Synthesis and Characterization of the Amidomanganates Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis
2.3. Characterisation
3. Results and Discussion
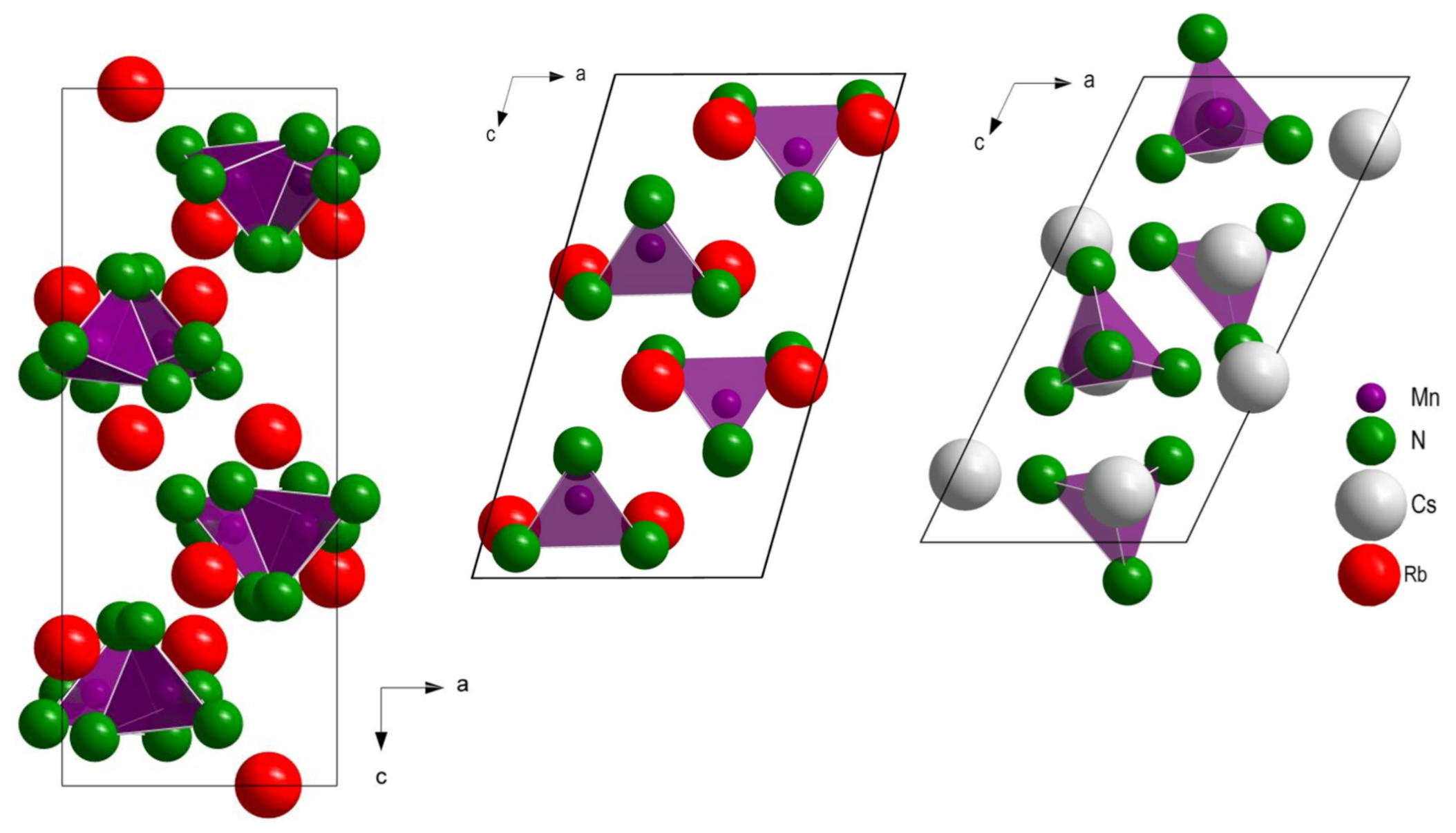
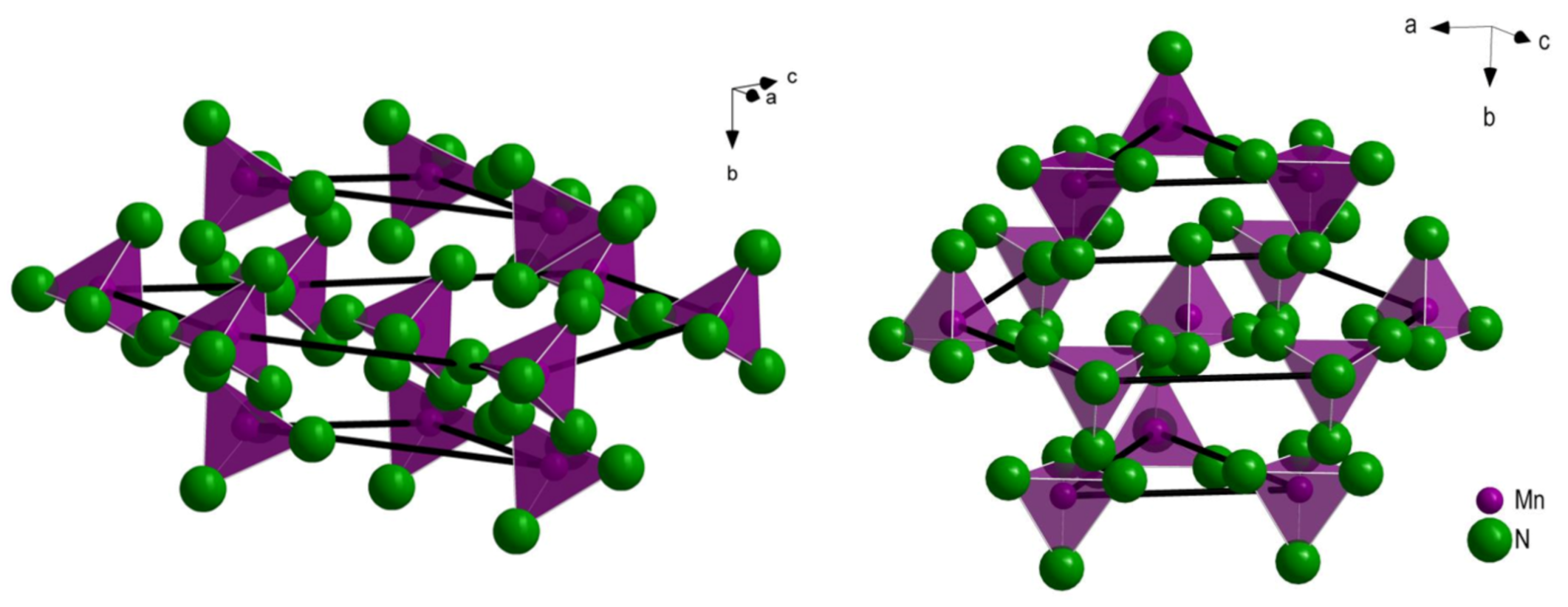
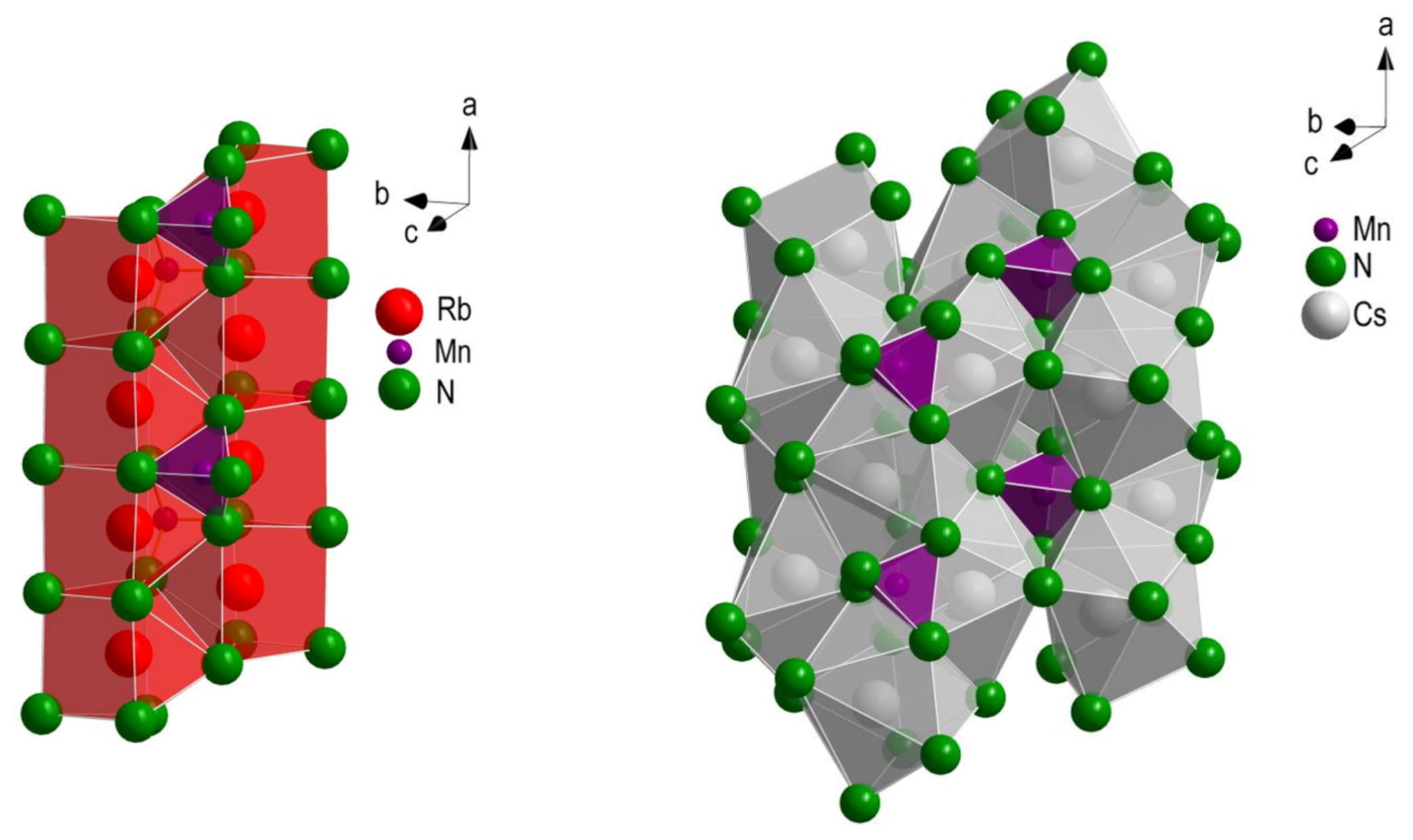
3.1. Crystal Structures of HT-Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]
3.2. Comparison of Structures of Known Alkali Metal Amidomanganates
3.3. Vibrational Spectroscopy
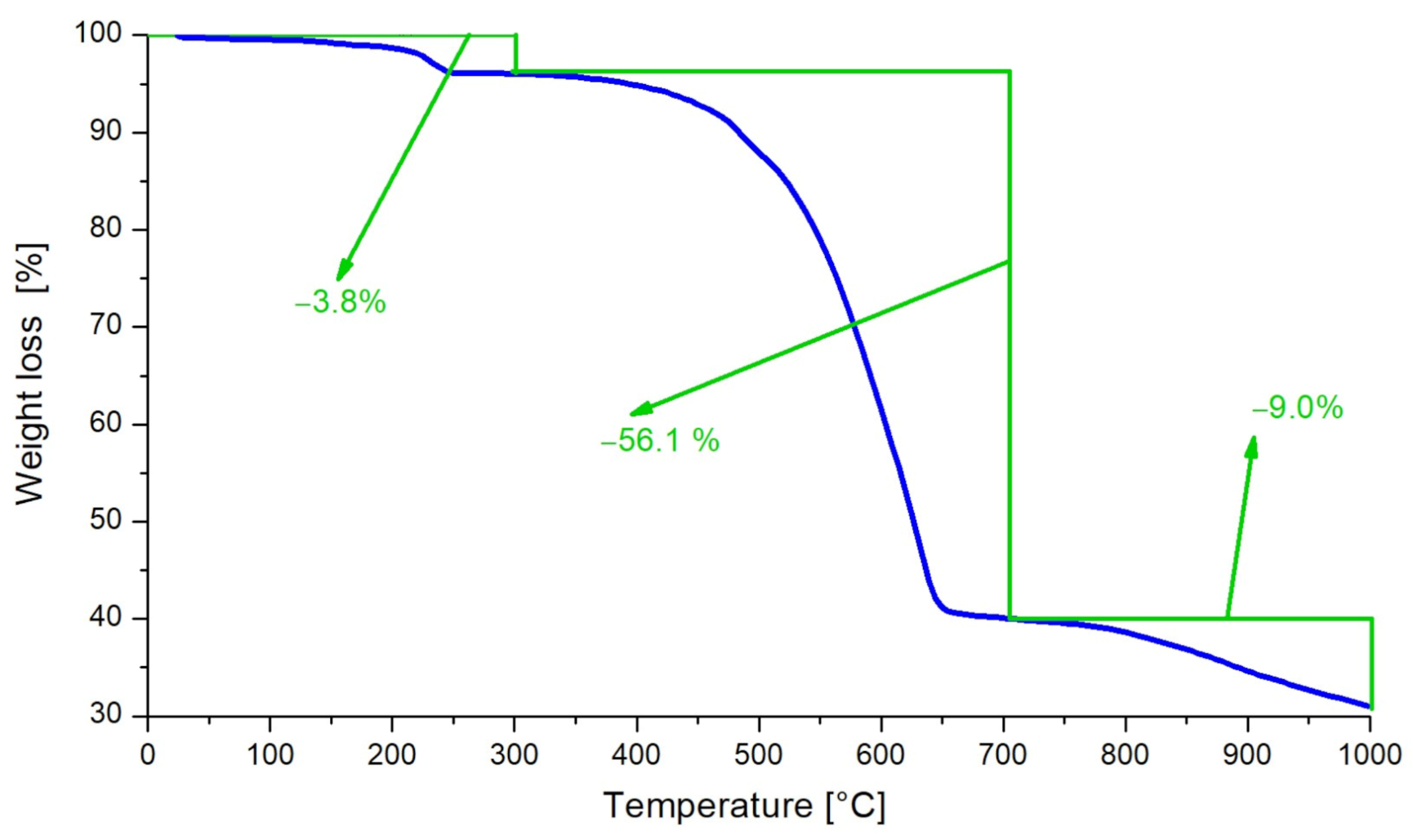
3.4. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lan, Y.C.; Chen, X.L.; Cao, Y.G.; Xu, Y.P.; Xun, L.D.; Xu, T.; Liang, J.K. Low-temperature synthesis and photoluminescence of AlN. J. Cryst. Growth 1999, 207, 247–250. [Google Scholar] [CrossRef]
- Dwiliński, R.; Baranowski, J.M.; Kamińska, M.; Doradziński, R.; Garczyński, J.; Sierzputowski, L. On GaN crystallization by ammonothermal method. Acta Phys. Pol. A 1996, 90, 763–766. [Google Scholar] [CrossRef]
- Hertrampf, J.; Becker, P.; Widenmeyer, M.; Weidenkaff, A.; Schlücker, E.; Niewa, R. Ammonothermal Crystal Growth of Indium Nitride. Cryst. Growth Des. 2018, 18, 2365–2369. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Niewa, R. Chemistry of Ammonothermal Synthesis. Inorganics 2014, 2, 29–78. [Google Scholar] [CrossRef]
- Maruska, H.Á.P.; Tietjen, J.J. The preparation and properties of vapor-deposited single-crystalline GaN. Appl. Phys. Lett. 1969, 15, 327–329. [Google Scholar] [CrossRef]
- Tietjen, J.J.; Amick, J.A. The preparation and properties of vapor-deposited epitaxial GaAs1−xPx using arsine and phosphine. J. Electrochem. Soc. 1966, 113, 724–728. [Google Scholar] [CrossRef]
- Fröhling, B.; Jacobs, H. Na2[Mn(NH2)4]: Ein neuer Schichtenstrukturtyp. Z. Anorg. Allg. Chem. 1997, 623, 1108–1112. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Ammonothermal Synthesis and Characterization of Cs2[Zn(NH2)4]. Z. Anorg. Allg. Chem. 2016, 642, 1207–1211. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Strobel, S.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Ammonothermal Synthesis and Crystal Structures of Diamminetriamidodizinc Chloride [Zn2(NH3)2(NH2)3]Cl and Diamminemonoamidozinc Bromide [Zn(NH3)2(NH2)]Br. Inorganics 2016, 4, 41. [Google Scholar] [CrossRef]
- Kreiner, G.; Jacobs, H. Magnetische Struktur von η-Mn3N2. J. Alloys Compd. 1992, 183, 345–362. [Google Scholar] [CrossRef]
- Jacobs, H.; Stüve, C. Hochdrucksynthese der η-phase im System Mn-N: Mn3N2. J. Less Common Met. 1984, 96, 323–329. [Google Scholar] [CrossRef]
- Juza, R.; Jacobs, H.; Gerke, H. Ammonothermalsynthese von Metallamiden und Metallnitriden. Ber. Bunsenges. Phys. Chem. 1966, 70, 1103–1105. [Google Scholar] [CrossRef]
- Zhang, S. Intermediates during the Formation of GaN under Ammonothermal Conditions. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2014. [Google Scholar]
- Wang, B.; Callahan, M.J. Transport growth of GaN crystals by the ammonothermal technique using various nutrients. J. Cryst. Growth 2006, 291, 455–460. [Google Scholar] [CrossRef]
- Peters, D. Ammonothermal synthesis of aluminum nitride. J. Cryst. Growth 1990, 104, 411–418. [Google Scholar] [CrossRef]
- Wang, B.; Callahan, M.; Rakes, K.; Bouthillette, L.; Wang, S.Q.; Bliss, D.; Kolis, J. Ammonothermal growth of GaN crystals in alkaline solutions. J. Cryst. Growth 2006, 287, 376–380. [Google Scholar] [CrossRef]
- Cao, H.; Guo, J.; Chang, F.; Pistidda, C.; Zhou, W.; Zhang, X.; Santoru, A.; Wu, H.; Schell, N.; Niewa, R.; et al. Transition and Alkali Metal Complex Ternary Amides for Ammonia Synthesis and Decomposition. Chem. Eur. J. 2017, 23, 9766–9771. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Zhang, S.; Niewa, R. Ammonothermal synthesis of dimorphic K2[Zn(NH2)4]. Z. Kristallogr. Cryst. Mater. 2013, 228, 351–358. [Google Scholar] [CrossRef]
- Drew, M.; Guémas, L.; Chevalier, P.; Palvadeau, P.; Rouxel, J. Etude structurale de l’amidozincate de rubidium Rb2Zn(NH2)4 et de l’amidomanganite de potassium K2Mn(NH2)4. Rev. Chim. Min. 1975, 12, 419–426. [Google Scholar]
- Bäucker, C.; Niewa, R. A New Modification of Rb[Al(NH2)4] and Condensation in Solid State. Crystals 2020, 10, 1018. [Google Scholar] [CrossRef]
- Cao, H.; Santoru, A.; Pistidda, C.; Richter, T.M.; Chaudhary, A.L.; Gizer, G.; Niewa, R.; Chen, P.; Klassen, T.; Dornheim, M. New synthesis route for ternary transition metal amides as well as ultrafast amide–hydride hydrogen storage materials. Chem. Commun. 2016, 52, 5100–5103. [Google Scholar] [CrossRef]
- Alt, N.S.A.; Meissner, E.; Schluecker, E. Development of a novel in situ monitoring technology for ammonothermal reactors. J. Cryst. Growth 2012, 350, 2–4. [Google Scholar] [CrossRef]
- Hüttig, G.F. Apparat zur gleichzeitigen Druck- und Raummessung von Gasen.(Tensi-Eudiometer.). Z. Anorg. Allg. Chem. 1920, 114, 161–173. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.; Birkenbeul, J.; Schmitz, D. Strukturverwandtschaft des Dicaesiumamidomagnesats, Cs2[Mg(NH2)4], zum β-K2SO4-Typ. J. Less Common Met. 1982, 85, 79–86. [Google Scholar] [CrossRef]
- Jacobs, H.; Birkenbeul, J.; Kockelkorn, J. Darstellung und Eigenschaften der Amidomagnesate des Kaliums und Rubidiums K2[Mg(NH2)4]- und Rb2[Mg(NH2)4]-Verbindungen mit isolierten [Mg(NH2)4]2−-Tetraedern. J. Less Common Met. 1984, 97, 205–214. [Google Scholar] [CrossRef]
- Hertrampf, J.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Three Solid Modifications of Ba[Ga(NH2)4]2: A Soluble Intermediate in Ammonothermal GaN Crystal Growth. Eur. J. Inorg. Chem. 2017, 2017, 902–909. [Google Scholar] [CrossRef]
- Liu, A.; Song, Y. In Situ High-Pressure Study of Sodium Amide by Raman and Infrared Spectroscopies. J. Phys. Chem. B 2011, 115, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.; Jänichen, K. Darstellung und Kristallstruktur von Tetraamidoaluminaten des Rubidiums und Caesiums. J. Less Common Met. 1990, 159, 315–325. [Google Scholar] [CrossRef]
- Jacobs, H.; Nöcker, B. Neubestimmung von Struktur und Eigenschaften isotyper Natriumtetraamidometallate des Aluminiums und Galliums. Z. Anorg. Allg. Chem. 1993, 619, 381–386. [Google Scholar] [CrossRef]

| Ref. [17] | HT-Rb2[Mn(NH2)4] | Cs2[Mn(NH2)4] | |
|---|---|---|---|
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | |||
| a/pm | 785.36(5) | 779.45(3) | 704.25(2) |
| b/pm | 694.49(5) | 702.04(2) | 942.06(2) |
| c/pm | 1384.11(11) | 1408.48(5) | 1396.04(4) |
| /deg. | 106.389(7) | 106.004(2) | 115.590(2) |
| V/ pm | 724.24(12) | 740.86(4) | 819.19(4) |
| Density/g cm | 2.600 | 3.121 | |
| Z | 4 | 4 | |
| F(000) | 540 | 684 | |
| range | −10–9/−7–9/−16–18 | // | |
| 2/deg. | 54.93 | 54.99 | |
| /mm | 14.74 | 10.29 | |
| Refl. meas./independent | 12333/1690 | 21819/1876 | |
| 0.0489/0.1028/1.091 | 0.0369/0.0840/1.106 | ||
| / | 0.0748/0.0375 | 0.0497/0.0192 | |
| Refined parameters | 65 | 65 | |
| Extinction coefficient | 0.00340 | 0.00614 | |
| /K | 429 | 293 | 293 |
| Largest electron | −0.70/0.67 | −1.69/1.66 | |
| difference min/max/Å |
| Atom | Wyckoff Site | ||||
|---|---|---|---|---|---|
| Rb(1) | 0.07762(6) | 0.16605(7) | 0.89877(4) | 0.0412(2) | |
| Rb(2) | 0.56877(6) | 0.15528(7) | 0.89187(3) | 0.0399(2) | |
| Mn | 0.70622(9) | 0.19650(9) | 0.65510(5) | 0.0286(2) | |
| N(1) | 0.4345(5) | 0.2200(6) | 0.5646(3) | 0.0363(9) | |
| N(2) | 0.8787(5) | 0.2224(6) | 0.5601(3) | 0.0361(9) | |
| N(3) | 0.7565(6) | 0.0679(6) | 0.2525(3) | 0.0478(12) | |
| N(4) | 0.2453(6) | 0.0517(6) | 0.2587(3) | 0.0532(13) | |
| Cs(1) | 0.04594(6) | 0.03197(4) | 0.35455(3) | 0.0433(2) | |
| Cs(2) | 0.34299(8) | 0.15821(5) | 0.10823(4) | 0.0573(2) | |
| Mn | 0.65076(13) | 0.24799(9) | 0.42175(7) | 0.0325(2) | |
| N(1) | 0.6317(8) | 0.0223(5) | 0.4028(4) | 0.0401(11) | |
| N(2) | 0.7880(9) | 0.1695(7) | 0.8243(5) | 0.0558(15) | |
| N(3) | 0.3435(9) | 0.1677(6) | 0.8648(5) | 0.0565(15) | |
| N(4) | 0.8481(9) | 0.1940(6) | 0.0827(5) | 0.0507(13) |
| Distance | Rb2[Mn(NH2)4] | Cs2[Mn(NH2)4] | Angle | Rb2[Mn(NH2)4] | Cs2[Mn(NH2)4] | ||
|---|---|---|---|---|---|---|---|
| Rb(1) | Rb(2) | Cs(1) | Cs(2) | ||||
| A–N | 316.9(4) | 300.5(4) | 315.2(5) | 333.5(7) | N(1)–Mn–N(2) | 107.542(2) | 108.174(1) |
| 318.2(4) | 303.0(4) | 325.9(5) | 336.6(6) | N(1)–Mn–N(3) | 107.435(1) | 109.152(1) | |
| 320.1(4) | 311.8(4) | 327.5(6) | 344.1(5) | N(1)–Mn–N(4) | 113.428(1) | 111.297(1) | |
| 322.9(5) | 319.0(4) | 329.8(6) | 346.0(6) | N(2)–Mn–N(3) | 107.734(1) | 107.763(1) | |
| 324.2(5) | 322.6(5) | 348.7(6) | 363.3(7) | N(2)–Mn–N(4) | 112.875(1) | 106.921(1) | |
| 330.9(5) | 336.9(5) | 360.1(6) | 370.6(6) | N(3)–Mn–N(4) | 107.558(1) | 113.329(1) | |
| 334.0(4) | 339.1(5) | 366.6(6) | 373.7(6) | ||||
| 395.1(1) | 401.3(1) | 369.4(6) | 386.7(6) | ||||
| 388.7(6) | 387.6(7) | ||||||
| 407.5(1) | |||||||
| 415.6(1) | |||||||
| Mn–N(4) | 209.8(4) | 210.7(5) | |||||
| Mn–N(3) | 211.6(4) | 211.3(5) | |||||
| Mn–N(2) | 215.0(4) | 210.5(6) | |||||
| Mn–N(1) | 215.6(4) | 213.9(5) | |||||
| HT-Rb2[Mn(NH2)4] | Cs2[Mn(NH2)4] | |
|---|---|---|
| Valence modes | ||
| 3342 | 3297 | |
| 3327 | 3248 | |
| 3275 | 3236 | |
| 3217 | ||
| Bending modes | ||
| 1549 | 1538 | |
| 1529 | 1525 | |
| 1516 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bäucker, C.; Bauch, S.; Niewa, R. Synthesis and Characterization of the Amidomanganates Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]. Crystals 2021, 11, 676. https://doi.org/10.3390/cryst11060676
Bäucker C, Bauch S, Niewa R. Synthesis and Characterization of the Amidomanganates Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]. Crystals. 2021; 11(6):676. https://doi.org/10.3390/cryst11060676
Chicago/Turabian StyleBäucker, Christian, Soeren Bauch, and Rainer Niewa. 2021. "Synthesis and Characterization of the Amidomanganates Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]" Crystals 11, no. 6: 676. https://doi.org/10.3390/cryst11060676
APA StyleBäucker, C., Bauch, S., & Niewa, R. (2021). Synthesis and Characterization of the Amidomanganates Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]. Crystals, 11(6), 676. https://doi.org/10.3390/cryst11060676