Conductivity Transitions of La0.7Sr0.3MnO3±δ and La0.6Sr0.4Co0.2Fe0.8O3−δ in Ce0.9Gd0.1O2−δ Matrix for Dual-Phase Oxygen Transport Membranes
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
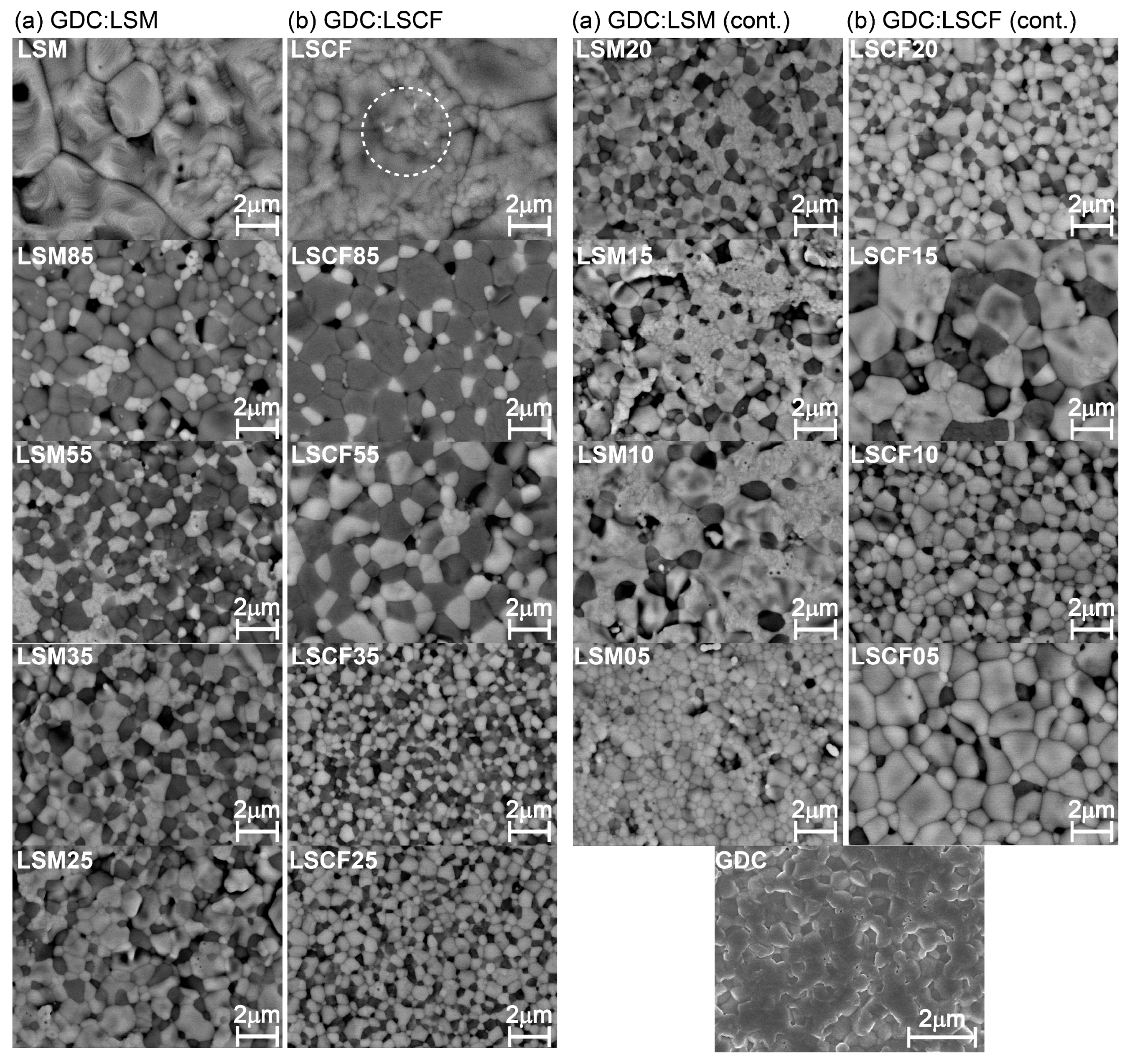
3.1. Microstructure Characterization
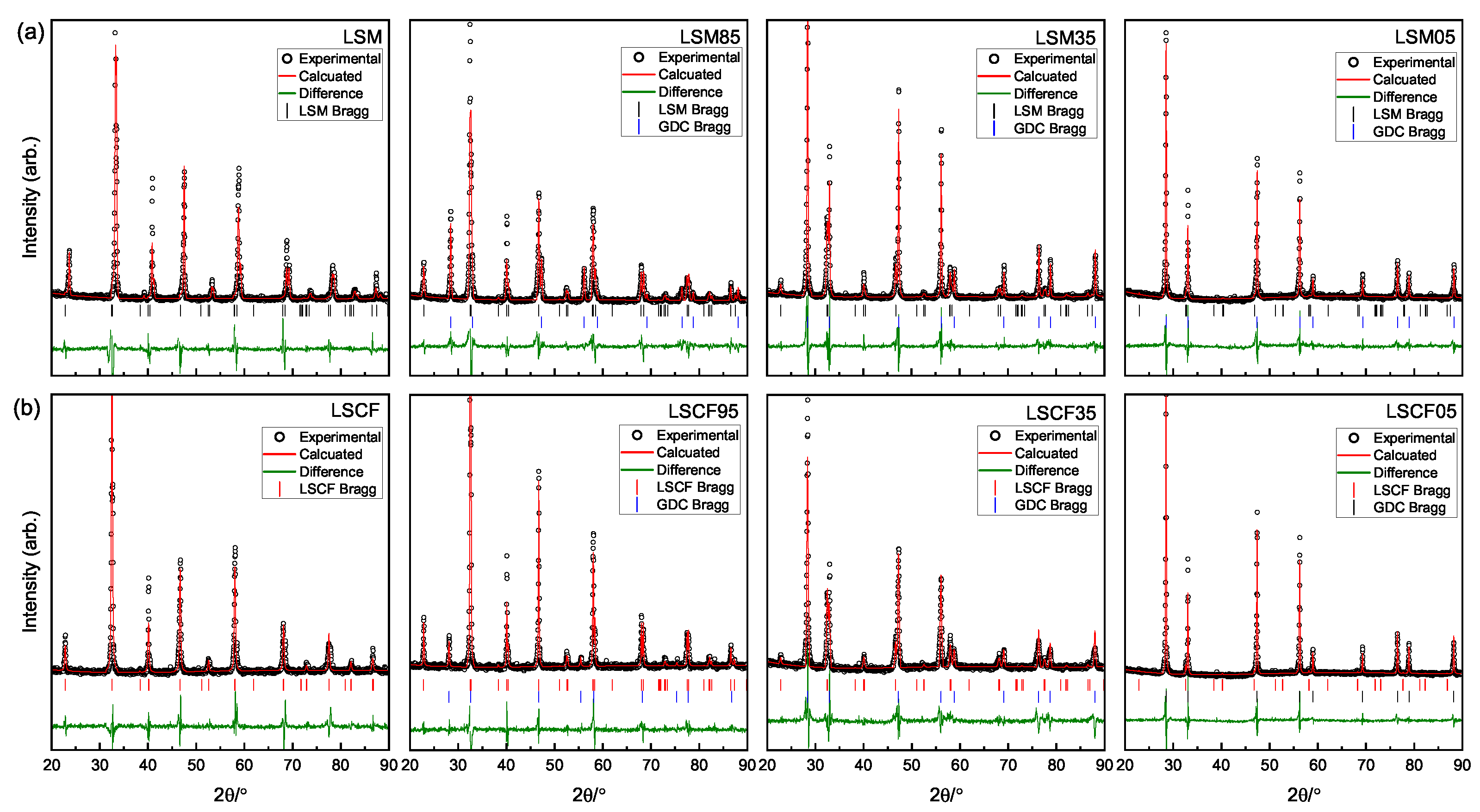
3.2. X-ray Diffraction Analysis
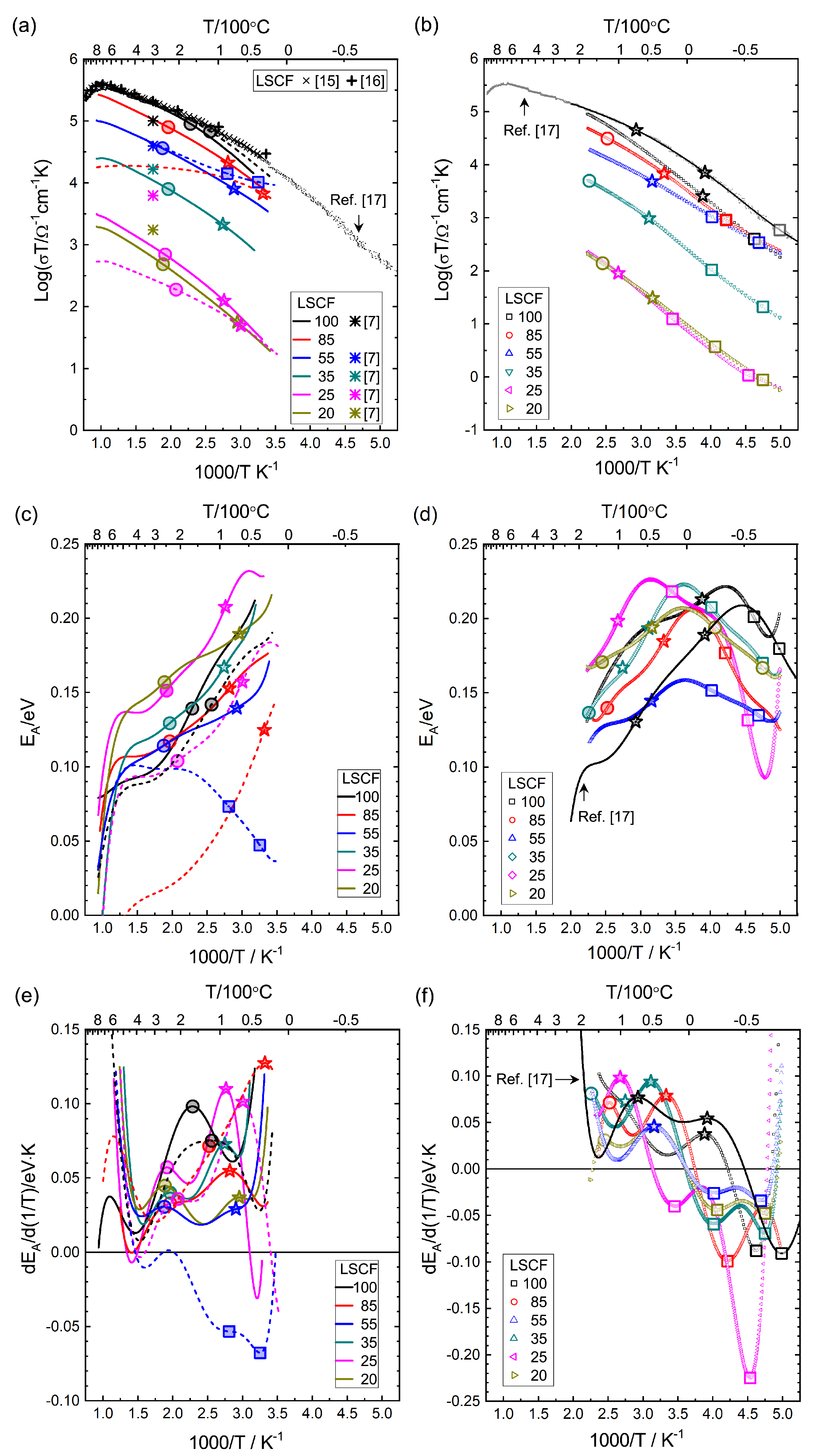
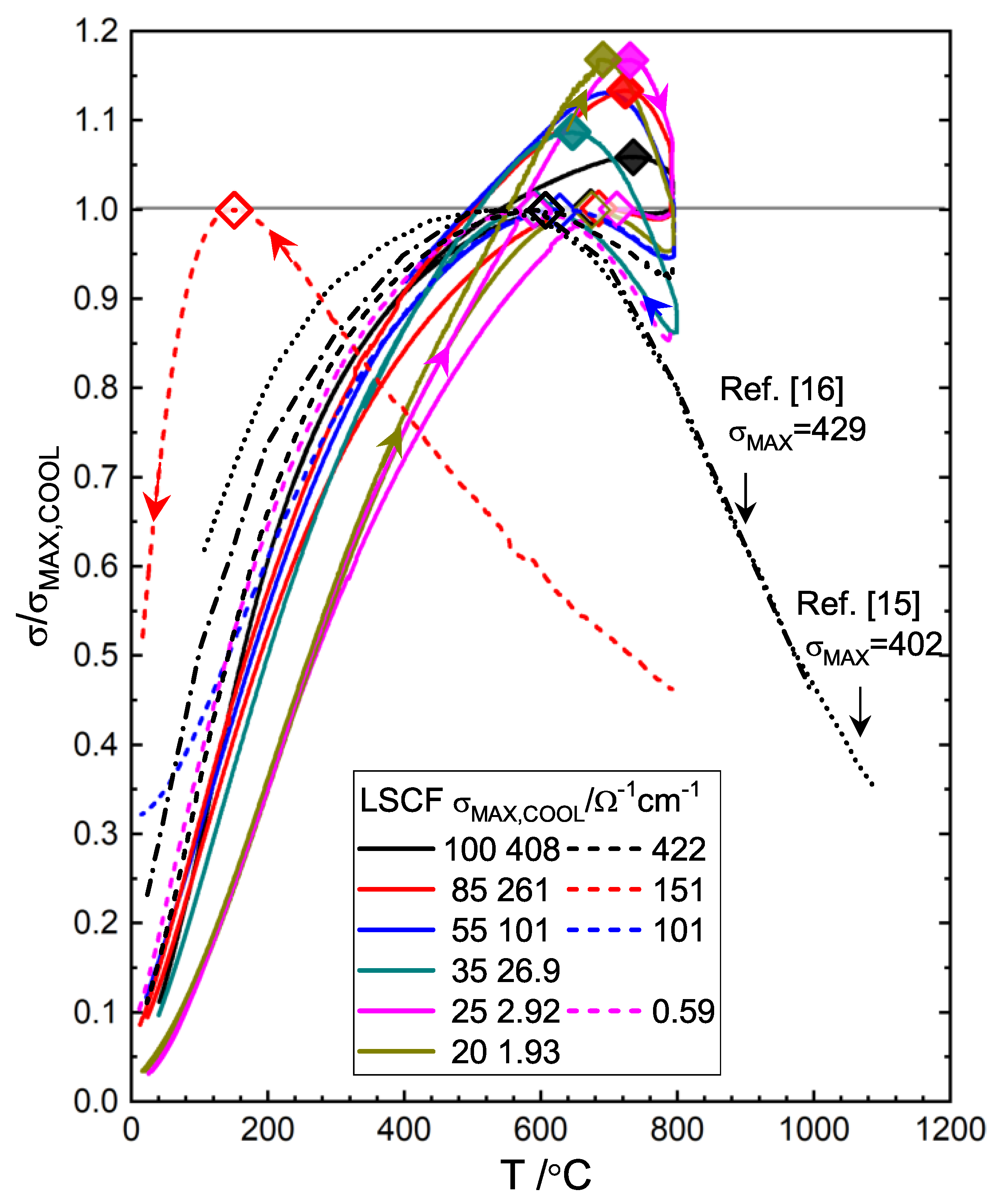
3.3. Electrical Conductivity
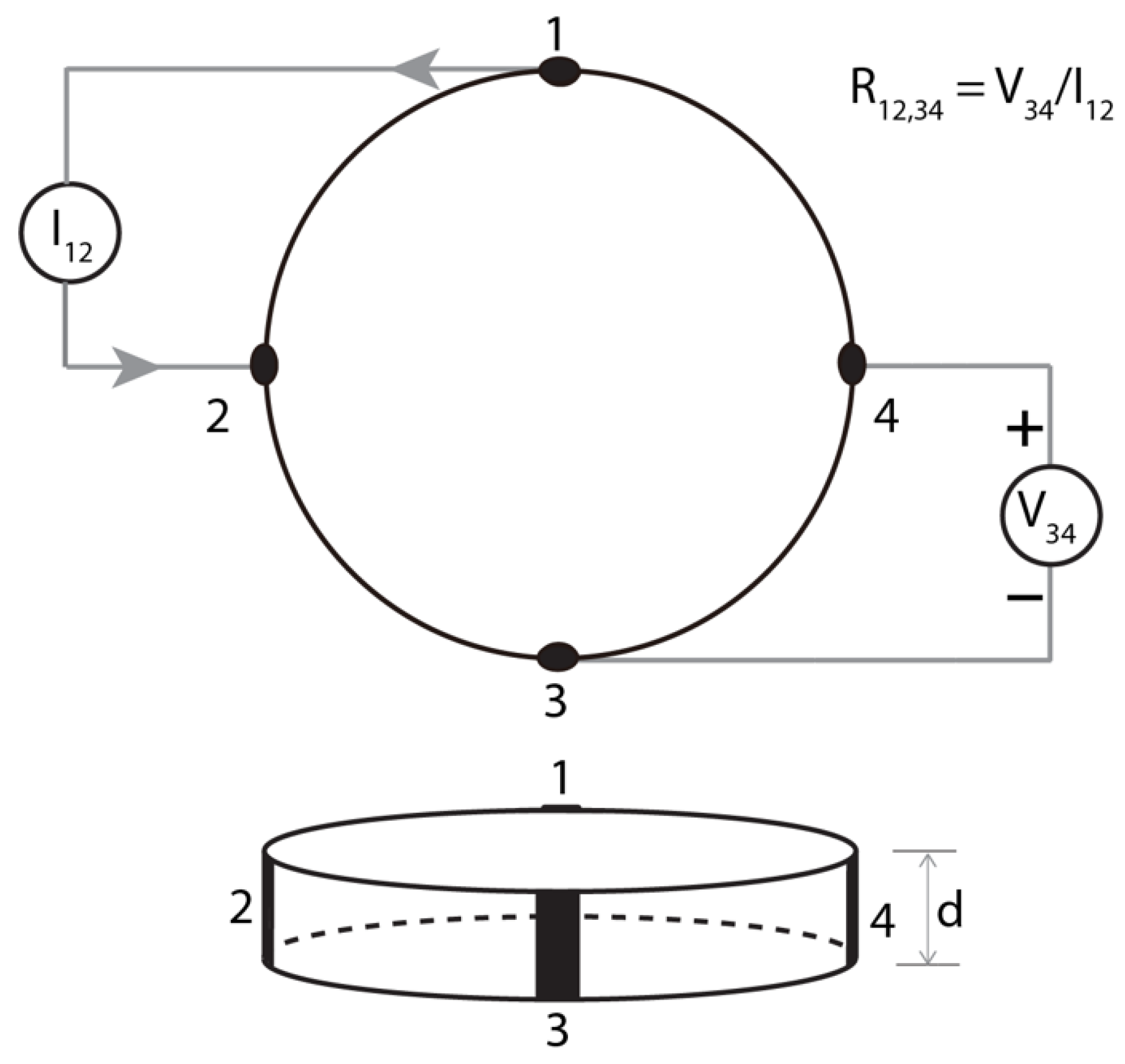
3.3.1. van Der Pauw Method
3.3.2. GDC:LSM Composites
3.3.3. GDC:LSCF Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, S.J.; Thomson, W.J. Stability of La0.6Sr0.4Co0.2Fe0.8O3−δ perovskite membranes in reducing and nonreducing environments. Ind. Eng. Chem. Res. 1998, 37, 1290–1299. [Google Scholar] [CrossRef]
- Xu, S.J.; Thomson, W.J. Oxygen permeation rates through ion-conducting perovskite membranes. Chem. Eng. Sci. 1999, 54, 3839–3850. [Google Scholar] [CrossRef]
- Kharton, V.; Kovalevsky, A.; Viskup, A.; Figueiredo, F.; Yaremchenko, A.; Naumovich, E.; Marques, F. Oxygen Permeability of Ce0.8Gd0.2O2−δ-La0.7Sr0.3MnO3−δ Composite Membranes. J. Electrochem. Soc. 2000, 147, 2814. [Google Scholar] [CrossRef]
- Kharton, V.; Kovalevsky, A.; Viskup, A.; Figueiredo, F.; Yaremchenko, A.; Naumovich, E.; Marques, F. Oxygen permeability and Faradaic efficiency of Ce0.8Gd0.2O2−δ-La0.7Sr0.3MnO3−δ composites. J. Eur. Ceram. Soc. 2001, 21, 1763–1767. [Google Scholar] [CrossRef]
- Shaula, A.; Kharton, V.; Marques, F.; Kovalevsky, A.; Viskup, A.; Naumovich, E. Oxygen permeability of mixed-conducting composite membranes: Effects of phase interaction. J. Solid State Electrochem. 2006, 10, 28–40. [Google Scholar] [CrossRef]
- Joo, J.H.; Park, G.S.; Yoo, C.Y.; Yu, J.H. Contribution of the surface exchange kinetics to the oxygen transport properties in Ce0.9Gd0.1O2−δ-La0.6Sr0.4Co0.2Fe0.8O3−δ dual-phase membrane. Solid State Ionics 2013, 253, 64–69. [Google Scholar] [CrossRef]
- Joo, J.H.; Yun, K.S.; Lee, Y.; Jung, J.; Yoo, C.Y.; Yu, J.H. Dramatically enhanced oxygen fluxes in fluorite-rich dual-phase membrane by surface modification. Chem. Mater. 2014, 26, 4387–4394. [Google Scholar] [CrossRef]
- Raju, K.; Kim, S.; Kang, E.J.; Yun, K.S.; Seong, Y.H.; Han, I.S.; Lee, H.K.; Yu, J.H. Two-step sintering technique for enhancing mechanical and oxygen permeation properties of dual-phase oxygen transport membranes. J. Eur. Ceram. Soc. 2021, 41, 4884–4895. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.H.; Lee, K.S.; Yu, J.H.; Seong, Y.H.; Han, I.S. Mechanical properties of LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)-GDC (Ce0.9Gd0.1O2−δ) for oxygen transport membranes. Ceram. Int. 2017, 43, 1916–1921. [Google Scholar] [CrossRef]
- Raju, K.; Kim, S.; Yu, J.H.; Kim, S.H.; Seong, Y.H.; Han, I.S. Rietveld refinement and estimation of residual stress in GDC-LSCF oxygen transport membrane ceramic composites. Ceram. Int. 2018, 44, 10293–10298. [Google Scholar] [CrossRef]
- Joo, J.H.; Yun, K.S.; Kim, J.H.; Lee, Y.; Yoo, C.Y.; Yu, J.H. Substantial oxygen flux in dual-phase membrane of ceria and pure electronic conductor by tailoring the surface. ACS Appl. Mater. Interfaces 2015, 7, 14699–14707. [Google Scholar] [CrossRef] [PubMed]
- Na, B.T.; Park, J.H.; Park, J.H.; Yu, J.H.; Joo, J.H. Elucidation of the oxygen surface kinetics in a coated dual-phase membrane for enhancing oxygen permeation flux. ACS Appl. Mater. Interfaces 2017, 9, 19917–19924. [Google Scholar] [CrossRef]
- Mizusaki, J.; Yonemura, Y.; Kamata, H.; Ohyama, K.; Mori, N.; Takai, H.; Tagawa, H.; Dokiya, M.; Naraya, K.; Sasamoto, T.; et al. Electronic conductivity, Seebeck coefficient, defect and electronic structure of nonstoichiometric La1−xSrxMnO3. Solid State Ionics 2000, 132, 167–180. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.; Anderson, H.; Sparlin, D.; Sehlin, S. Structure and electrical properties of La1−xSrxCo1−yFeyO3. II: The system La1−xSrxFe0.8O3. Solid State Ionics 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Stevenson, J.; Armstrong, T.; Carneim, R.; Pederson, L.; Weber, W. Electrochemical properties of mixed conducting perovskites La1−xMxCo1−yFeyO3−δ (M = Sr, Ba, Ca). J. Electrochem. Soc. 1996, 143, 2722. [Google Scholar] [CrossRef]
- Araki, W.; Malzbender, J. Electrical conductivity of La0.58Sr0.4Co0.2Fe0.8O3−δ during ferroelastic deformation under uniaxial compressive loading. Solid State Ionics 2013, 233, 67–72. [Google Scholar] [CrossRef]
- Araki, W.; Arai, Y.; Malzbender, J. Transitions of Ba0.5Sr0.5Co0.8Fe0.2O3−δ and La0.58Sr0.4Co0.2Fe0.8O3−δ. Mater. Lett. 2014, 132, 295–297. [Google Scholar] [CrossRef]
- Zou, Y.; Ziegner, M.; Malzbender, J.; Krüger, M. Investigation of rhombohedral-cubic phase transition of La0.58Sr0.4Co0.2Fe0.8O3−δ using high temperature XRD. Ceram. Int. 2018, 44, 2822–2826. [Google Scholar] [CrossRef]
- Grande, T.; Tolchard, J.R.; Selbach, S.M. Anisotropic thermal and chemical expansion in Sr-substituted LaMnO3+δ: Implications for chemical strain relaxation. Chem. Mater. 2012, 24, 338–345. [Google Scholar] [CrossRef]
- Urushibara, A.; Moritomo, Y.; Arima, T.; Asamitsu, A.; Kido, G.; Tokura, Y. Insulator-metal transition and giant magnetoresistance in La1−xSrxMnO3. Phys. Rev. B 1995, 51, 14103. [Google Scholar] [CrossRef] [PubMed]
- Imada, M.; Fujimori, A.; Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 1998, 70, 1039. [Google Scholar] [CrossRef] [Green Version]
- Anane, A.; Dupas, C.; Le Dang, K.; Renard, J.; Veillet, P.; de Leon Guevara, A.; Millot, F.; Pinsard, L.; Revcolevschi, A. Transport properties and magnetic behaviour of La1−xSrxMnO3 single crystals. J. Phys. Condens. Matter 1995, 7, 7015. [Google Scholar] [CrossRef]
- Tsuda, N.; Nasu, K.; Fujimori, A.; Siratori, K. Electronic Conduction in Oxides; Springer Science & Business Media: New York, NY, USA, 2013; Volume 94. [Google Scholar]
- Huang, B.; Steinbrech, R.; Malzbender, J. Direct observation of ferroelastic domain effects in LSCF perovskites. Solid State Ionics 2012, 228, 32–36. [Google Scholar] [CrossRef]
- Sun, C.; Alonso, J.A.; Bian, J. Recent advances in perovskite-type oxides for energy conversion and storage applications. Adv. Energy Mater. 2021, 11, 2000459. [Google Scholar] [CrossRef]
- Kim, G.R.; Seo, H.H.; Jo, J.M.; Shin, E.C.; Yu, J.H.; Lee, J.S. Moving boundary diffusion problem for hydration kinetics evidenced in non-monotonic conductivity relaxations of proton conducting perovskites. Solid State Ionics 2015, 272, 60–73. [Google Scholar] [CrossRef]
- Araki, W.; Yamaguchi, T.; Arai, Y.; Malzbender, J. Strontium surface segregation in La0.58Sr0.4Co0.2Fe0.8O3−δ annealed under compression. Solid State Ionics 2014, 268, 1–6. [Google Scholar] [CrossRef]
- Tai, L.; Nasrallah, M.; Anderson, H. Thermochemical stability, electrical conductivity, and seebeck coefficient of Sr-doped LaCo0.2Fe0.8O3−δ. J. Solid State Chem. 1995, 118, 117–124. [Google Scholar] [CrossRef]
- Chen, X.; Grande, T. Anisotropic Chemical Expansion of La1−xSrxCoO3−δ. Chem. Mater. 2013, 25, 927–934. [Google Scholar] [CrossRef]
- Chen, X.; Grande, T. Anisotropic and Nonlinear Thermal and Chemical Expansion of La1−xSrxFeO3−δ (x = 0.3, 0.4, 0.5) Perovskite Materials. Chem. Mater. 2013, 25, 3296–3306. [Google Scholar] [CrossRef]
- Mizusaki, J.; Mori, N.; Takai, H.; Yonemura, Y.; Minamiue, H.; Tagawa, H.; Dokiya, M.; Inaba, H.; Naraya, K.; Sasamoto, T.; et al. Oxygen nonstoichiometry and defect equilibrium in the perovskite-type oxides La1−xSrxMnO3+d. Solid State Ionics 2000, 129, 163–177. [Google Scholar] [CrossRef]
- Kasl, C.; Hoch, M. Effects of sample thickness on the van der Pauw technique for resistivity measurements. Rev. Sci. Instrum. 2005, 76, 033907. [Google Scholar] [CrossRef]
- Preis, W. Modelling of surface exchange reaction, oxygen diffusion, and conductivity relaxation of solid oxides: Application to the van der Pauw method on disk-shaped samples. Comput. Mater. Sci. 2015, 103, 237–243. [Google Scholar] [CrossRef]
- Van der Pauw, L.J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Van der Pauw, L.J. A method of measuring the resistivity and Hall coefficient on lamellae of arbitrary shape. Philips Tech. Rev. 1959, 20, 220–224. [Google Scholar]
- Iterative Numeric Solution to Van Der Pauw Equation Using LabVIEW. 2009. Available online: https://forums.ni.com/t5/Example-Code/Iterative-Numeric-Solution-to-Van-Der-Pauw-Equation-using/ta-p/3518294?profile.language=en (accessed on 20 June 2021).
- Godoi, G.S.; de Souza, D.P. Electrical and microstructural characterization of La0.7Sr0.3MnO3 (LSM), Ce0.8Y0.2O2 (CY) and LSM–CY composites. Mater. Sci. Eng. B 2007, 140, 90–97. [Google Scholar] [CrossRef]
- Taran, S.; Chaudhuri, B.; Chatterjee, S.; Yang, H.; Neeleshwar, S.; Chen, Y. Critical exponents of the La0.7Sr0.3MnO3, La0.7Ca0.3MnO3, and Pr0.7Ca0.3MnO3 systems showing correlation between transport and magnetic properties. J. Appl. Phys. 2005, 98, 103903. [Google Scholar] [CrossRef]
- Snyder, G.J.; Hiskes, R.; DiCarolis, S.; Beasley, M.; Geballe, T. Intrinsic electrical transport and magnetic properties of La0.67Ca0.33MnO3 and La0.67Sr0.33MnO3 MOCVD thin films and bulk material. Phys. Rev. B 1996, 53, 14434. [Google Scholar] [CrossRef]
- Nikolaenko, Y.M.; Varyukhin, V.; Medvedev, Y.V.; Efros, N.; Zhikharev, I.; Kara-Murza, S.; Tikhii, A. Optimum oxygen content in La0.7Sr0.3MnO3 thin films. arXiv 2011, arXiv:1111.7180. [Google Scholar]
- Tikhii, A.; Gritskih, V.; Kara-Murza, S.; Korchikova, N.; Nikolaenko, Y.M.; Revenko, Y.F.; Reshidova, I.Y.; Zhikharev, I. Optical and magnetoresistive properties of polycrystalline La0.7Sr0.3MnO3 films on crystalline Al2O3 and Gd3Ga5O12 substrates. Low Temp. Phys. 2014, 40, 756–760. [Google Scholar] [CrossRef]
- Ripple, D. NIST 60. NIST ITS-90 Thermocouple Database. In World Wide Web-Internet and Web Information Systems; NIST: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Bishop, S.; Duncan, K.; Wachsman, E. Defect equilibria and chemical expansion in non-stoichiometric undoped and gadolinium-doped cerium oxide. Electrochim. Acta 2009, 54, 1436–1443. [Google Scholar] [CrossRef]
- Dalslet, B.T.; Søgaard, M.; Bouwmeester, H.J.; Hendriksen, P.V. Defect chemistry and oxygen transport of (La0.6Sr0.4−xMx)0.99Co0.2Fe0.8O3−δ, M=Ca (x = 0.05, 0.1), Ba (= 0.1, 0.2), Sr: Part I: Defect chemistry. Solid State Ionics 2009, 180, 1173–1182. [Google Scholar] [CrossRef]
- Araki, W.; Malzbender, J. Ferroelastic deformation of La0.58Sr0.4Co0.2Fe0.8O3−δ under uniaxial compressive loading. J. Eur. Ceram. Soc. 2013, 33, 805–812. [Google Scholar] [CrossRef]
- Le, P.G.; Tran, T.L.; Kim, H.P.; Jo, W.; Lee, J.S.; Fisher, J.G. Growth of single crystals of 0.75(Na0.5Bi0.5)TiO3-0.25(Sr0.7Ca0.3)TiO3 and characterisation of their electrical properties. Open Ceram. 2021, 6, 100099. [Google Scholar] [CrossRef]
- Ritzmann, A.M.; Dieterich, J.M.; Carter, E.A. Density functional theory+ U analysis of the electronic structure and defect chemistry of LSCF (La0.5Sr0.5Co0.25Fe0.75O3−δ). Phys. Chem. Chem. Phys. 2016, 18, 12260–12269. [Google Scholar] [CrossRef] [PubMed]
- Araki, W.; Abe, T.; Arai, Y. Anomalous variation of the mechanical behaviour of ferroelastic La0.6Sr0.4Co0.2Fe0.8O3−δ during the ferro-to-paramagnetic transition. Scr. Mater. 2015, 99, 9–12. [Google Scholar] [CrossRef]
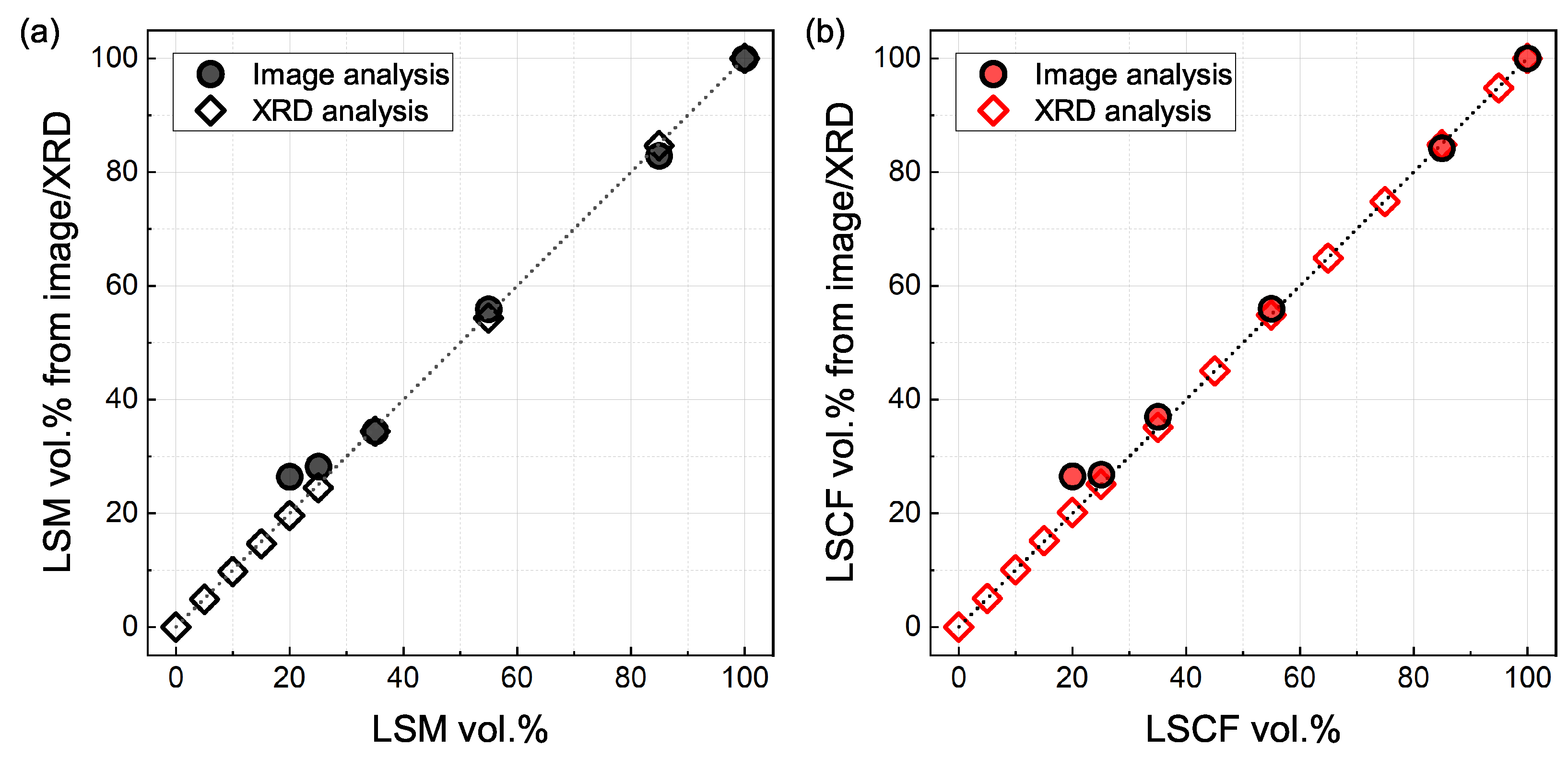
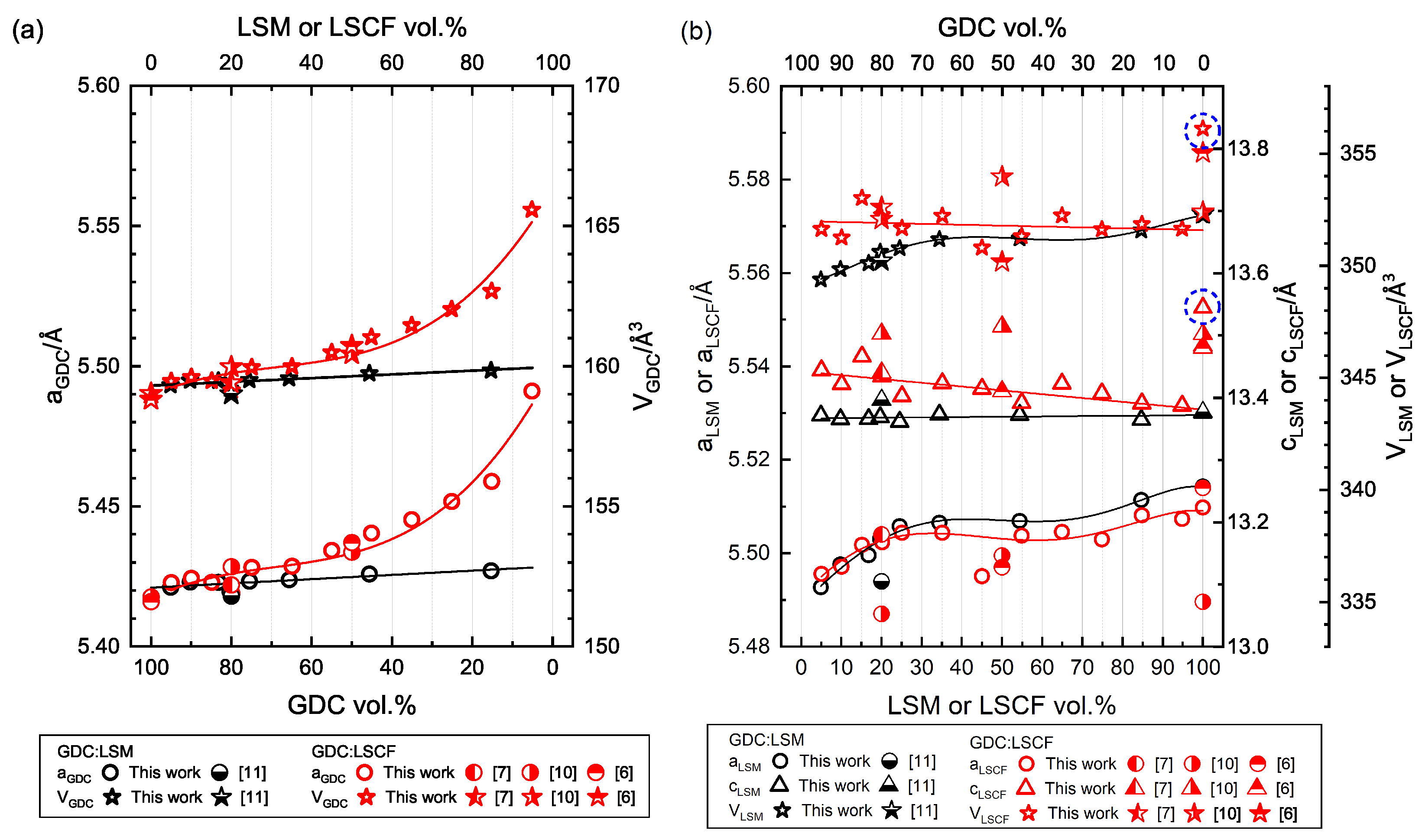
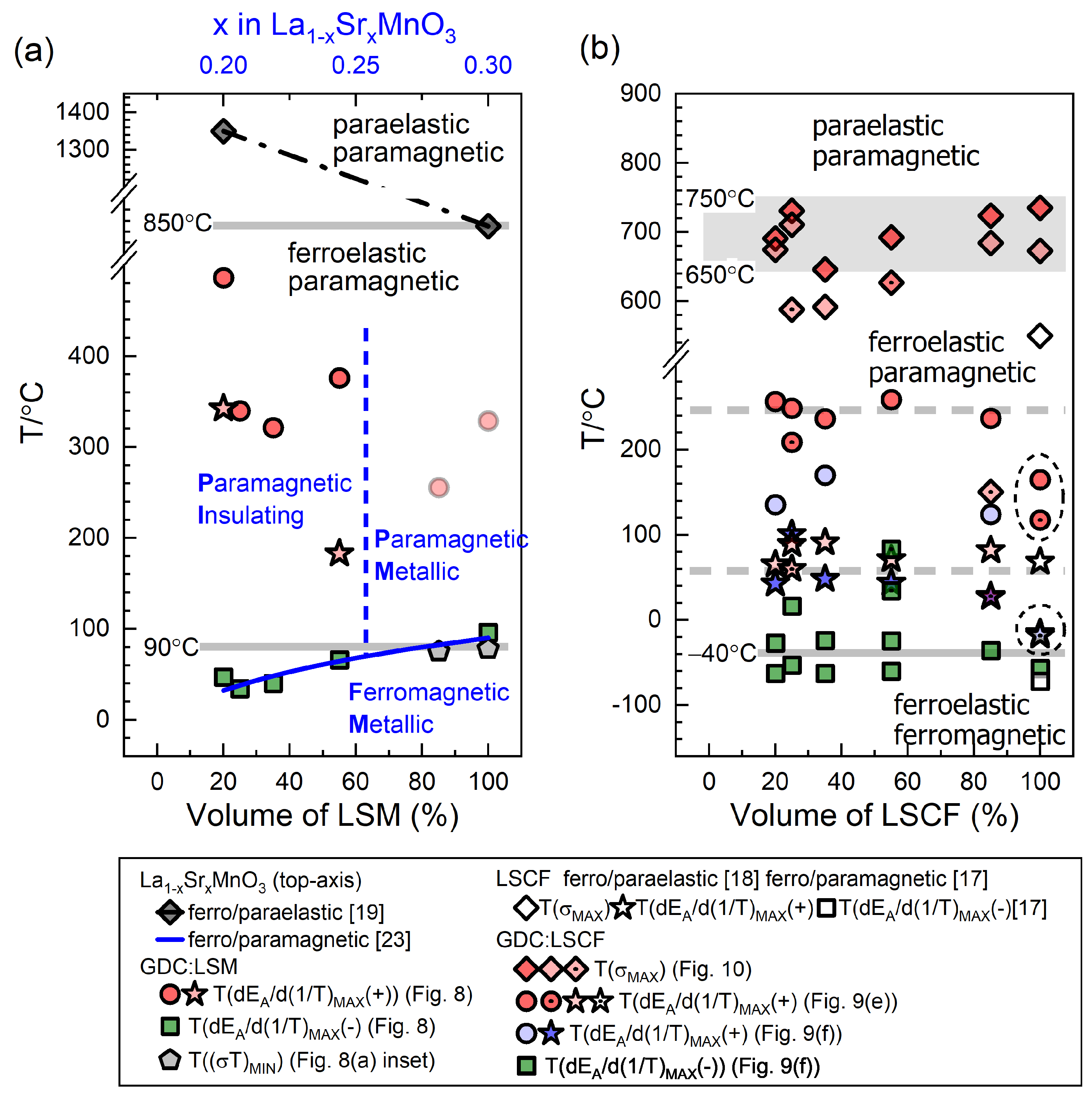
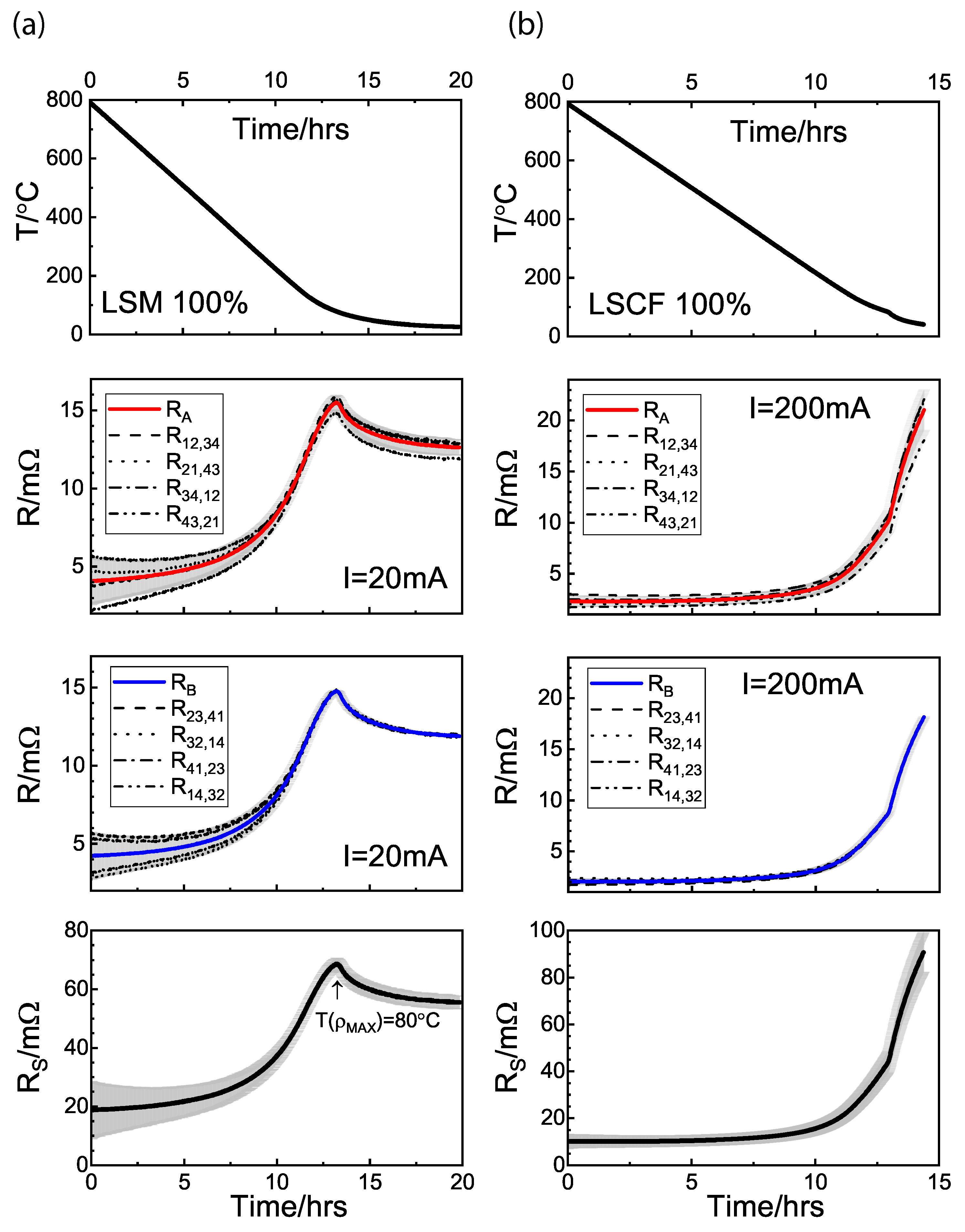
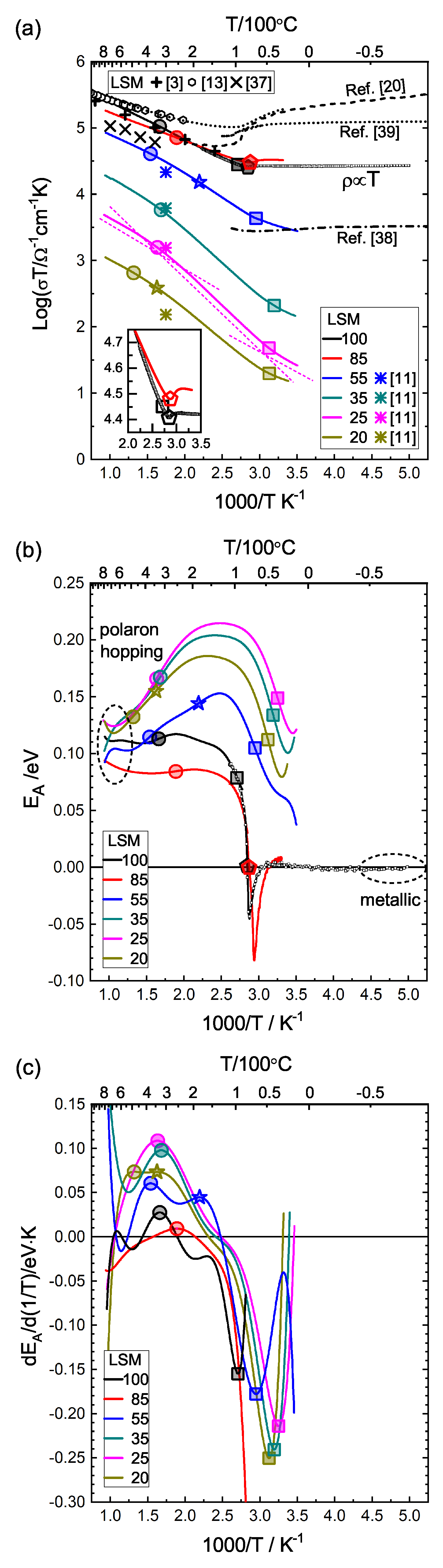
| Sample Name | LSM100 | LSM85 | LSM55 | LSM35 | LSM25 | LSM20 | LSM15 | LSM10 | LSM5 | LSM0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight % (exp) | 100 | 83.13 | 51.52 | 31.89 | 22.47 | 17.85 | 13.30 | 8.81 | 4.35 | 0 | |
| Weight % (Rietveld) | 100 | 85.4 | 55.6 | 34.3 | 23.7 | 20.4 | 15.3 | 10.2 | 3.4 | 0 | |
| Volume % (exp) * | 100 | 84.66 | 54.34 | 34.42 | 24.51 | 19.60 | 14.64 | 9.74 | 4.86 | 0 | |
| Volume % (Rietveld) | 100 | 86.76 | 58.38 | 36.92 | 25.81 | 22.33 | 16.81 | 11.26 | 3.78 | 0 | |
| GDC phase () | a (Å) | 5.4270 | 5.4259 | 5.4239 | 5.4232 | 5.4197 | 5.4229 | 5.4230 | 5.4211 | 5.4180 | |
| Volume of a unit cell () | 159.84 | 159.74 | 159.56 | 159.51 | 159.20 | 159.47 | 159.48 | 159.32 | 159.04 | ||
| Density | 7.1905 | 7.1950 | 7.2031 | 7.2055 | 7.2197 | 7.2070 | 7.2066 | 7.2140 | 7.2266 | ||
| LSM phase () | a (Å) | 5.5140 | 5.5114 | 5.5069 | 5.5065 | 5.5058 | 5.5031 | 5.4996 | 5.4976 | 5.4927 | |
| c (Å) | 13.3760 | 13.3644 | 13.3720 | 13.3728 | 13.3613 | 13.3683 | 13.3658 | 13.3652 | 13.3713 | ||
| Volume of a unit cell () | 352.23 | 351.56 | 351.20 | 351.16 | 350.77 | 350.60 | 350.10 | 349.83 | 349.37 | ||
| Density | 6.4057 | 6.4180 | 6.4246 | 6.4253 | 6.4325 | 6.4355 | 6.4448 | 6.4498 | 6.4582 | ||
| Reliability factors | 6.20 | 6.40 | 6.59 | 6.66 | 6.72 | 9.62 | 9.08 | 9.15 | 9.63 | ||
| 6.54 | 6.21 | 5.73 | 5.93 | 6.36 | 6.92 | 6.54 | 7.45 | 6.79 | |||
| 12.05 | 10.04 | 8.85 | 9.58 | 10.44 | 8.85 | 9.75 | 11.58 | 9.97 | |||
| 3.78 | 2.46 | 1.80 | 2.07 | 2.41 | 0.85 | 1.15 | 1.61 | 1.07 | |||
| Sample Name | LSCF100 | LSCF95 | LSCF85 | LSCF75 | LSCF65 | LSCF55 | LSCF45 | LSCF35 | LSCF25 | LSCF20 | LSCF15 | LSCF10 | LSCF5 | LSCF0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight % (exp) | 100 | 94.37 | 83.33 | 72.58 | 62.10 | 51.88 | 41.92 | 32.20 | 22.72 | 18.07 | 13.47 | 8.93 | 4.44 | 0 | |
| Weight % (Rietveld) | 100 | 95.9 | 85.2 | 74.2 | 65.7 | 56.6 | 45.3 | 35.2 | 22.7 | 19.5 | 12.6 | 9.7 | 3.9 | 0 | |
| Volume % (exp) * | 100 | 94.85 | 84.84 | 74.83 | 64.92 | 54.90 | 44.95 | 35.12 | 25.07 | 20.17 | 15.14 | 10.04 | 5.03 | 0 | |
| Volume % (Rietveld) | 100 | 96.26 | 86.57 | 76.36 | 68.38 | 59.56 | 45.30 | 38.23 | 25.05 | 21.73 | 14.18 | 10.90 | 4.43 | 0 | |
| GDC phase () | a (Å) | 5.4912 | 5.4589 | 5.4517 | 5.4453 | 5.4405 | 5.4343 | 5.4287 | 5.4283 | 5.4189 | 5.4229 | 5.4243 | 5.4228 | 5.4180 | |
| Volume of a unit cell () | 165.57 | 162.68 | 162.03 | 161.46 | 161.03 | 160.48 | 159.98 | 159.95 | 159.12 | 159.47 | 159.60 | 159.46 | 159.04 | ||
| Density | 6.9415 | 7.0651 | 7.0932 | 7.1185 | 7.1373 | 7.1616 | 7.1840 | 7.1855 | 7.2230 | 7.2070 | 7.2011 | 7.2075 | 7.2266 | ||
| LSCF phase () | a (Å) | 5.5100 | 5.5073 | 5.5081 | 5.5030 | 5.5046 | 5.5038 | 5.4951 | 5.5044 | 5.5044 | 5.5023 | 5.5018 | 5.4971 | 5.4956 | |
| c (Å) | 13.5450 | 13.3869 | 13.3909 | 13.4069 | 13.4226 | 13.3920 | 13.4143 | 13.4231 | 13.4020 | 13.4343 | 13.4660 | 13.4217 | 13.4440 | ||
| Volume of a unit cell () | 356.10 | 351.64 | 351.84 | 351.61 | 352.22 | 351.31 | 350.79 | 352.21 | 351.66 | 352.24 | 353.00 | 351.24 | 351.62 | ||
| Density | 6.2353 | 6.3145 | 6.3108 | 6.3152 | 6.3040 | 6.3204 | 6.3296 | 6.3042 | 6.3140 | 6.3036 | 6.2900 | 6.3216 | 6.3147 | ||
| Reliability factors | 5.28 | 5.31 | 5.77 | 3.25 | 3.53 | 6.03 | 3.53 | 7.60 | 6.44 | 8.23 | 9.50 | 8.96 | 8.97 | ||
| 4.81 | 3.77 | 3.61 | 4.50 | 4.03 | 4.44 | 4.92 | 5.81 | 5.80 | 6.47 | 6.67 | 6.12 | 6.48 | |||
| 8.56 | 6.33 | 5.18 | 7.44 | 6.21 | 6.65 | 7.96 | 8.89 | 10.01 | 11.77 | 10.20 | 8.94 | 9.61 | |||
| 2.63 | 1.42 | 0.81 | 5.24 | 3.10 | 1.22 | 5.08 | 1.37 | 2.41 | 2.05 | 1.15 | 1.00 | 1.15 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.L.; Yu, J.H.; Lee, J.-S. Conductivity Transitions of La0.7Sr0.3MnO3±δ and La0.6Sr0.4Co0.2Fe0.8O3−δ in Ce0.9Gd0.1O2−δ Matrix for Dual-Phase Oxygen Transport Membranes. Crystals 2021, 11, 712. https://doi.org/10.3390/cryst11060712
Pham TL, Yu JH, Lee J-S. Conductivity Transitions of La0.7Sr0.3MnO3±δ and La0.6Sr0.4Co0.2Fe0.8O3−δ in Ce0.9Gd0.1O2−δ Matrix for Dual-Phase Oxygen Transport Membranes. Crystals. 2021; 11(6):712. https://doi.org/10.3390/cryst11060712
Chicago/Turabian StylePham, Thuy Linh, Ji Haeng Yu, and Jong-Sook Lee. 2021. "Conductivity Transitions of La0.7Sr0.3MnO3±δ and La0.6Sr0.4Co0.2Fe0.8O3−δ in Ce0.9Gd0.1O2−δ Matrix for Dual-Phase Oxygen Transport Membranes" Crystals 11, no. 6: 712. https://doi.org/10.3390/cryst11060712
APA StylePham, T. L., Yu, J. H., & Lee, J.-S. (2021). Conductivity Transitions of La0.7Sr0.3MnO3±δ and La0.6Sr0.4Co0.2Fe0.8O3−δ in Ce0.9Gd0.1O2−δ Matrix for Dual-Phase Oxygen Transport Membranes. Crystals, 11(6), 712. https://doi.org/10.3390/cryst11060712







