Effect of Batchelor Flow on Polymorphic Crystallization in a Rotating Disk Crystallizer
Abstract
1. Introduction
2. Materials and Methods
3. Result and Discussion
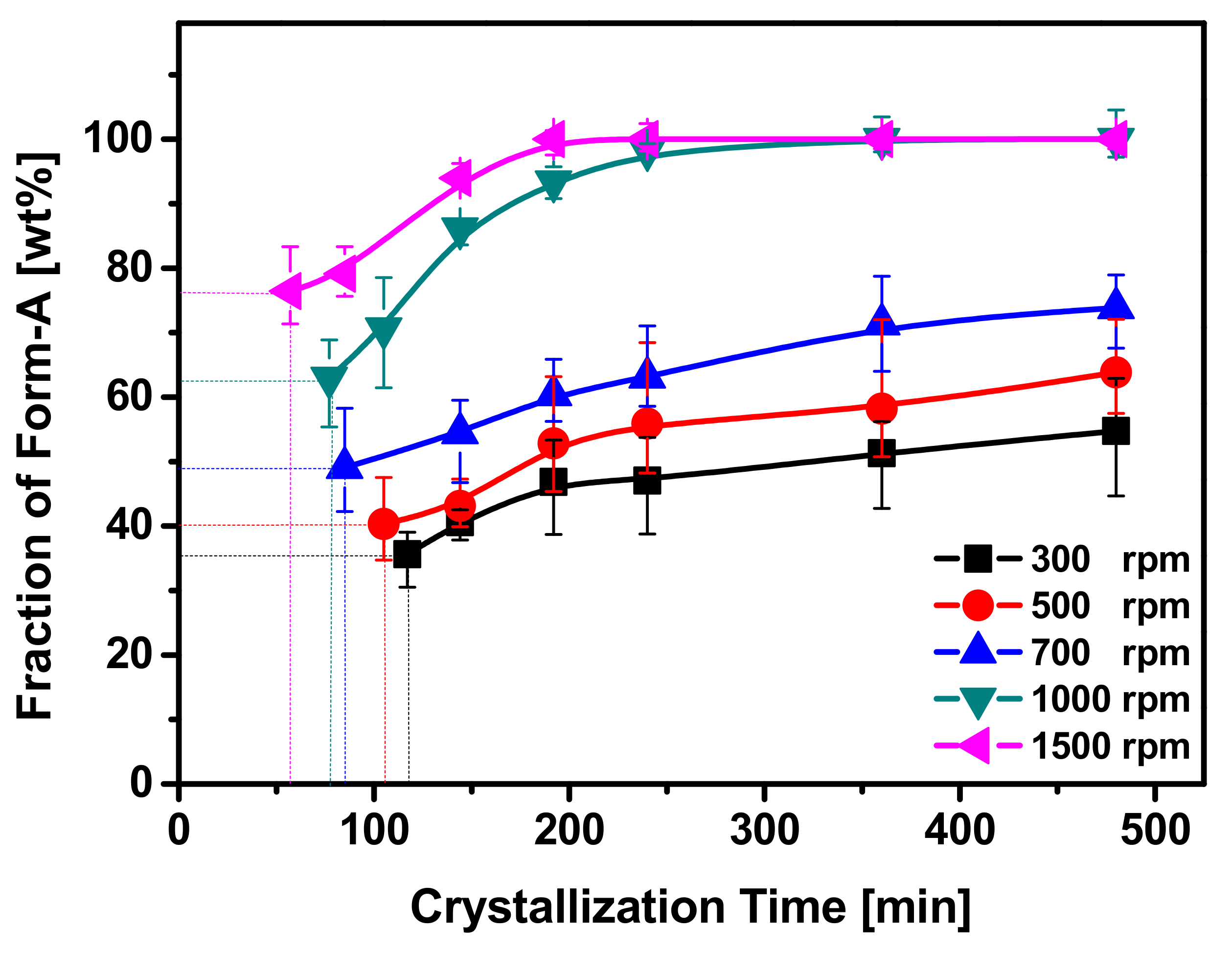
3.1. Effect of Rotation Speed
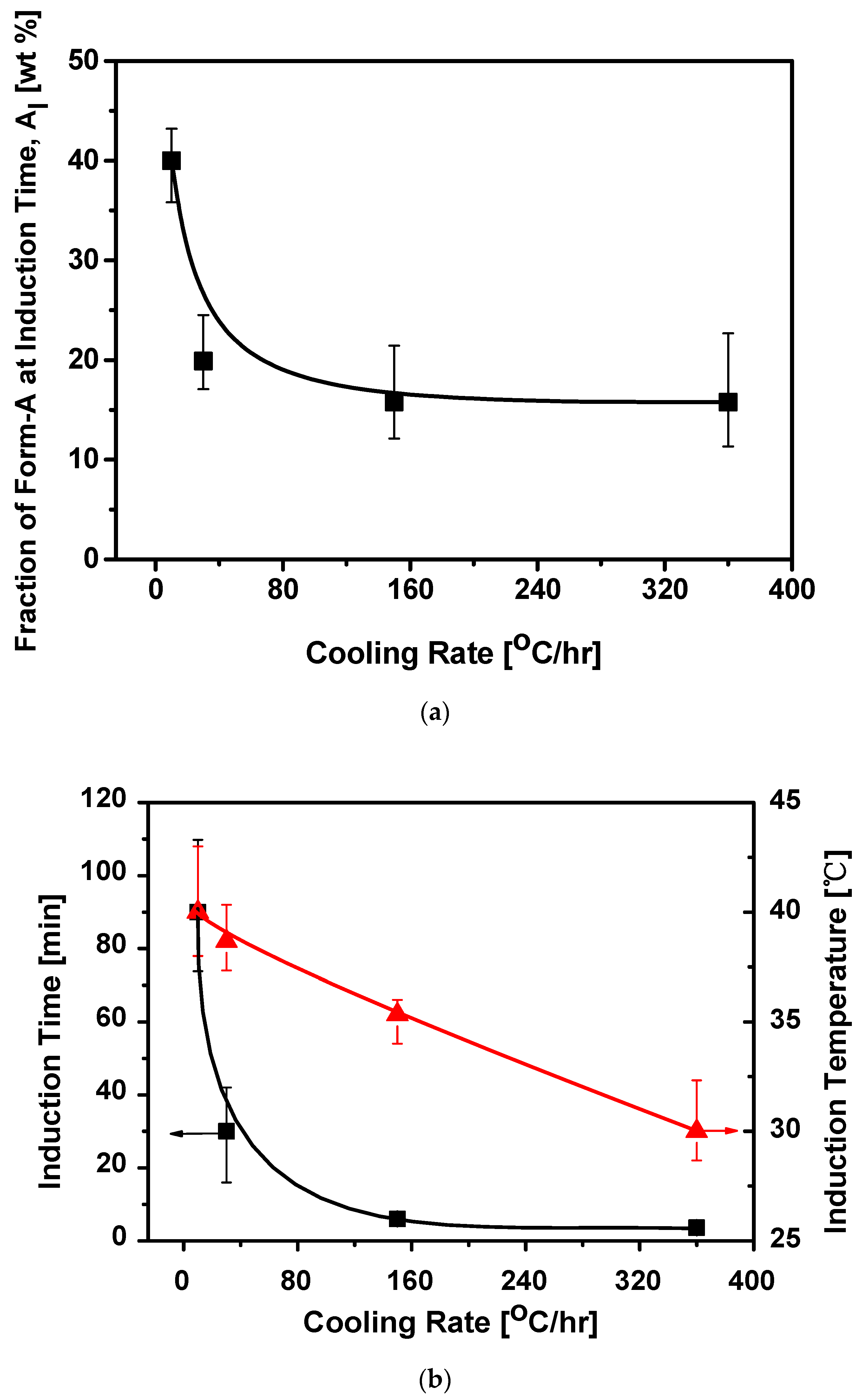
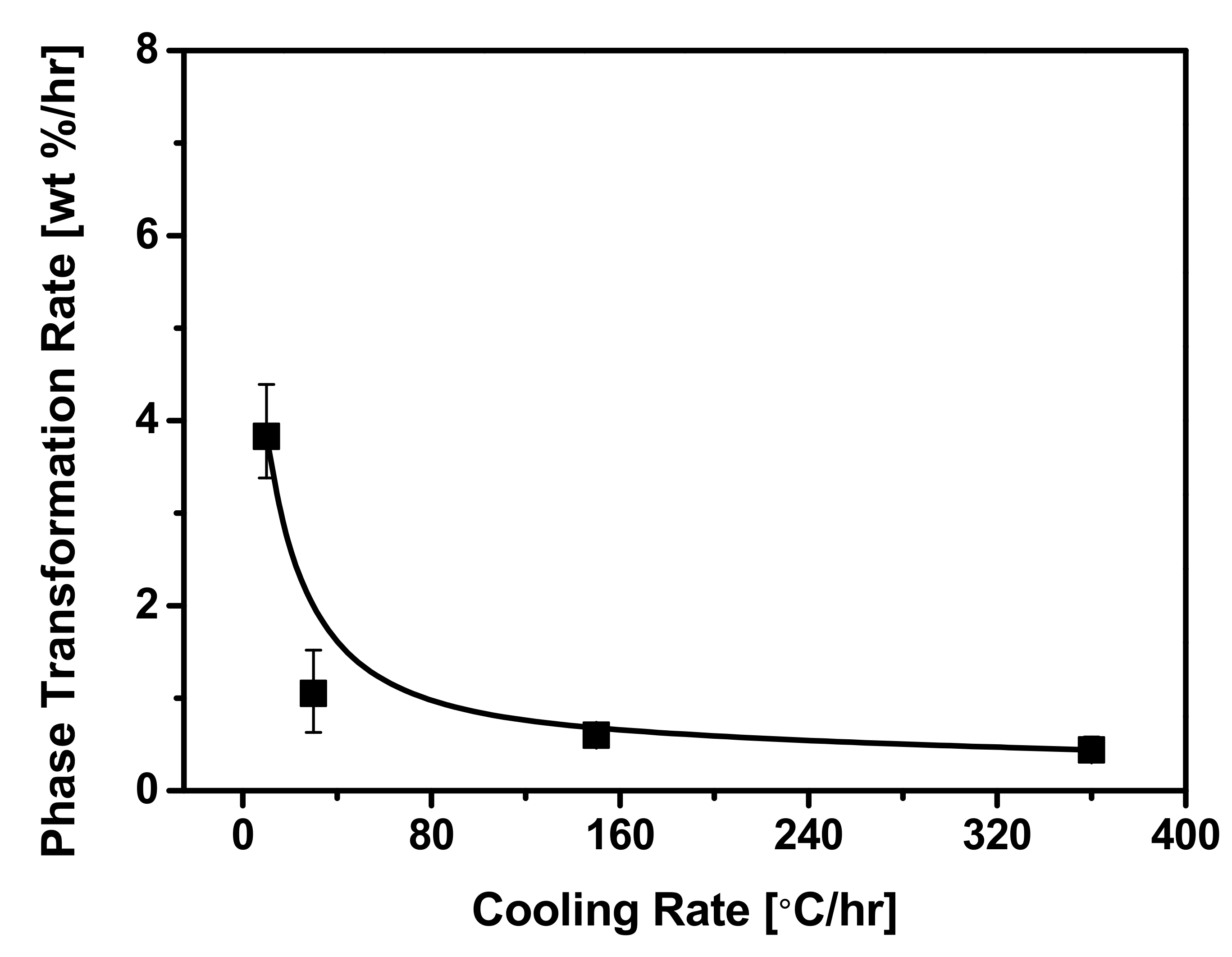
3.2. Effect of Cooling Rate
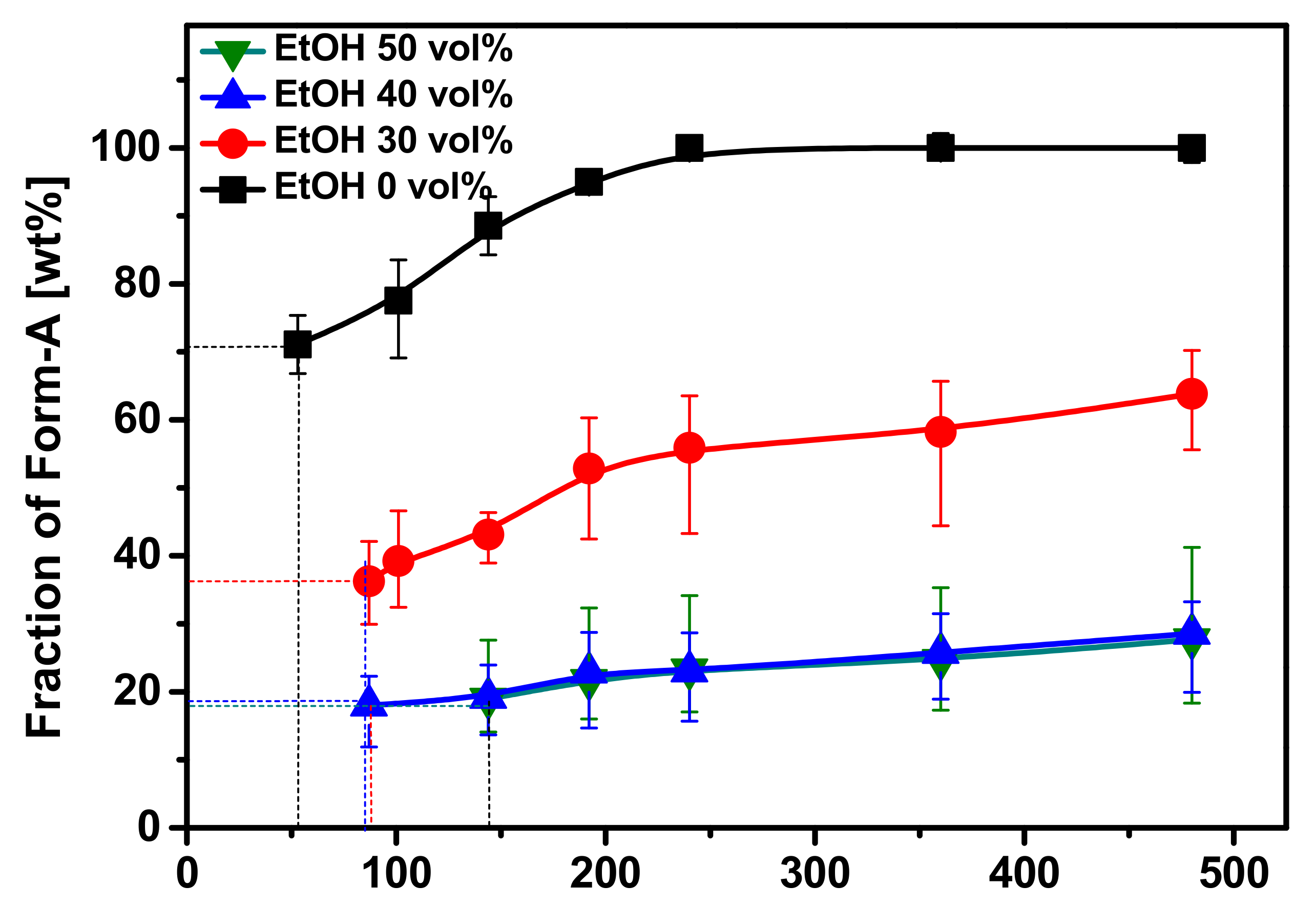
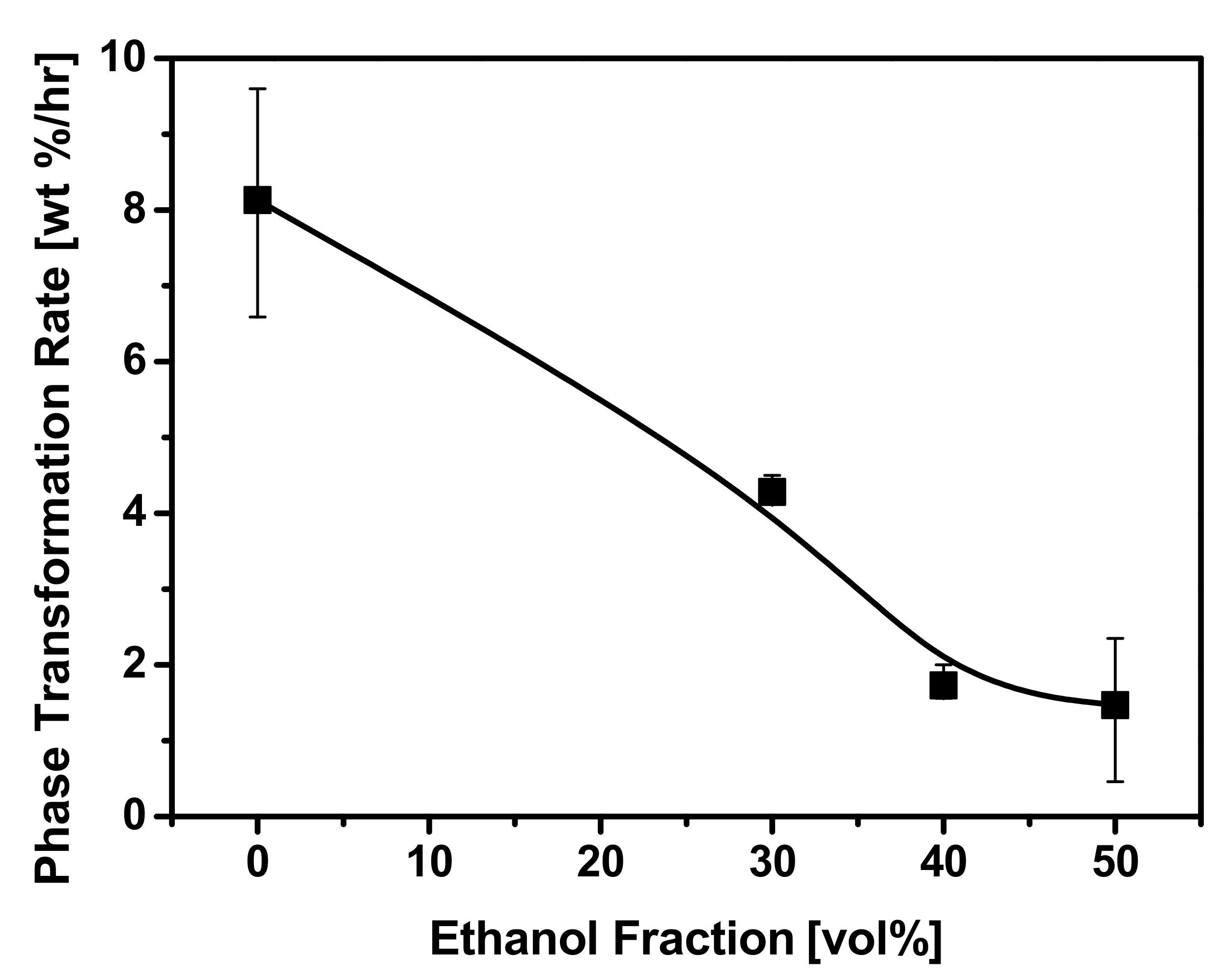
3.3. Effect of Ethanol Fraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, D.J.W. Polymorphism in Pharmaceutical Solids; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Yang, L.; Yin, Q.; Hou, B.; Wang, Y.; Bao, Y.; Wang, J.; Hao, H. Solubility and Thermodynamic Stability of the Enantiotropic Polymorphs of 2,3,5-Trimethyl-1,4-Diacetoxybenzene. Ind. Eng. Chem. Res. 2013, 52, 2477–2485. [Google Scholar] [CrossRef]
- Chong, H.; Victor, Y.J.R.; Grant, D.J.W. Polymorphic Screening: Influence of Solvent on the Rate of Solvent-Mediated Polymorphic Transformation. J. Pharm. Sci. 2001, 90, 1878–1890. [Google Scholar]
- Kitamura, M. Controlling Factor of Polymorphism in Crystallization Process. J. Cryst. Growth 2002, 237–239, 2205–2214. [Google Scholar] [CrossRef]
- Lo, E.; Mattas, E.; Wei, C.; Kacsur, D.; Chen, C.-K. Simultaneous API Particle Size Reduction and Polymorph Transformation Using High Shear. Org. Process Res. Dev. 2012, 16, 102–108. [Google Scholar] [CrossRef]
- Mazzanti, G.; Guthrie, S.E.; Sirota, E.B.; Marangoni, A.G.; Idziak, S.H.J. Orientation and Phase Transitions of Fat Crystals under Shear. Cryst. Growth Des. 2003, 3, 721–725. [Google Scholar] [CrossRef]
- Gracin, S.; Uusi-Penttilä, M.; Rasmuson, Å.C. Influence of Ultrasound on the Nucleation of Polymorphs of p-Aminobenzoic Acid. Cryst. Growth Des. 2005, 5, 1787–1794. [Google Scholar] [CrossRef]
- Caridi, A.; Kulkarni, S.A.; Di Profio, G.; Curcio, E.; ter Horst, J.H. Template-Induced Nucleation of Isonicotinamide Polymorphs. Cryst. Growth Des. 2014, 14, 1135–1141. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Srinivasan, K. Nucleation Control, Separation and Bulk Growth of Metastable α-L-Glutamic Acid Single Crystals in the Presence of L-Tyrosine. J. Cryst. Growth 2013, 364, 23–29. [Google Scholar] [CrossRef]
- Sypek, K.; Burns, I.S.; Florence, A.J.; Sefcik, J. In Situ Monitoring of Stirring Effects on Polymorphic Transformations during Cooling Crystallization of Carbamazepine. Cryst. Growth Des. 2012, 12, 4821–4828. [Google Scholar] [CrossRef]
- Liang, K.; White, G.; Wilkinson, D.; Ford, L.J.; Roberts, K.J.; Wood, W.M.L. An Examination into the Effect of Stirrer Material and Agitation Rate on the Nucleation of L-Glutamic Acid Batch Crystallized from Supersaturated Aqueous Solutions. Cryst. Growth Des. 2004, 4, 1039–1044. [Google Scholar] [CrossRef]
- Croker, D.; Hodnett, B.K. Mechanistic Features of Polymorphic Transformations: The Role of Surfaces. Cryst. Growth Des. 2010, 10, 2806–2816. [Google Scholar] [CrossRef]
- Liu, J.; Svärd, M.; Rasmuson, Å.C. Influence of Agitation and Fluid Shear on Nucleation of m-Hydroxybenzoic Acid Polymorphs. Cryst. Growth Des. 2014, 14, 5521–5531. [Google Scholar] [CrossRef]
- Li, Z.-H.; Yu, T.; Lee, T.; Kim, W.-S. Cocrystallization of Caffeine−Maleic Acid in a Batchelor Vortex Flow. Cryst. Growth Des. 2020, 20, 1618–1627. [Google Scholar] [CrossRef]
- Sun, X.; Kim, J.; Kim, W.-S. Spherical agglomeration of nickel-manganese-cobalt hydroxide in turbulent Batchelor vortex flow. Chem. Eng. J. 2021, 421, 129924. [Google Scholar] [CrossRef]
- Jung, W.; Hoon Kang, S.; Kim, K.; Kim, W.; Kyun Choi, C. Precipitation of Calcium Carbonate Particles by Gas–Liquid Reaction: Morphology and Size Distribution of Particles in Couette-Taylor and Stirred Tank Reactors. J. Cryst. Growth 2010, 312, 3331–3339. [Google Scholar] [CrossRef]
- Jung, T.; Kim, W.-S.; Choi, C.K. Effect of Non-Stoichiometry of Calcium Carbonate Precipitation in a Couette-Taylor Reactor. Cryst. Growth Des. 2004, 4, 491–495. [Google Scholar] [CrossRef]
- Mayra, Q.-P.; Kim, W.-S. Agglomeration of Ni-Rich Hydroxide in Reaction Crystallization: Effect of Taylor Vortex Dimension and Intensity. Cryst. Growth Des. 2015, 15, 1726–1734. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.H.; Kim, W.-S. Anti-Solvent Crystallization of L-Threonine in Taylor Crystallizers and MSMPR Crystallizer: Effect of Hydrodynamic Fluid Motions on Crystal Size, Shape and Recovery. J. Cryst. Growth 2017, 469, 119–127. [Google Scholar] [CrossRef]
- Nguyen, A.-T.; Kim, J.-M.; Chang, S.-M.; Kim, W.-S. Taylor Vortex Effect on Phase Transformation of Guanosine 5-Monophosphate in Drowning-Out Crystallization. Ind. Eng. Chem. Res. 2010, 49, 4865–4872. [Google Scholar] [CrossRef]
- Wu, Z.; Kim, D.H.; Kim, W.-S. Batch Cooling Crystallization in Non-Isothermal Taylor Vortex Flow: Effective Method for Controlling Crystal Size Distribution. Cryst. Growth Des. 2017, 17, 28–36. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Kim, W.-S. Polymorphic Crystallization of Sulfamerazine in Taylor Vortex Flow: Polymorphic Nucleation and Phase Transformation. Cryst. Growth Des. 2015, 15, 3617–3627. [Google Scholar] [CrossRef]
- Nguyen, A.-T.; Kim, W.-S. Influence of Feeding Mode on Cooling Crystallization of L-Lysine in Couette-Taylor Crystallizer. Korean J. Chem. Eng. 2017, 34, 2002–2010. [Google Scholar] [CrossRef]
- Nguyen, A.-T.; Kim, W.-S. Effect of Sinusoidal Taylor Vortex Flow on Cooling Crystallization of L-Lysine. Korean J. Chem. Eng. 2017, 34, 1896–1904. [Google Scholar] [CrossRef]
- Park, S.; Kim, W.-S. Influence of Fluid Motions on Polymorphic Crystallization of l-Histidine: Taylor Vortex Flow and Turbulent Eddy Flow. Cryst. Growth Des. 2018, 18, 710–722. [Google Scholar] [CrossRef]
- Batchelor, G.K. Note on a Class of Solutions of the Navier-Stokes Equations Representing Steady Rotationally Symmetric Flow. Q. J. Mech. Appl. Math. 1951, 4, 29–41. [Google Scholar] [CrossRef]
- Bödewadt, U.T. Die Drehströmung über Festem Grunde. Z. Angew. Math. Mech. 1940, 20, 241–253. [Google Scholar] [CrossRef]
- VoKármán, T.V.T. Über Laminare und Turbulente Reibung. Z. Angew. Math. Mech. 1921, 1, 233–252. [Google Scholar] [CrossRef]
- Daily, J.W.; Nece, R.E. Chamber Dimension Effects on Induced Flow and Frictional Resistance of Enclosed Rotating Disks. J. Basic Eng. 1960, 82, 217–230. [Google Scholar] [CrossRef]
- Cros, A.; Ali, R.; Le Gal, P.; Thomas, P.J.; Schouveiler, L.; Carpenter, P.W.; Chauve, M.P. Effects of Wall Compliance on the Laminar-Turbulent Transition of Torsional Couette Flow. J. Fluid Mech. 2003, 481, 177–186. [Google Scholar] [CrossRef]
- Cros, A.; Floriani, E.; Le Gal, P.; Lima, R. Transition to Turbulence of the Batchelor Flow in a Rotor/Stator Device. Eur. J. Mech. B Fluids 2005, 24, 409–424. [Google Scholar] [CrossRef]
- Visscher, F.; van der Schaaf, J.; de Croon, M.H.J.M.; Schouten, J.C. Liquid–liquid mass transfer in a rotor–stator spinning disc reactor. Chem. Eng. J. 2012, 185–186, 267–273. [Google Scholar] [CrossRef]
- Meeuwse, M.; Lempers, S.; van der Schaaf, J.; Schouten, J.C. Liquid-solid mass transfer and reaction in a rotor-stator spinning disc reactor. Ind. Eng. Chem. Res. 2010, 49, 10751–10757. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-H.; Kim, J.; Kim, W.-S. Effect of Batchelor Flow on Polymorphic Crystallization in a Rotating Disk Crystallizer. Crystals 2021, 11, 701. https://doi.org/10.3390/cryst11060701
Li Z-H, Kim J, Kim W-S. Effect of Batchelor Flow on Polymorphic Crystallization in a Rotating Disk Crystallizer. Crystals. 2021; 11(6):701. https://doi.org/10.3390/cryst11060701
Chicago/Turabian StyleLi, Zun-Hua, Jinsoo Kim, and Woo-Sik Kim. 2021. "Effect of Batchelor Flow on Polymorphic Crystallization in a Rotating Disk Crystallizer" Crystals 11, no. 6: 701. https://doi.org/10.3390/cryst11060701
APA StyleLi, Z.-H., Kim, J., & Kim, W.-S. (2021). Effect of Batchelor Flow on Polymorphic Crystallization in a Rotating Disk Crystallizer. Crystals, 11(6), 701. https://doi.org/10.3390/cryst11060701






