.; Hossain, M.; Dalai, S. Synthesis, Characterization and Crystal Structure of a New 3D Cadmium(II) Coordination Polymer: Binding Interaction with DNA and Double Stranded RNA. J. Inorg. Organomet. Polym. 2016, 26, 806–818. [Google Scholar] [CrossRef]
- Zianna, A.; Ristović, M.Š.; Psomas, G.; Hatzidimitriou, A.; Coutouli-Argyropoulou, E.; Lalia-Kantouri, M. Cadmium(II) complexes of 5-bromo-salicylaldehyde and α-diimines: Synthesis, structure and interaction with calf-thymus DNA and albumins. Polyhedron 2016, 107, 136–147. [Google Scholar] [CrossRef]
- Wazeer, M.I.M.; Isab, A.A.; Fettouhi, M. New cadmium chloride complexes with imidazolidine-2-thione and its derivatives: X-ray structures, solid state and solution NMR and antimicrobial activity studies. Polyhedron 2007, 26, 1725–1730. [Google Scholar] [CrossRef]
- Ruíz, M.; Perelló, L.; Server-Carrió, J.; Ortiz, R.; García-Granda, S.; Díaz, M.R.; Cantón, E. Cinoxacin complexes with divalent metal ions. Spectroscopic characterization. Crystal structure of a new dinuclear Cd(II) complex having two chelate-bridging carboxylate groups. Antibacterial studies. J. Inorg. Biochem. 1998, 69, 231–239. [Google Scholar] [CrossRef]
- Dubler, E.; Gyr, E. New metal complexes of the antitumor drug 6-mercaptopurine. Syntheses and x-ray structural characterizations of dichloro(6-mercaptopurinium)copper(I), dichlorotetrakis(6-mercaptopurine)cadmium(II), and bis(6-mercaptopurinato)cadmium(II) dehydrate. Inorg. Chem. 1988, 27, 1466–1473. [Google Scholar] [CrossRef]
- Perez, J.M.; Cerrillo, V.; Matesanz, A.I.; Millan, J.M.; Navaro, P.; Alonso, C.; Souza, P. DNA Interstrand Cross-Linking Efficiency and Cytotoxic Activity of Novel Cadmium(II)–Thiocarbodiazone Complexes. ChemBioChem 2001, 2, 119–123. [Google Scholar] [CrossRef]
- Ignatius, I.C.; Rajathi, S.; Kirubavathi, K.; Selvaraju, K. Synthesis, crystal growth and characterization of novel semiorganic nonlinear optical crystal: Dichloro(beta-alanine)cadmium(II). Optik 2014, 125, 5144–5147. [Google Scholar] [CrossRef]
- Saghatforoush, L.; Khoshtarkib, Z.; Keypour, H.; Hakimi, M. Mononuclear, tetranuclear and polymeric cadmium(II) complexes with the 3,6-bis(2-pyridyl)-1,2,4,5-tetrazine ligand: Synthesis, crystal structure, spectroscopic and DFT studies. Polyhedron 2016, 119, 160–174. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, J.; Wang, L.; Duan, L.; Zhang, D.; Yang, W.; Qiu, Y. Synthesis, Structures, and Optical Properties of Cadmium Iodide/Phenethylamine Hybrid Materials with Controlled Structures and Emissions. Inorg.Chem. 2007, 46, 10252–10260. [Google Scholar] [CrossRef]
- Biswas, F.B.; Roy, T.G.; Rahman, A.; Emran, T.B. An in vitro antibacterial and antifungal effects of cadmium (II) complexes of hexamethyltetraazacyclotetradecadiene and isomers of its saturated analogue Asian Pac. J. Trop. Med. 2014, 7, S534–S539. [Google Scholar]
- Yi, X.C.; Huang, M.X.; Qi, Y.; Gao, E.Q. Synthesis, structure, luminescence and catalytic properties of cadmium(II) coordination polymers with 9H-carbazole-2,7-dicarboxylic acid. Dalton Trans. 2014, 43, 3691–3697. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.G.; Li, D.D.; Gu, C.; Dai, Y.C.; Zhou, Y.H.; Yuan, G.; Sheng, E.H. Two cadmium(II) complexes with oxazoline-based ligands as effective catalysts for C–N cross-coupling reactions. Inorg. Chim. Acta 2015, 427, 226–231. [Google Scholar] [CrossRef]
- Tronic, T.A. Copper (I) Cyanide Networks: Synthesis, Luminescence Behavior and Thermal Analysis. Part 1. Diimine Ligands. Inorg. Chem. 2007, 46, 8897–8912. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Escuer, A.; Langer, V. New Polymeric Manganese Azide Derivatives with Quinazoline. Eur. J. Inorg. Chem. 2005, 2005, 4659–4664. [Google Scholar] [CrossRef]
- Machura, B.; Nawrot, I.; Kruszynski, R.; Dulski, M. Syntheses, structures, thermal and luminescent properties of cadmium(II) complexes based on quinazoline and phthalazine. Polyhedron 2013, 54, 272–284. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A. Low temperature X-ray structure analyses combined with NBO studies of a new heteroleptic octa-coordinated Holmium(III) complex with N,N,N-tridentate hydrazono-phthalazine-type ligand. J. Mol. Struct. 2018, 1157, 222–229. [Google Scholar] [CrossRef]
- Soliman, S.M.; Albring, J.H.; El-Faham, A. Cu(II)-promoted cyclization of hydrazonophthalazine to triazolophthalazine; Synthesis and structure diversity of six novel Cu(II)-triazolophthalazine complexes. Appl. Organomet. Chem. 2019, 33, e4992. [Google Scholar] [CrossRef]
- Roy, S.; Bauzá, A.; Frontera, A.; Chattopadhyay, S. A combined experimental and theoretical study on the synthesis and characterization of two new dinuclear cadmium (II) Schiff base complexes with selenocyanate-κ-Se. Inorg. Chim. Acta 2016, 453, 51–61. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09.Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sheldrick, G.M. Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; University of Western Australia: Perth, Australia, 2017. Available online: http://hirshfeldsurface.net (accessed on 12 June 2017).
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Commun. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M.; Halasyamani, P.S.; Casanova, D.; Llunell, M.; Alvarez, S. Distortions in Octahedrally Coordinated d0 Transition Metal Oxides: A Continuous Symmetry Measures Approach. Chem. Mater. 2006, 18, 3176–3183. [Google Scholar] [CrossRef]
- Santiaqo, A.; David, A.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar]
- Hagit, Z.; Shmuel, P.; David, A. Continuous symmetry measures. J. Am. Chem. Soc. 1992, 114, 7843–7851. [Google Scholar]
- Keinan, S.; Avnir, D. Quantitative Symmetry in Structure−Activity Correlations: The Near C2 Symmetry of Inhibitor/HIV Protease Complexes. J. Am. Chem. Soc. 2000, 122, 4378–4384. [Google Scholar] [CrossRef]









| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C13H13CdCl3N4OS | C11H8CdN6OS3 | C8H10N4Cl3Cd1.5O |
| Formula weight (g/mol) | 492.08 | 448.81 | 453.15 |
| Temperature (K) | 293(2) | 115(2) | 293.15 |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 |
| Unit cell dimensions | |||
| a (Å) | a = 7.277(3) | a = 7.0680(6) | 6.972(3) |
| b (Å) | b = 11.451(5) | b = 10.0072(9) | 10.706(4) |
| c (Å) | c = 12.042(5) | c = 10.9572(9) | 11.887(5) |
| α(°) | α = 110.994(9) | α = 83.385(5) | 67.966(10) |
| β(°) | β = 99.076(9) | β = 84.500(5) | 77.907(11) |
| γ(°) | γ = 107.083(8) | γ = 80.595(5) | 81.146(12) |
| Volume (Å3) | 855.7(6) | 757.16(11) | 801.4(6) |
| Z | 2 | 2 | 2 |
| Density (calculated, g/cm3) | 1.910 | 1.969 | 1.878 |
| Absorption coefficient (mm−1) | 1.873 | 1.864 | 2.499 |
| F(000) | 484 | 440 | 434 |
| Crystal size, mm3 | 0.15 × 0.17 × 0.27 | 0.143 × 0.176 × 0.299 | 0.3 × 0.23 × 0.12 |
| θ range | 2.12 to 25.00° | 2.65 to 25.00° | 5.996 to 49.992° |
| Index ranges | −8 ≤ h ≤ 8, −13 ≤ k ≤ 13, −14 ≤ l ≤ 14 | −8 ≤ h ≤ 8, −11 ≤ k ≤ 11, −13 ≤ l ≤ 13 | −8 ≤ h ≤ 8, −12 ≤ k ≤ 12, −14 ≤ l ≤ 13 |
| Reflections collected | 31488 | 10710 | 7897 |
| Independent reflections | 3023 [R(int) = 0.0360] | 2660 [R(int) = 0.0497] | 2805 [Rint = 0.0523] |
| Completeness to theta | 99.90% | 99.60% | 99.4% |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3023/3/224 | 2660/0/207 | 2805/0/162 |
| Goodness-of-fit on F2 | 1.061 | 1.077 | 1.092 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0295, wR2 = 0.0712 | R1 = 0.0364, wR2 = 0.1003 | R1 = 0.0427, wR2 = 0.1094 |
| R indices (all data) | R1 = 0.0366, wR2 = 0.0751 | R1 = 0.0409, wR2 = 0.1054 | R1 = 0.0503, wR2 = 0.1167 |
| Largest diff. peak and hole | 0.916/−0.661 Å−3 | 1.729/−1.325 Å−3 | 0.94/−1.25 Å−3 |
| CCDC No. | 2093653 | 2093654 | 2093655 |
| Atoms | Distance | Atoms | Distance |
|---|---|---|---|
| Cd1-N2 | 2.773(4) | Cd1-Cl1 | 2.563(1) |
| Cd1-Cl3 | 2.497(1) | Cd1-Cl1 # | 2.677(1) |
| Cd1-Cl2 | 2.517(1) | Cd1-Cl2 ## | 2.858(1) |
| Atoms | Angle | Atoms | Angle |
| Cl3-Cd1-Cl2 | 147.56(4) | Cl2-Cd1-Cl2 ## | 79.43(4) |
| Cl3-Cd1-Cl1 | 103.19(5) | Cl1-Cd1-Cl2 ## | 89.46(4) |
| Cl2-Cd1-Cl1 | 106.08(5) | N2-Cd1-Cl1 | 157.21(8) |
| Cl3-Cd1-Cl1 # | 99.06(4) | N2-Cd1-Cl1 # | 74.32(8) |
| Cl2-Cd1-Cl1 # | 98.03(4) | N2-Cd1-Cl2 | 80.70(8) |
| Cl1-Cd1-Cl1 # | 83.12(4) | N2-Cd1-Cl2 ## | 113.29(8) |
| Cl3-Cd1-Cl2 ## | 87.22(4) | N2-Cd1-Cl3 | 77.68(8) |
| D-H…A | D-H (Å) | H…A (Å) | D…A (Å) | D-H…A(°) | Symm. Code |
|---|---|---|---|---|---|
| O1-H1A…Cl3 | 0.83(8) | 2.62(9) | 3.289(5) | 140(7) | 1−x,1−y,1−z |
| O1-H1B …Cl2 | 0.84(4) | 2.39(4) | 3.210(4) | 167(6) | x,y,−1+z |
| N3-H5A…O1 | 0.91(5) | 1.87(5) | 2.762(6) | 167(4) | x,y,z |
| C6-H6…O1 | 0.93 | 2.51 | 3.372(6) | 154 | x,y,z |
| C11-H11…Cl1 | 0.93 | 2.76 | 3.563(4) | 145 | x,y,−1+z |
| Atoms | Distance | Atoms | Distance |
|---|---|---|---|
| Cd1-N2 | 2.256(4) | Cd1-S2 | 2.636(1) |
| Cd1-N6 | 2.338(4) | Cd1-S3 | 2.741(1) |
| Cd1-N4 | 2.480(3) | Cd1-S1 | 2.749(1) |
| Atoms | Angle | Atoms | Angle |
| N2-Cd1-N6 | 93.95(13) | N4-Cd1-S3 | 83.06(8) |
| N2-Cd1-N4 | 175.83(11) | S2-Cd1-S3 | 91.40(3) |
| N6-Cd1-N4 | 90.05(12) | N2-Cd1-S1 | 97.61(9) |
| N2-Cd1-S2 | 98.49(10) | N6-Cd1-S1 | 88.41(9) |
| N6-Cd1-S2 | 167.56(10) | N4-Cd1-S1 | 83.68(8) |
| N4-Cd1-S2 | 77.51(8) | S2-Cd1-S1 | 90.06(3) |
| N2-Cd1-S3 | 95.93(9) | S3-Cd1-S1 | 166.02(3) |
| N6-Cd1-S3 | 87.18(9) |
| D-H…A | D-H (Å) | H…A (Å) | D…A (Å) | D-H…A(°) | Symm. Code |
|---|---|---|---|---|---|
| N3-H3A…O1 | 0.88 | 1.80 | 2.665(5) | 166.0 | |
| O1-H15…N6 | 0.73(7) | 2.30(6) | 3.005(5) | 163(6) | x,y,1+z |
| O1-H16…S3 | 0.78(7) | 2.67(7) | 3.445(4) | 168(6) | x,y,1+z |
| Atoms | Distance | Atoms | Distance |
|---|---|---|---|
| Cd1-Cl1 | 2.6320(17) | Cd2-N1 | 2.432(5) |
| Cd1-Cl1(I) | 2.6320(17) | Cd2-Cl1 | 2.6039(17) |
| Cd1-Cl3(I) | 2.6918(17) | Cd2-Cl2 | 2.5223(18) |
| Cd1-Cl3 | 2.6918(17) | Cd2-Cl3 | 2.6569(18) |
| Cd1-N3 | 2.331(5) | Cd2-N4 | 2.318(5) |
| Cd1-N3(I) | 2.331(5) | Cd2-O5 | 2.427(5) |
| Atoms | Angle | Atoms | Angle |
| Cl1 1-Cd1Cl1 | 180.00 | O5-Cd2-N1 | 81.54(19) |
| Cl1-Cd1-Cl3 1 | 98.11(6) | O5-Cd2-Cl1 | 80.33(13) |
| Cl1 1-Cd1-Cl3 | 98.11(6) | O5-Cd2-Cl2 | 88.36(12) |
| Cl1 1-Cd1-Cl3 1 | 81.89(6) | O5-Cd2-Cl3 | 163.40(13) |
| Cl1-Cd1-Cl3 | 81.89(6) | N1-Cd2-Cl1 | 151.27(14) |
| Cl3-Cd1-Cl3 1 | 180.00(7) | N1-Cd2-Cl2 | 94.45(13) |
| N3 1-Cd1-Cl1 | 91.04(13) | N1-Cd2-Cl3 | 114.06(15) |
| N3 1-Cd1-Cl1 1 | 88.96(13) | Cl1-Cd2-Cl3 | 83.09(5) |
| N3-Cd1-Cl1 1 | 91.04(13) | Cl2-Cd2-Cl1 | 107.08(7) |
| N3-Cd1-Cl1 | 88.96(13) | Cl2-Cd2-Cl3 | 95.70(6) |
| N3-Cd1-Cl3 | 87.26(12) | N4-Cd2-N1 | 68.71(17) |
| N3 1-Cd1-Cl3 1 | 87.26(12) | N4-Cd2-Cl1 | 91.87(13) |
| N3 1-Cd1-Cl3 | 92.74(12) | N4-Cd2-Cl2 | 160.85(13) |
| N3-Cd1-Cl3 1 | 92.74(12) | N4-Cd2-Cl3 | 83.67(12) |
| N3 1-Cd1-N3 | 180.00 | N4-Cd2-O5 | 97.70(16) |
| D-H…A | D-H (Å) | H…A (Å) | D…A (Å) | D-H…A(°) | Symm. Code |
|---|---|---|---|---|---|
| N1-H1A…Cl2 | 0.89 | 2.58 | 3.464(7) | 173 | 1−x,2−y,−z |
| N1-H1B…O5 | 0.89 | 2.37 | 3.047(7) | 133 | 2−x,2−y,−z |
| O5-H5A…Cl2 | 0.89 | 2.44 | 3.093(6) | 131 | 2−x,2−y,−z |
| O5-H5B…Cl3 | 0.89 | 2.39 | 3.227(5) | 157 | 1+x,y,z |
| Parameter | HL1 | HL2 | H2L+ |
|---|---|---|---|
| E | −1118.6190 | −1118.5948 | −1119.0100 |
| ZPVE | 0.2105 | 0.2086 | 0.2233 |
| Ecorr a | −1118.4085 | −1118.3861 | −1118.7867 |
| H | −1118.3933 | −1118.3705 | −1118.771078 |
| G | −1118.4517 | −1118.4308 | −1118.830192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.M.; Massoud, R.A.; Al-Rasheed, H.H.; El-Faham, A. Molecular and Supramolecular Structures of Cd(II) Complexes with Hydralazine-Based Ligands; A New Example for Cyclization of Hydrazonophthalazine to Triazolophthalazine. Crystals 2021, 11, 823. https://doi.org/10.3390/cryst11070823
Soliman SM, Massoud RA, Al-Rasheed HH, El-Faham A. Molecular and Supramolecular Structures of Cd(II) Complexes with Hydralazine-Based Ligands; A New Example for Cyclization of Hydrazonophthalazine to Triazolophthalazine. Crystals. 2021; 11(7):823. https://doi.org/10.3390/cryst11070823
Chicago/Turabian StyleSoliman, Saied M., Raghdaa A. Massoud, Hessa H. Al-Rasheed, and Ayman El-Faham. 2021. "Molecular and Supramolecular Structures of Cd(II) Complexes with Hydralazine-Based Ligands; A New Example for Cyclization of Hydrazonophthalazine to Triazolophthalazine" Crystals 11, no. 7: 823. https://doi.org/10.3390/cryst11070823
APA StyleSoliman, S. M., Massoud, R. A., Al-Rasheed, H. H., & El-Faham, A. (2021). Molecular and Supramolecular Structures of Cd(II) Complexes with Hydralazine-Based Ligands; A New Example for Cyclization of Hydrazonophthalazine to Triazolophthalazine. Crystals, 11(7), 823. https://doi.org/10.3390/cryst11070823