DFT Study of Electronic Structure and Optical Properties of Kaolinite, Muscovite, and Montmorillonite
Abstract
1. Introduction
2. Methods
3. Results and Discussion
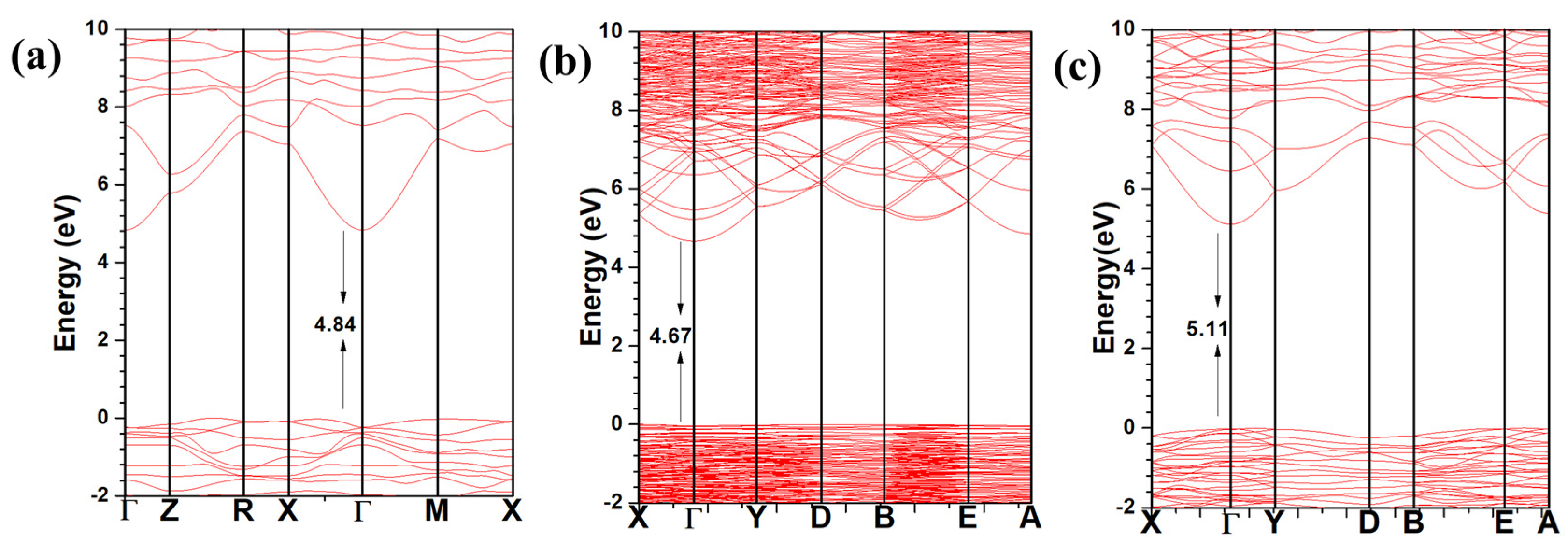
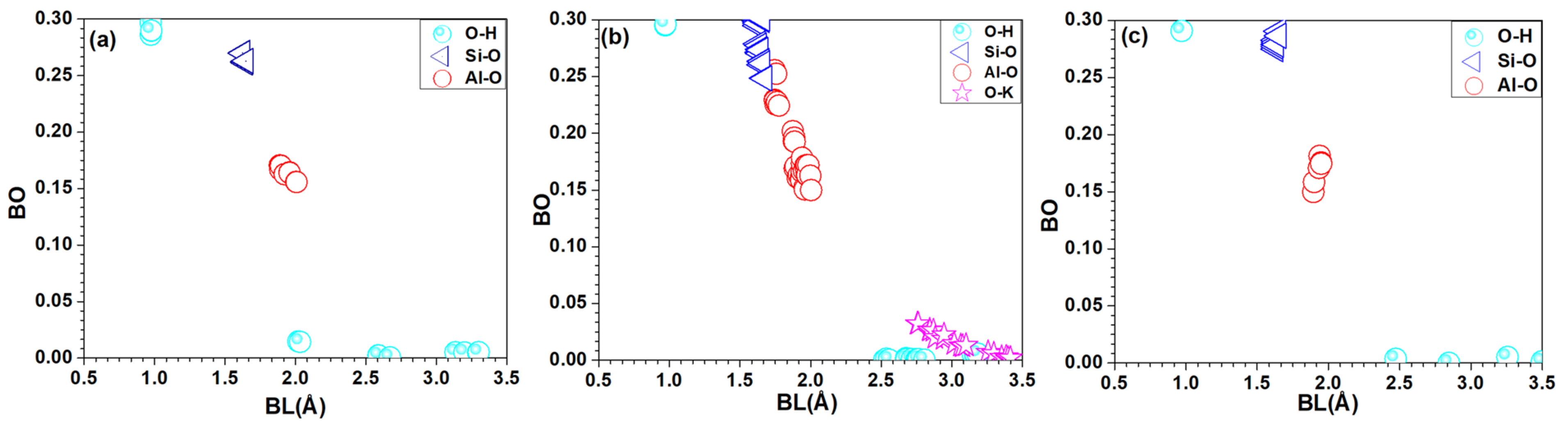
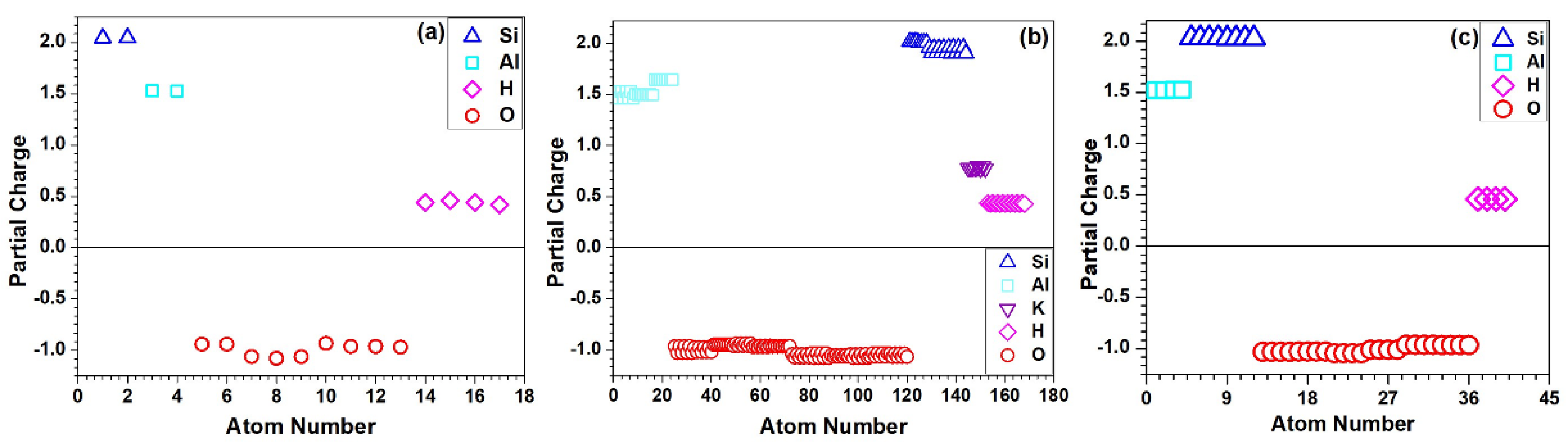
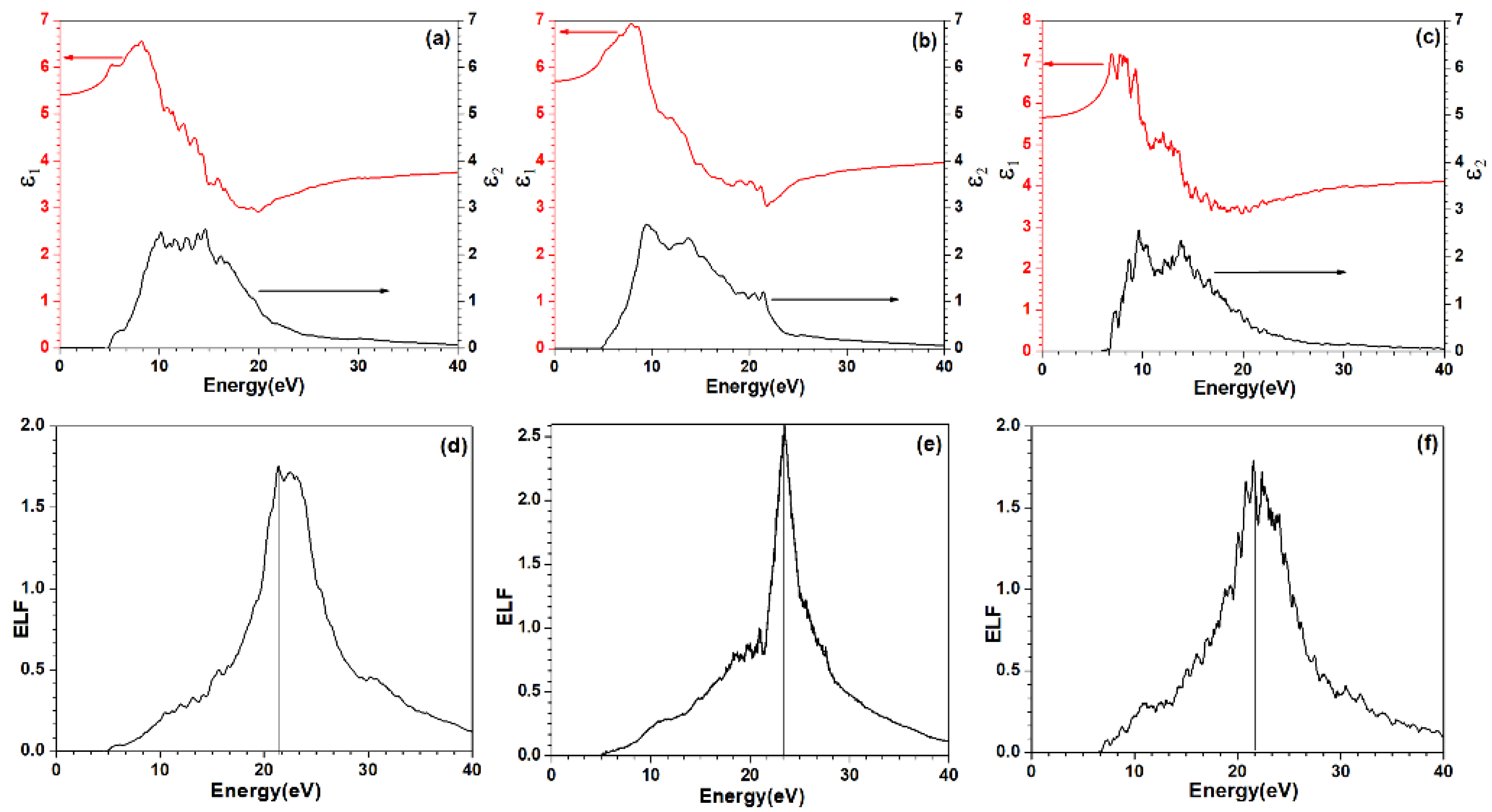
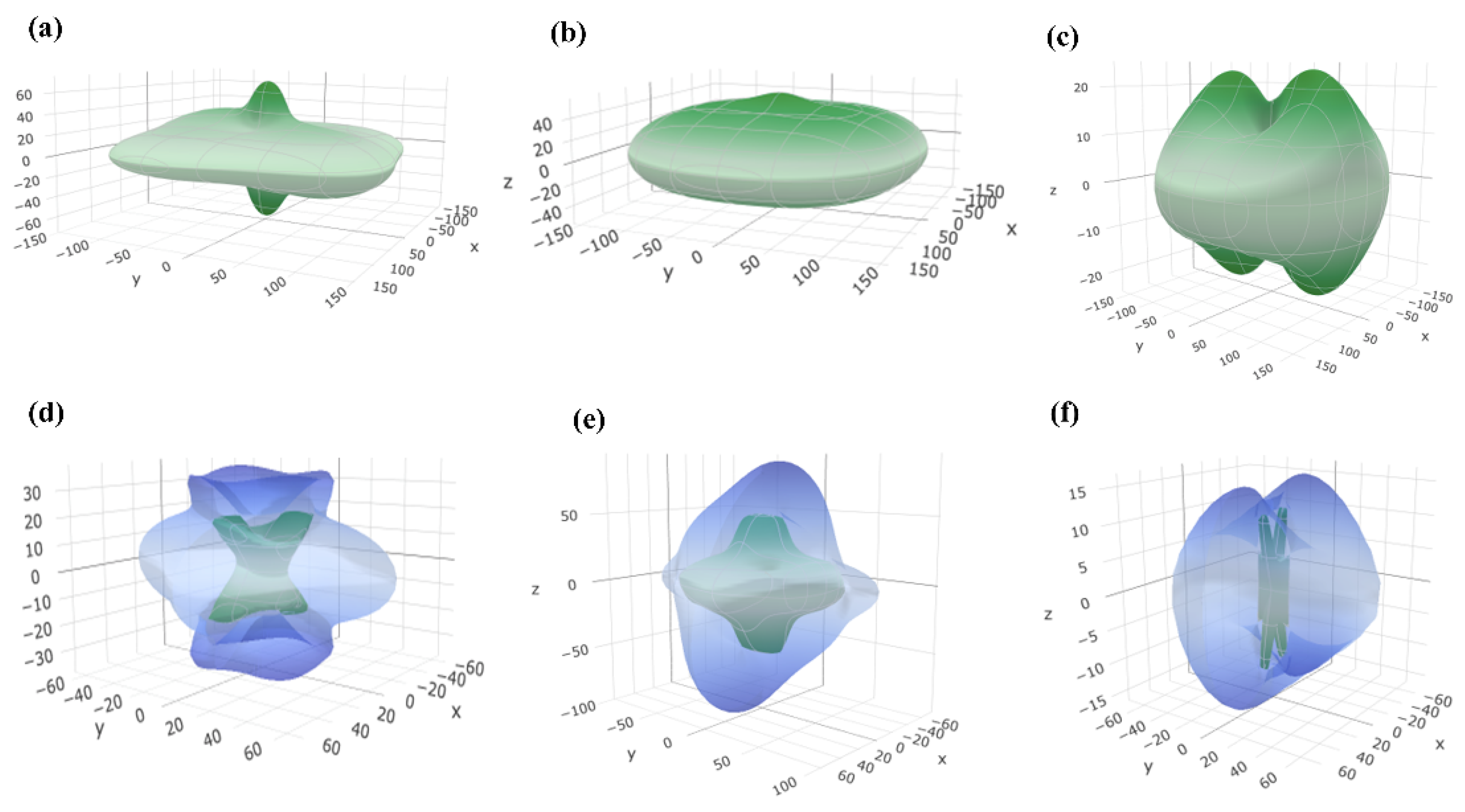
3.1. Electronic Structure and Interatomic Bonding
3.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brindley, G.W. CLAYS, CLAY MINERALSClays, clay minerals. In Mineralogy; Springer: Boston, MA, USA, 1983; pp. 69–80. [Google Scholar]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Detellier, C. Functional kaolinite. Chem. Rec. 2018, 18, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Ö.; Altunkaynak, Y.; Güzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Gehr, P.; Heyder, J. Particle-Lung Interactions; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Gianni, E.; Avgoustakis, K.; Papoulis, D. Kaolinite group minerals: Applications in cancer diagnosis and treatment. Eur. J. Pharm. Biopharm. 2020, 154, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Trevino, J.C.; Coles, C.A. Kaolinite properties, structure and influence of metal retention on pH. Appl. Clay Sci. 2003, 23, 133–139. [Google Scholar] [CrossRef]
- Liang, J.-J.; Hawthorne, F.C. Rietveld refinement of micaceous materials; muscovite-2M 1, a comparison with single-crystal structure refinement. Can. Mineral. 1996, 34, 115–122. [Google Scholar]
- Wang, J.; Kalinichev, A.G.; Kirkpatrick, R.J.; Cygan, R.T. Structure, energetics, and dynamics of water adsorbed on the muscovite (001) surface: A molecular dynamics simulation. J. Phys. Chem. B 2005, 109, 15893–15905. [Google Scholar] [CrossRef]
- Man-Chao, H.; Zhi-Jie, F.; Ping, Z. Atomic and electronic structures of montmorillonite in soft rock. Chin. Phys. B 2009, 18, 2933. [Google Scholar] [CrossRef]
- Murray, H.H. Overview—clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- Toth, R.; Voorn, D.-J.; Handgraaf, J.-W.; Fraaije, J.G.; Fermeglia, M.; Pricl, S.; Posocco, P. Multiscale computer simulation studies of water-based montmorillonite/poly (ethylene oxide) nanocomposites. Macromolecules 2009, 42, 8260–8270. [Google Scholar] [CrossRef]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Cambridge, MA, USA, 2019; pp. 113–127. [Google Scholar]
- Hess, A.C.; Saunders, V.R. Periodic ab initio Hartree-Fock calculations of the low-symmetry mineral kaolinite. J. Phys. Chem. 1992, 96, 4367–4374. [Google Scholar] [CrossRef]
- Radoslovich, E. The structure of muscovite, KAl2(Si3Al)O10(OH)2. Acta Crystallogr. 1960, 13, 919–932. [Google Scholar] [CrossRef]
- Subramanian, N.; Whittaker, M.L.; Ophus, C.; Lammers, L.N. Structural Implications of Interfacial Hydrogen Bonding in Hydrated Wyoming-Montmorillonite Clay. J. Phys. Chem. C 2020, 124, 8697–8705. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Vienna Ab-Initio Aimulation Package (vasp). Available online: https://www.vasp.at/ (accessed on 26 May 2021).
- Ching, W.-Y.; Rulis, P. Electronic Structure Methods for Complex Materials: The Orthogonalized Linear Combination of Atomic Orbitals; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Nielsen, O.; Martin, R.M. First-principles calculation of stress. Phys. Rev. Lett. 1983, 50, 697. [Google Scholar] [CrossRef]
- Yao, H.; Ouyang, L.; Ching, W.Y. Ab initio calculation of elastic constants of ceramic crystals. J. Am. Ceram. Soc. 2007, 90, 3194–3204. [Google Scholar] [CrossRef]
- Reuß, A. Berechnung der fließgrenze von mischkristallen auf grund der plastizitätsbedingung für einkristalle. Zamm J. Appl. Math. Mech. /Z. Für Angew. Math. Und Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Richard, D.; Rendtorff, N.M. First principles study of structural properties and electric field gradients in kaolinite. Appl. Clay Sci. 2019, 169, 67–73. [Google Scholar] [CrossRef]
- Yu, C.-J.; Choe, S.-H.; Jang, Y.-M.; Jang, G.-H.; Pae, Y.-H. First-principles study of organically modified muscovite mica with ammonium () or methylammonium (CH3) ion. J. Mater. Sci. 2016, 51, 10806–10818. [Google Scholar] [CrossRef]
- Davidson, A.; Vickers, A. The optical properties of mica in the vacuum ultraviolet. J. Phys. C Solid State Phys. 1972, 5, 879. [Google Scholar] [CrossRef]
- Fang, Z.-J.; Gou, K.-Y.; Mo, M.; Zeng, J.-S.; He, H.; Zhou, X.; Li, H. First-principle study of electronic structure of montmorillonite at high pressure. Mod. Phys. Lett. B 2020, 34, 2050263. [Google Scholar] [CrossRef]
- Dharmawardhana, C.; Misra, A.; Ching, W.-Y. Quantum mechanical metric for internal cohesion in cement crystals. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Mondol, N.; Jahren, J.; Bjørlykke, K.; Brevik, I. Elastic properties of clay minerals. Geophysics 2008, 27, 758–770. [Google Scholar] [CrossRef]
- Vanorio, T.; Prasad, M.; Nur, A. Elastic properties of dry clay mineral aggregates, suspensions and sandstones. Geophys. J. Int. 2003, 155, 319–326. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Cates, M.E. Effective elastic properties of solid clays. Geophysics 2001, 66, 428–440. [Google Scholar] [CrossRef]
- Tan, B.T.; Wu, S.; Anariba, F.; Wu, P. A DFT study on brittle-to-ductile transition of D022-TiAl3 using multi-doping and strain-engineered effects. J. Mater. Sci. Technol. 2020, 51, 180–192. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Zhao, Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Gaillac, R.; Pullumbi, P.; Coudert, F.-X. ELATE: An open-source online application for analysis and visualization of elastic tensors. J. Phys. Condens. Matter 2016, 28, 275201. [Google Scholar] [CrossRef]


| Crystal | Chemical Formula | No. Atoms (Space Group) | a, b, c (Å) α, β, γ |
|---|---|---|---|
| Kaolinite | Al2Si2O5(OH)4 | 17 (P1) | 5.19, 5.18, 7.54 77.84°, 84.31°, 60.10° a. 5.15, 5.15, 7.41 75.14°, 84.12°, 60.18° |
| Muscovite | 8[KAl2(Si3AlO10)(OH)2] | 168 (C12/c1) | 10.47, 9.10, 20.68 90°, 96.20°, 90° b. 5.19, 9.00 20.10 90°, 95.18°, 90° |
| MMT | 2[Al2Si4O10(OH)2] | 40 (C121) | 5.21, 9.06, 10.27 90°, 99.46°, 90° c. 5.18, 8.97, 10.07 90°, 99.50°, 90° |
| Crystal | Vol (Å3) | Bond | PBOD (Electron/Å3) | TBO | TBOD |
|---|---|---|---|---|---|
| Kaolinite | 171.91 | O-H | 0.007 | 5.446 | 0.032 |
| Si-O | 0.013 | ||||
| Al-O | 0.012 | ||||
| Muscovite | 1957.66 | O-H | 0.003 | 57.096 | 0.031 |
| Si-O | 0.015 | ||||
| Al-O | 0.013 | ||||
| O-K | 0.001 | ||||
| Al-H | 0.000 | ||||
| MMT | 478.36 | O-H | 0.003 | 14.273 | 0.030 |
| Si-O | 0.019 | ||||
| Al-O | 0.008 |
| Crystal | K(GPa) | G(GPa) | E(GPa) | η | G/K | HV(GPa) |
|---|---|---|---|---|---|---|
| Kaolinite | 46.93 | 31.83 | 77.88 | 0.2235 | 0.6782 | 6.853 |
| Muscovite | 53.47 | 38.24 | 92.64 | 0.2112 | 0.7152 | 8.293 |
| MMT | 31.85 | 23.74 | 57.049 | 0.2015 | 0.7453 | 6.198 |
| Crystal | |||
|---|---|---|---|
| Kaolinite | 2.58 | 5885 | 3512 |
| Muscovite | 2.71 | 6208 | 3756 |
| MMT | 2.76 | 4797 | 2933 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafei, L.; Adhikari, P.; Ching, W.-Y. DFT Study of Electronic Structure and Optical Properties of Kaolinite, Muscovite, and Montmorillonite. Crystals 2021, 11, 618. https://doi.org/10.3390/cryst11060618
Shafei L, Adhikari P, Ching W-Y. DFT Study of Electronic Structure and Optical Properties of Kaolinite, Muscovite, and Montmorillonite. Crystals. 2021; 11(6):618. https://doi.org/10.3390/cryst11060618
Chicago/Turabian StyleShafei, Layla, Puja Adhikari, and Wai-Yim Ching. 2021. "DFT Study of Electronic Structure and Optical Properties of Kaolinite, Muscovite, and Montmorillonite" Crystals 11, no. 6: 618. https://doi.org/10.3390/cryst11060618
APA StyleShafei, L., Adhikari, P., & Ching, W.-Y. (2021). DFT Study of Electronic Structure and Optical Properties of Kaolinite, Muscovite, and Montmorillonite. Crystals, 11(6), 618. https://doi.org/10.3390/cryst11060618






