Effect of Ni2+ on Lithium-Ion Diffusion in Layered LiNi1−x−yMnxCoyO2 Materials
Abstract
1. Introduction
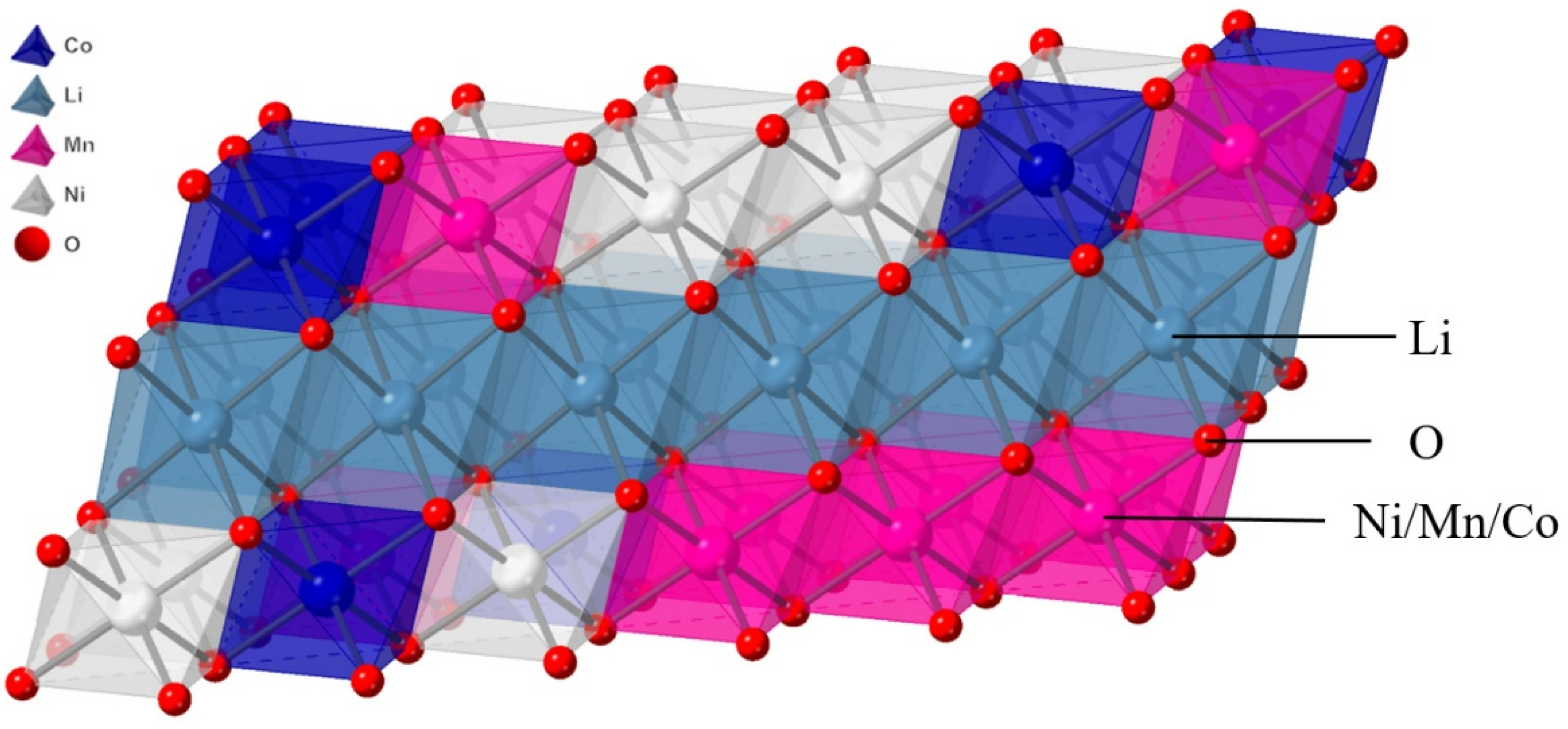
2. Simulation Method
3. Results and Discussion
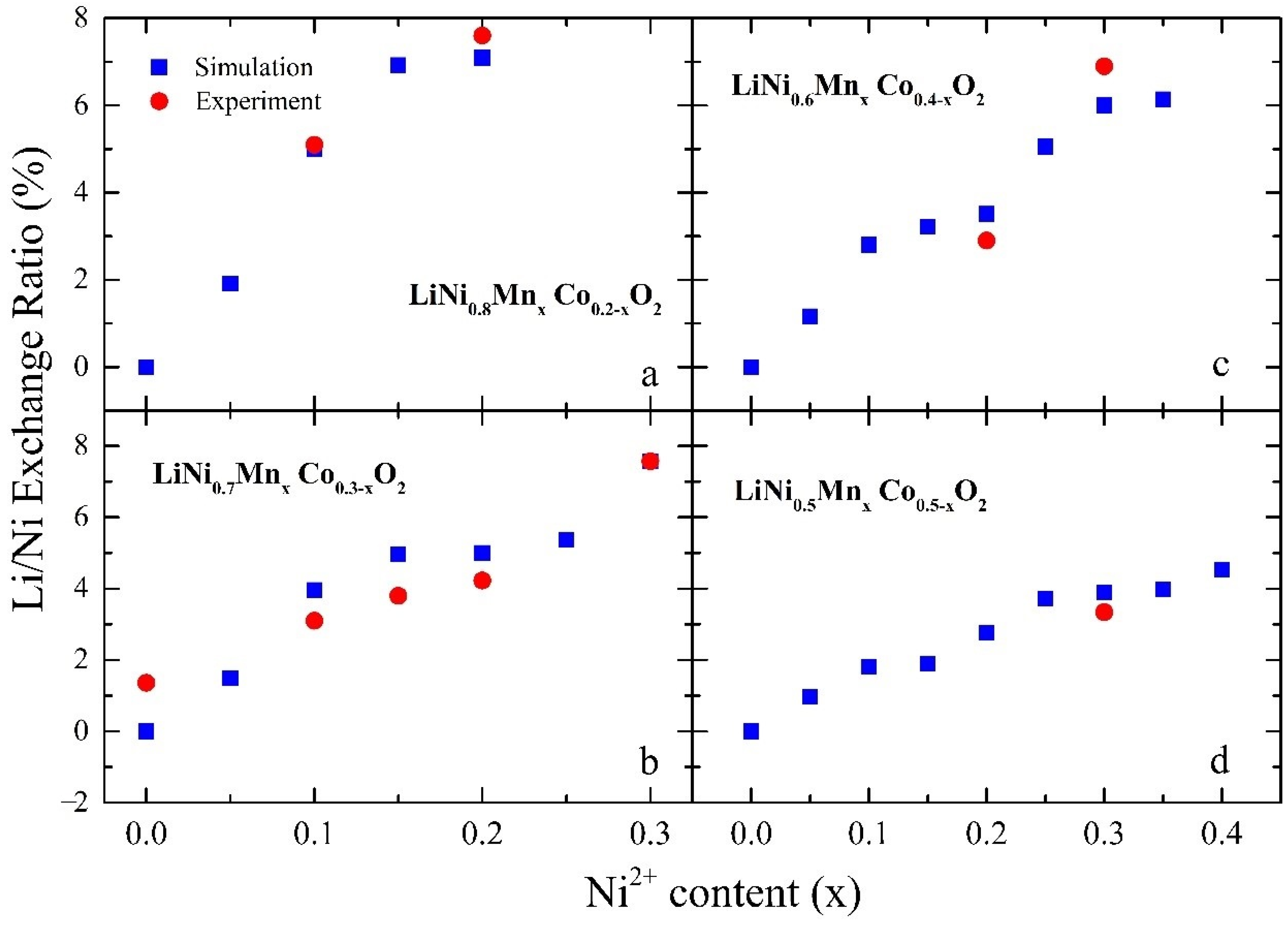
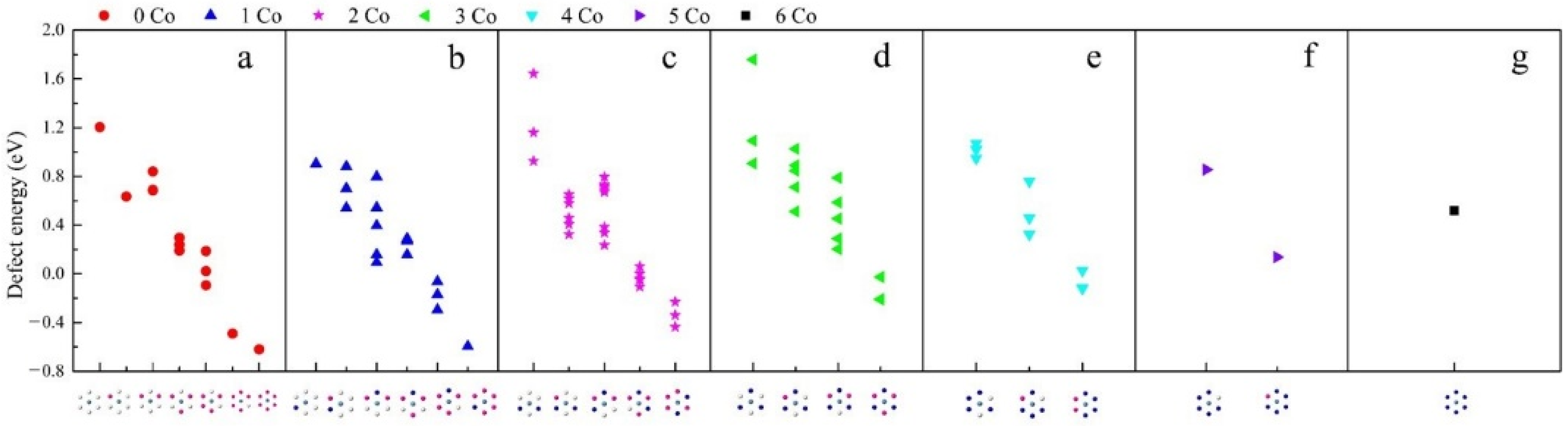
3.1. Effect of on Li-Ion Diffusion
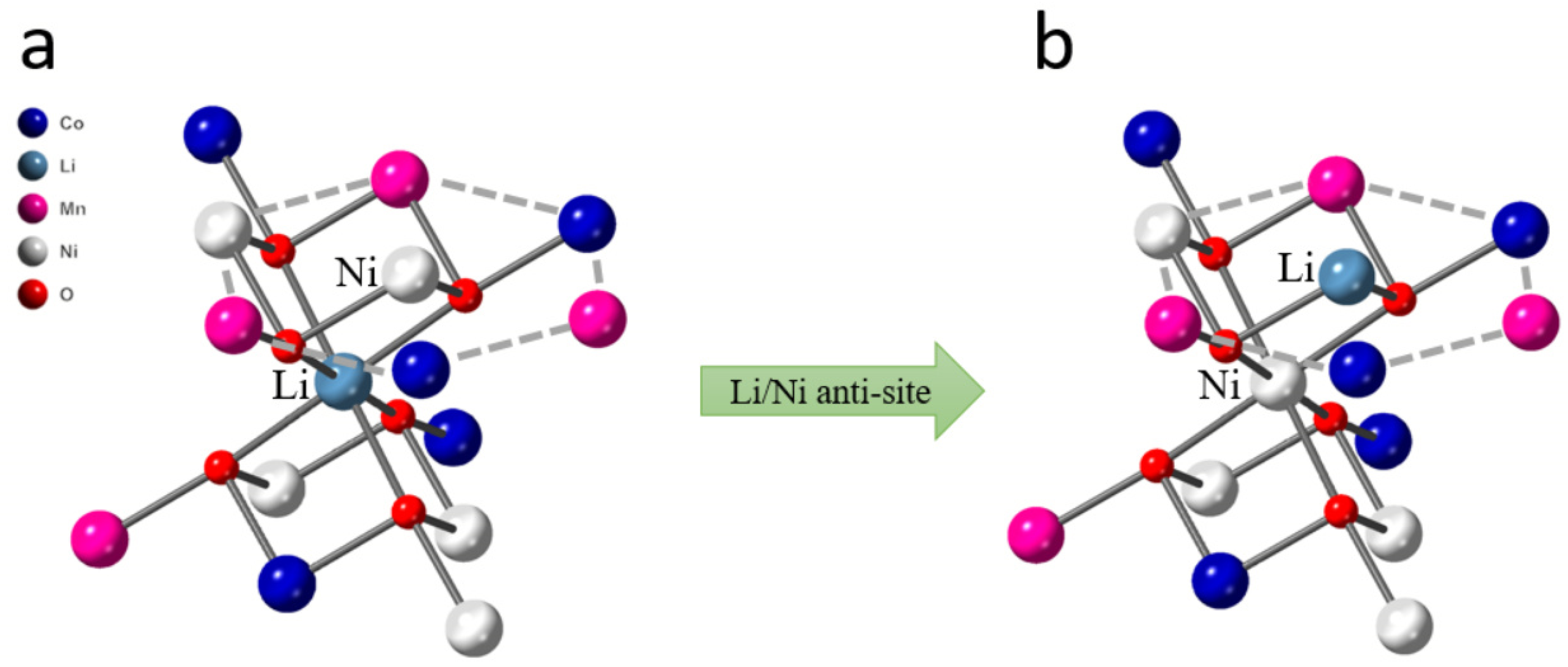
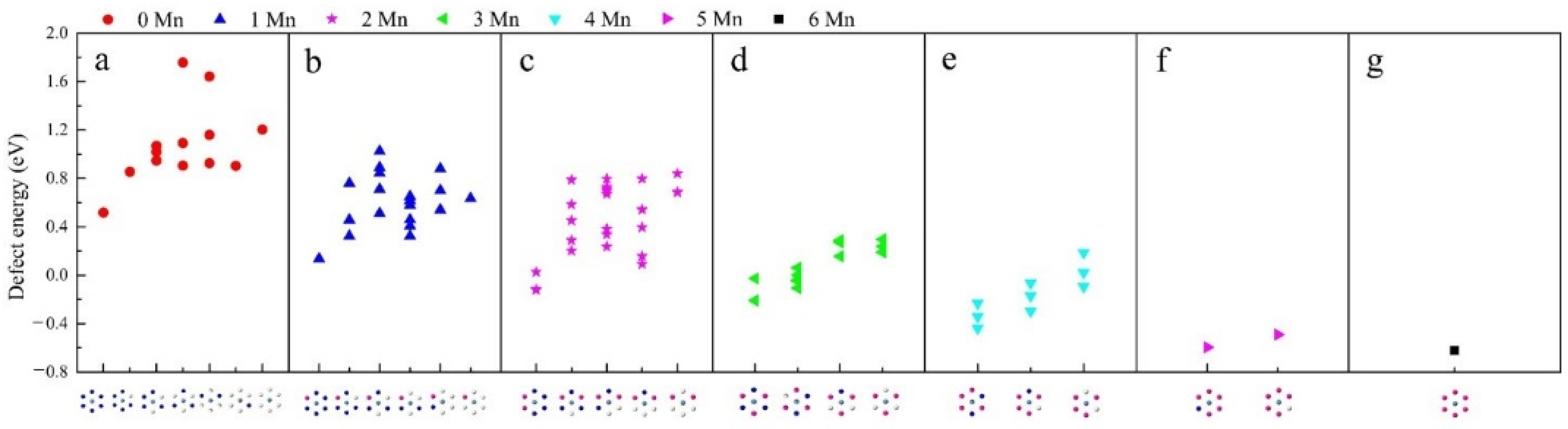
3.2. Local Coordination Structure of Li/Ni Anti-Site
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Source 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Zhao, E.; He, L.; Wang, B.; Li, X.; Zhang, J.; Wu, Y.; Chen, J.; Zhang, S.; Liang, T.; Chen, Y.; et al. Structural and mechanistic revelations on high capacity cation-disordered Li-rich oxides for rechargeable Li-ion batteries. Energy Storage Mater. 2019, 16, 354–363. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Chen, C.-L.; Pu, N.-W.; Wu, C.-H.; Liu, Y.-M.; Chen, Y.-H.; Youh, M.-J.; Ger, M.-D. Experimental and Modeling Analysis of Holey Graphene Electrodes for High-Power-Density Li-Ion Batteries. Crystals 2020, 10, 1063. [Google Scholar] [CrossRef]
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes I. Nickel-Rich, LiNixCoyMnzO2. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Tuccillo, M.; Palumbo, O.; Pavone, M.; Muñoz-García, A.B.; Paolone, A.; Brutti, S. Analysis of the Phase Stability of LiMO2 Layered Oxides (M = Co, Mn, Ni). Crystals 2020, 10, 526. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wang, Y.; Guo, Y.-Z.; Liu, C.-W.; Dan, L.-L. Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met. 2014, 40, 78–83. [Google Scholar] [CrossRef]
- Satyanarayana, M.; James, J.; Varadaraju, U.V. Electrochemical performance of LiNi0.4Co0.2Mn0.4O2 prepared by different molten salt flux: LiNO3-LiCl and LiNO3-KNO3. Appl. Surf. Sci. 2017, 418, 72–78. [Google Scholar]
- Zhao, X.; Liu, B.; Yang, J.; Hou, J.; Wang, Y.; Zhu, Y.; Zhao, X.; Liu, B.; Yang, J.; Hou, J.; et al. Synthesizing LiNi0.5 Co0.2Mn0.3O2 with microsized peanut-like structure for enhanced electrochemical properties of lithium ion batteries. J. Alloys Compd. 2020, 832, 154464. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Deng, S.; Lei, T.; Li, Y.; Li, W.; Cao, G.; Zhu, J.; Zhang, J. Chemical coupling constructs amorphous silica modified LiNi0.6Co0.2Mn0.2O2 cathode materials and its electrochemical performances. J. Power Source 2019, 431, 8–16. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Yang, L.; Chen, Y.; Guo, J.; Zhu, J.; Cao, G.L. Enhancing high-voltage electrochemical performance of LiNi0.7Mn0.15Co0.15O2 cathode materials with SiO2 coatings via electrostatic attraction forces method. Ionics 2020, 26, 5393–5403. [Google Scholar] [CrossRef]
- Liang, J.; Lu, Y.; Wang, J.; Liu, X.; Chen, K.; Ji, W.; Zhu, Y.; Wang, D. Well-ordered layered LiNi0.8Co0.1Mn0.1O2 submicron sphere with fast electrochemical kinetics for cathodic lithium storage. J. Energy Chem. 2020, 47, 188–195. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Source 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Yang, X.; Bai, Y.; Liu, Z.; Shu, H.; Wei, Q. Determination of the chemical diffusion coefficient of lithium ions in spherical Li[Ni0.5Mn0.3Co0.2]O2. Electrochim. Acta 2012, 66, 88–93. [Google Scholar] [CrossRef]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Source 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Li, Z.; Ban, C.; Chernova, N.A.; Wu, Z.; Upreti, S.; Dillon, A.; Whittingham, M.S. Towards understanding the rate capability of layered transition metal oxides. J. Power Source 2014, 268, 106–112. [Google Scholar] [CrossRef]
- Gao, A.; Sun, Y.; Zhang, Q.; Zheng, J.; Lu, X. Evolution of Ni/Li antisites under the phase transition of a layered LiNi1/3Co1/3Mn1/3O2 cathode. J. Mater. Chem. A 2020, 8, 6337–6348. [Google Scholar] [CrossRef]
- Cui, S.; Wei, Y.; Liu, T.; Deng, W.; Hu, Z.; Su, Y.; Li, H.; Li, M.; Guo, H.; Duan, Y.; et al. Optimized Temperature Effect of Li-Ion Diffusion with Layer Distance in Li(NixMnyCoz)O2 Cathode Materials for High Performance Li-Ion Battery. Adv. Energy Mater. 2016, 6, 1501309. [Google Scholar] [CrossRef]
- Yamada, A.; Tanaka, M.; Tanaka, K.; Sekai, K. Jahn–Teller instability in spinel Li–Mn–O. J. Power Source 1999, 81–82, 73–78. [Google Scholar] [CrossRef]
- Ellis, B.L.; Lee, K.T.; Nazar, L.F. Positive Electrode Materials for Li-Ion and Li-Batteries. Chem. Mater. 2010, 22, 691–714. [Google Scholar] [CrossRef]
- Guo, J.; Jiao, L.F.; Yuan, H.T.; Li, H.X.; Zhang, M.; Wang, Y.M. Effect of synthesis condition on the structural and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 prepared by the metal acetates decomposition method. Electrochim. Acta 2006, 51, 3731–3735. [Google Scholar] [CrossRef]
- Hwang, B.J.; Santhanam, R.; Chen, C.H. Effect of synthesis conditions on electrochemical properties of LiNi1−yCoyO2 cathode for lithium rechargeable batteries. J. Power Source 2003, 114, 244–252. [Google Scholar] [CrossRef]
- Ren, H.; Huang, Y.; Wang, Y.; Li, Z.; Cai, P.; Peng, Z.; Zhou, Y. Effects of different carbonate precipitators on LiNi1/3Co1/3Mn1/3O2 morphology and electrochemical performance. Mater. Chem. Phys. 2009, 117, 41–45. [Google Scholar] [CrossRef]
- Kang, K.; Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 2006, 74, 094105. [Google Scholar] [CrossRef]
- Yu, H.; Qian, Y.; Otani, M.; Tang, D.; Guo, S.; Zhu, Y.; Zhou, H. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: Experimental and firstprinciples calculations. Energy Environ. Sci. 2014, 7, 1068–1078. [Google Scholar] [CrossRef]
- Yan, P.; Nie, A.; Zheng, J.; Zhou, Y.; Lu, D.; Zhang, X.; Xu, R.; Belharouak, I.; Zu, X.; Xiao, J.; et al. Evolution of Lattice Structure and Chemical Composition of the Surface Reconstruction Layer in Li1.2Ni0.2Mn0.6O2 Cathode Material for Lithium Ion Batteries. Nano Lett. 2015, 15, 514–522. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Lv, D.; Wei, Y.; Zheng, J.; Wang, Z.; Kuppan, S.; Yu, J.; Luo, L.; Edwards, D.; et al. Atomic-Resolution Visualization of Distinctive Chemical Mixing Behavior of Ni, Co, and Mn with Li in Layered Lithium Transition-Metal Oxide Cathode Materials. Chem. Mater. 2015, 27, 5393–5401. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Breger, J.; Grey, C.P.; Ceder, G. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics Tuning of Li-Ion Diffusion in Layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef]
- Lychagin, D.; Dmitriev, A.; Nikonov, A. Alfyorova, Crystallographic and Geometric Factors in the Shear Development in <001> FCC Single Crystals: Molecular Dynamics Simulation and Experimental Study. Crystals 2020, 10, 666. [Google Scholar] [CrossRef]
- Wang, B.-B.; Xiao, Y.; Xu, Z.-M. Variation in Properties of Pre-Nucleation Calcium Carbonate Clusters Induced by Aggregation: A Molecular Dynamics Study. Crystals 2021, 11, 102. [Google Scholar] [CrossRef]
- Hu, B.; Tao, G. Molecular Dynamics Simulations on Lithium Diffusion in LiFePO4: The effect of anti-site defects. J. Mater. Chem. A 2015, 3, 20399–20407. [Google Scholar] [CrossRef]
- Tateishi, K.; du Boulay, D.; Ishizawa, N. The effect of mixed Mn valences on Li migration in LiMn2O4 spinel: A molecular dynamics study. Appl. Phys. Lett. 2004, 84, 529–531. [Google Scholar] [CrossRef]
- Smith, W.; Forester, T.R.; Todorov, I.T. The DL_POLY_4 User Manual; Version 4.08; STFC Daresbury Laboratory Daresbury: Warrington, UK, 2016. [Google Scholar]
- CrystalMaker Software Ltd., Centre for Innovation & Enterprise, Begbroke Science Park, Woodstock Road, Begbroke, Oxfordshire, OX5 1PF, UK. Available online: http://www.crystalmaker.com (accessed on 15 April 2021).
- Ahn, W.; Lim, S.N.; Jung, K.-N.; Yeon, S.-H.; Kim, K.-B.; Song, H.S.; Shin, K.-H. Combustion-synthesized LiNi0.6Mn0.2Co0.2O2 as cathode material for lithium ion batteries. J. Alloys Compd. 2014, 609, 143–149. [Google Scholar] [CrossRef]
- Guo, J.; Hu, C.Y. Synthesis of nanosized LiNi0.7Mn0.2Co0.1O2 cathode material for lithium ion batteries by combination method of forced hydrolytic and hydroxide coprecipitation. Mater. Res. Innov. 2014, 19, 238–243. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Kaichev, V.V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni,Mn,Co)O2 cathodes for better electrochemistry. J. Power Source 2007, 174, 965–969. [Google Scholar] [CrossRef]
- Ewald, P.P. The calculation of optical and electrostatic grid potential. Ann Phys. 1921, 64, 253–287. [Google Scholar] [CrossRef]
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Soo, S.P.; Idris, M.S.; Osman, R.A.; Rahmat, A. The Effect of Synthesis Temperature on Interlayer Mixing in Layered Rock Salt Cathode Materials LiNi0.7Mn0.1Co0.2O2 for Li-ion Batteries Application. Mater. Sci. Forum. 2015, 819, 155–160. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, T.; Liu, J.; He, L.; Chen, J.; Zhang, J.; Luo, P.; Lu, H.; Wang, R.; Zhu, W.; et al. Insight into the origin of lithium/nickel ions exchange in layered Li(NixMnyCoz)O2 cathode materials. Nano Energy 2018, 49, 77–85. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Menziani, M.C.; Cormack, A.N.; Segre, U. A New Self-Consistent Empirical Interatomic Potential Model for Oxides, Silicates, and Silica-Based Glasses. J. Phys. Chem. B 2006, 110, 11780–11795. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, L.; Gao, M. Molecular dynamics study on the Li diffusion mechanism and delithiation process of Li2MnO3. Solid State Ion. 2020, 346, 115195. [Google Scholar] [CrossRef]
- Gale, J.D. Gulp: A Computer Program for the Symmetry-Adapted Simulation of Solids. J. Chem. Soc. Faraday Trans. 1997, 93, 629–637. [Google Scholar] [CrossRef]
- Asadi, A.; Aghamiri, S.F.; Talaie, M.R. Molecular dynamics simulation of a LixMn2O4 spinel cathode material in Li-ion batteries. RSC Adv. 2016, 6, 115354–115363. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Prieto, V.M.H.; Islam, M.S. Lithium Battery Materials LiMPO4(M = Mn, Fe, Co, and Ni): Insights into Defect Association, Transport Mechanisms, and Doping Behavior. Chem. Mater. 2008, 20, 5907–5915. [Google Scholar] [CrossRef]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.J.; Slater, P.R. Atomic-Scale Investigation of Defects, Dopants, and Lithium Transport in the LiFePO4 Olivine-Type Battery Material. Chem. Mater. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Kuganathan, N.; Islam, M.S.; Bruce, P.G. Structure and Lithium Transport Pathways in Li2FeSiO4, Cathodes for Lithium Batteries. J. Am. Chem. Soc. 2011, 133, 13031–13035. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Kuganathan, N.; Islam, M.S. Defect chemistry and lithium-ion migration in polymorphs of the cathode material Li2MnSiO4. J. Mater. Chem. A 2013, 1, 4207–4214. [Google Scholar] [CrossRef]
- Tripathi, R.; Gardiner, G.R.; Islam, M.S.; Nazar, L.F. Alkali-ion Conduction Paths in LiFeSO4F and NaFeSO4F Tavorite-Type Cathode Materials. Chem. Mater. 2011, 23, 2278–2284. [Google Scholar] [CrossRef]
- Perera, D.; Ganeshalingam, S.; Kuganathan, N.; Chroneos, A. A Computational Study of Defects, Li-Ion Migration and Dopants in Li2ZnSiO4 Polymorphs. Crystals 2019, 9, 563. [Google Scholar] [CrossRef]
- Read, M.S.D.; Jackson, R.A. Derivation of enhanced potentials for uranium dioxide and the calculation of lattice and intrinsic defect properties. J. Nucl. Mater. 2010, 406, 293–303. [Google Scholar] [CrossRef]
- Lee, E.; Lee, K.-R.; Lee, B.-J. An interatomic potential for the Li-Co-O ternary system. Comput. Mater. Sci. 2018, 142, 47–58. [Google Scholar] [CrossRef]
- Van der Ven, A.; Ceder, G.; Asta, M.; Tepesch, P.D. First-principles theory of ionic diffusion with nondilute carriers. Phys. Rev. B 2001, 64, 184307. [Google Scholar] [CrossRef]
- Ngala, J.K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalij, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214–220. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Zaghib, K.; Groult, H. Optimization of Layered Cathode Materials for Lithium-Ion Batteries. Materials 2016, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, C.; Zhang, M.; Bai, J.; Zheng, J.; Kou, R.; Ko, J.Y.P.; Huq, A.; Zhong, G.; Sun, C.-J.; et al. Intrinsic Role of Cationic Substitution in Tuning Li/Ni Mixing in High-Ni Layered Oxides. Chem. Mater. 2019, 31, 2731–2740. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, Electrochemical, and Thermal Properties of Nickel-Rich LiNixMnyCozO2 Materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Zheng, J.; Teng, G.; Xin, C.; Zhuo, Z.; Liu, J.; Li, Q.; Hu, Z.; Xu, M.; Yan, S.; Yang, W.; et al. Role of Superexchange Interaction on Tuning of Ni/Li Disordering in Layered Li(NixMnyCoz)O2. J. Phys. Chem. Lett. 2017, 8, 5537–5542. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.S. Atomistic Simulation Study of Mixed-Metal Oxide (LiNi1⁄3Mn1⁄3Co1⁄3O2) Cathode Material for Lithium Ion Battery. J. Phys. Chem. C 2012, 116, 6484–6489. [Google Scholar] [CrossRef]


| Interaction | |||
|---|---|---|---|
| 0.001114 | 2.681360 | 3.429506 | |
| 0.029356 | 2.500754 | 2.679137 | |
| 0.042395 | 3.358701 | 1.659316 | |
| 0.029658 | 2.440000 | 3.012000 | |
| 0.010958 | 2.400628 | 3.461272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Huang, Y.; Du, R.; Tang, M.; Wang, B.; Zhang, J. Effect of Ni2+ on Lithium-Ion Diffusion in Layered LiNi1−x−yMnxCoyO2 Materials. Crystals 2021, 11, 465. https://doi.org/10.3390/cryst11050465
Zhu Y, Huang Y, Du R, Tang M, Wang B, Zhang J. Effect of Ni2+ on Lithium-Ion Diffusion in Layered LiNi1−x−yMnxCoyO2 Materials. Crystals. 2021; 11(5):465. https://doi.org/10.3390/cryst11050465
Chicago/Turabian StyleZhu, Yuanyuan, Yang Huang, Rong Du, Ming Tang, Baotian Wang, and Junrong Zhang. 2021. "Effect of Ni2+ on Lithium-Ion Diffusion in Layered LiNi1−x−yMnxCoyO2 Materials" Crystals 11, no. 5: 465. https://doi.org/10.3390/cryst11050465
APA StyleZhu, Y., Huang, Y., Du, R., Tang, M., Wang, B., & Zhang, J. (2021). Effect of Ni2+ on Lithium-Ion Diffusion in Layered LiNi1−x−yMnxCoyO2 Materials. Crystals, 11(5), 465. https://doi.org/10.3390/cryst11050465








