Structure-Properties Correlation of Cross-Linked Penicillin G Acylase Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant Protein Production
2.2. Protein Purification
2.3. Crystallization
2.4. Production of Crystal Seeds
2.5. Generation of Cross-Linked Enzyme Crystals (CLECs)
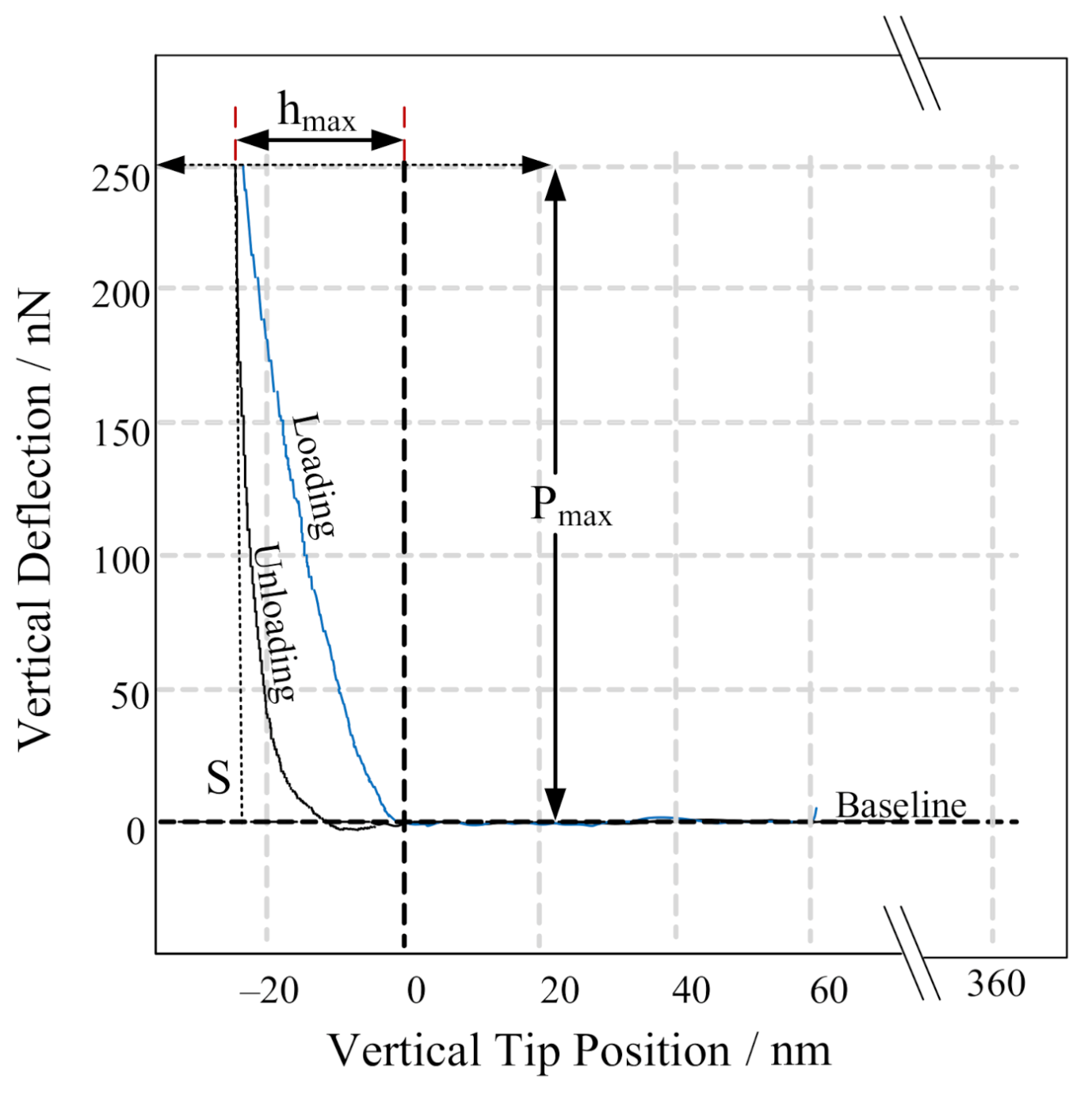
2.6. Mechanical Measurements
2.7. NIPAB Assay for Activity Determination of PGA CLECs
3. Results and Discussion
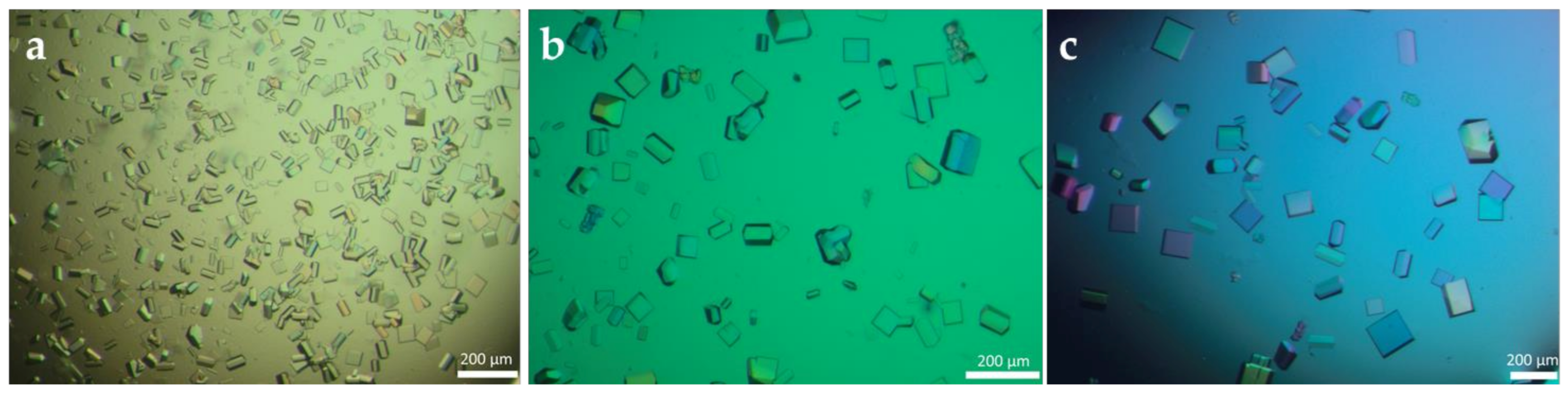
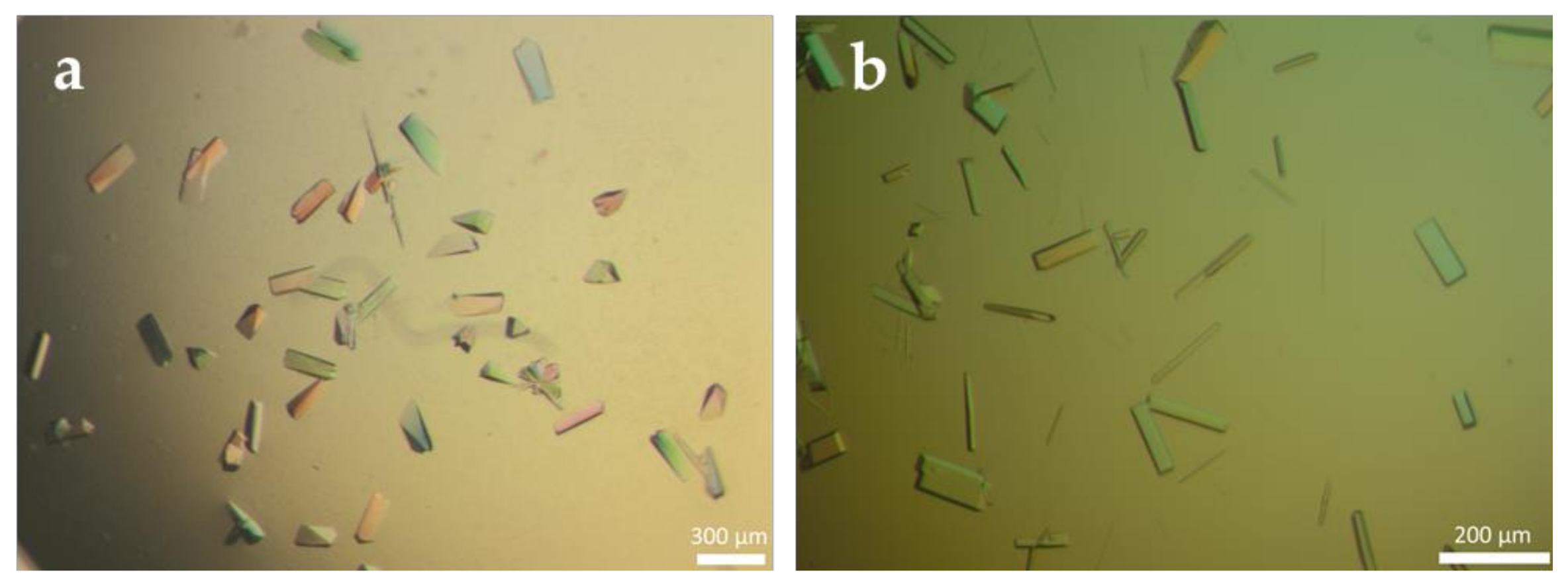
3.1. Production of Cross-Linked Enzyme Crystals (CLECs) of Different PGAs
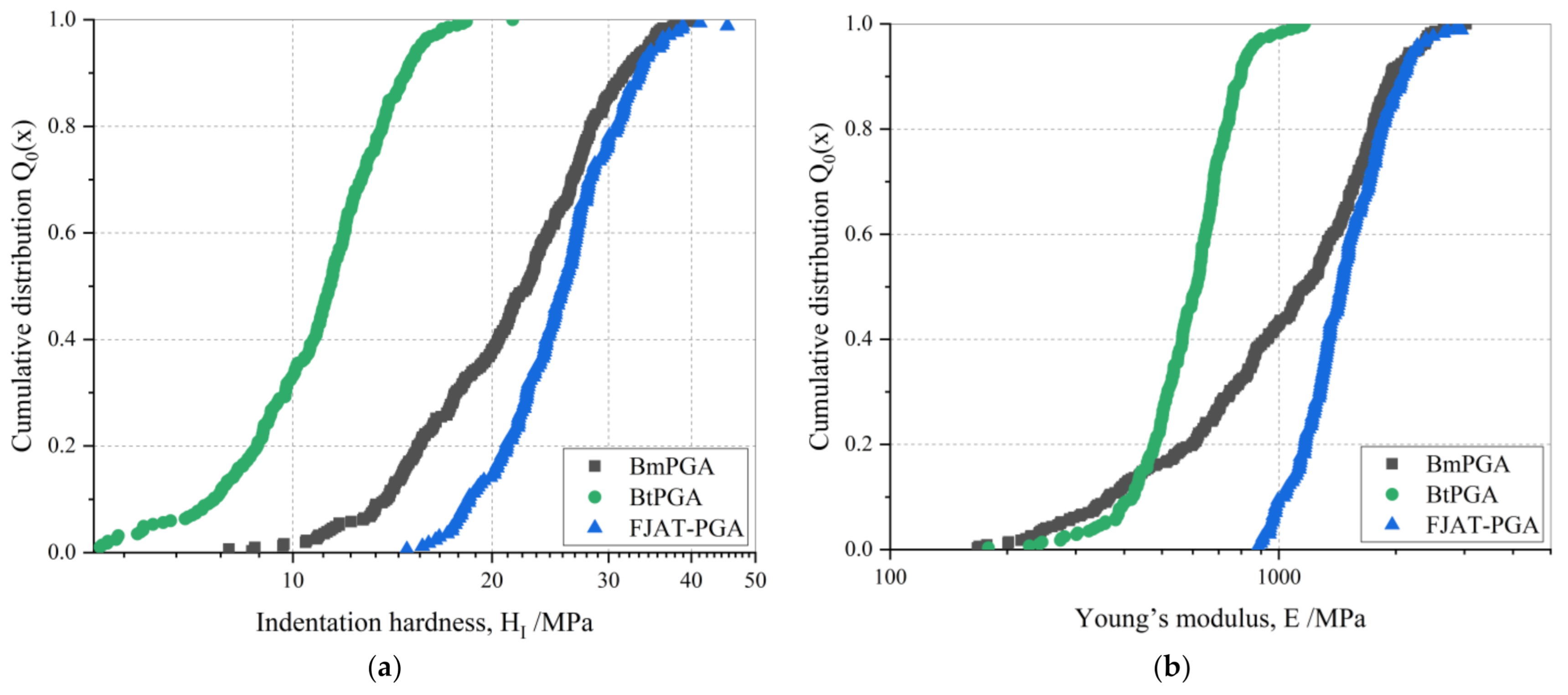
3.2. Mechanical Characterization of PGA CLECs
3.3. Activity of PGA CLECs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marešová, H.; Plačková, M.; Grulich, M.; Kyslík, P. Current state and perspectives of penicillin G acylase-based biocatalyses. Appl. Microbiol. Biotechnol. 2014, 98, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Rajendhran, J.; Gunasekaran, P. Recent biotechnological interventions for developing improved penicillin G acylases. J. Biosci. Bioeng. 2004, 97, 1–13. [Google Scholar] [CrossRef]
- Cobos-Puc, L.; Rodríguez-Herrera, R.; Cano-Cabrera, J.C.; Aguayo-Morales, H.; Silva-Belmares, S.Y.; Flores Gallegos, A.C.; Martínez Hernández, J.L. Classical and New Pharmaceutical Uses of Bacterial Penicillin G Acylase. Curr. Pharm. Biotechnol. 2020, 21, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Pippel, J.; Günther, G.; Müller, C.; Lauermann, A.; Knuuti, T.; Blankenfeldt, W.; Jahn, D.; Biedendieck, R. Crystal structures and protein engineering of three different penicillin G acylases from Gram-positive bacteria with different thermostability. Appl. Microbiol. Biotechnol. 2019, 103, 7537–7552. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Margolin, A.L. Novel crystalline catalysts. Trends Biotechnol. 1996, 14, 223–230. [Google Scholar] [CrossRef]
- Margolin, A.L.; Navia, M.A. Protein Crystals as Novel Catalytic Materials. Angew. Chem. Int. Ed. 2001, 40, 2204–2222. [Google Scholar] [CrossRef]
- Koizumi, H.; Kawamoto, H.; Tachibana, M.; Kojima, K. Effect of intracrystalline water on micro-Vickers hardness in tetragonal hen egg-white lysozyme single crystals. J. Phys. D Appl. Phys. 2008, 41, 074019. [Google Scholar] [CrossRef]
- Tachibana, M.; Kobayashi, Y.; Shimazu, T.; Ataka, M.; Kojima, K. Growth and mechanical properties of lysozyme crystals. J. Cryst. Growth 1999, 198–199, 661–664. [Google Scholar] [CrossRef]
- Suzuki, R.; Kishi, T.; Tsukashima, S.; Tachibana, M.; Wako, K.; Kojima, K. Hardness and slip systems of orthorhombic hen egg-white lysozyme crystals. Philos. Mag. 2016, 96, 2930–2942. [Google Scholar] [CrossRef]
- Kishi, T.; Suzuki, R.; Shigemoto, C.; Murata, H.; Kojima, K.; Tachibana, M. Microindentation Hardness of Protein Crystals under Controlled Relative Humidity. Crystals 2017, 7, 339. [Google Scholar] [CrossRef]
- Tait, S.; White, E.T.; Litster, J.D. Mechanical Characterization of Protein Crystals. Part. Part. Syst. Charact. 2008, 25, 266–276. [Google Scholar] [CrossRef]
- Morozov, V.N.; Morozova, T.Y. Viscoelastic properties of protein crystals: Triclinic crystals of hen egg white lysozyme in different conditions. Biopolymers 1981, 20, 451–467. [Google Scholar] [CrossRef]
- Kubiak, M.; Solarczek, J.; Kampen, I.; Schallmey, A.; Kwade, A.; Schilde, C. Micromechanics of anisotropic cross-linked enzyme crystals. Cryst. Growth Des. 2018, 18, 5885–5895. [Google Scholar] [CrossRef]
- Kubiak, M.; Storm, K.F.; Kampen, I.; Schilde, C. Relationship between Cross-Linking Reaction Time and Anisotropic Mechanical Behavior of Enzyme Crystals. Cryst. Growth Des. 2019, 19, 4453–4464. [Google Scholar] [CrossRef]
- Wittchen, K.D.; Meinhardt, F. Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl. Microbiol. Biotechnol. 1995, 42, 871–877. [Google Scholar] [CrossRef]
- Lakowitz, A.; Krull, R.; Biedendieck, R. Recombinant production of the antibody fragment D1.3 scFv with different Bacillus strains. Microb. Cell Fact. 2017, 16, 1–18. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tranchida, D.; Piccarolo, S.; Loos, J.; Alexeev, A. Mechanical Characterization of Polymers on a Nanometer Scale through Nanoindentation. A Study on Pile-up and Viscoelasticity. Macromolecules 2007, 40, 1259–1267. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. Nanoindentation, 3rd ed.; Mechanical Engineering Series; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9871-2. [Google Scholar]
- D’Arcy, A.; Bergfors, T.; Cowan-Jacob, S.W.; Marsh, M. Microseed matrix screening for optimization in protein crystallization: What have we learned? Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Labonte, D.; Lenz, A.-K.; Oyen, M.L. On the relationship between indentation hardness and modulus, and the damage resistance of biological materials. Acta Biomater. 2017, 57, 373–383. [Google Scholar] [CrossRef]
- Schilde, C.; Westphal, B.; Kwade, A. Effect of the primary particle morphology on the micromechanical properties of nanostructured alumina agglomerates. J. Nanopart. Res. 2012, 14, 745. [Google Scholar] [CrossRef]
- Schilde, C.; Kwade, A. Measurement of the micromechanical properties of nanostructured aggregates via nanoindentation. J. Mater. Res. 2012, 27, 672–684. [Google Scholar] [CrossRef]
- Arfsten, J.; Kampen, I.; Kwade, A. Mechanical testing of single yeast cells in liquid environment: Effect of the extracellular osmotic conditions on the failure behavior. Int. J. Mater. Res. 2009, 100, 978–983. [Google Scholar] [CrossRef]
- Arfsten, J.; Bradtmöller, C.; Kampen, I.; Kwade, A. Compressive testing of single yeast cells in liquid environment using a nanoindentation system. J. Mater. Res. 2008, 23, 3153–3160. [Google Scholar] [CrossRef]
- Xu, Z.; Cen, Y.K.; Zou, S.P.; Xue, Y.P.; Zheng, Y.G. Recent advances in the improvement of enzyme thermostability by structure modification. Crit. Rev. Biotechnol. 2020, 40, 83–98. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 2.0 Schrödinger; LLC: New York, NY, USA.
- Chruszcz, M.; Potrzebowski, W.; Zimmerman, M.D.; Grabowski, M.; Zheng, H.; Lasota, P.; Minor, W. Analysis of solvent content and oligomeric states in protein crystals-does symmetry matter? Protein Sci. 2008, 17, 623–632. [Google Scholar] [CrossRef]
- Yan, E.K.; Cao, H.L.; Zhang, C.Y.; Lu, Q.Q.; Ye, Y.J.; He, J.; Huang, L.J.; Yin, D.C. Cross-linked protein crystals by glutaraldehyde and their applications. RSC Adv. 2015, 5, 26163–26174. [Google Scholar] [CrossRef]
- Diamond, R. Real-space refinement of the structure of hen egg-white lysozyme. J. Mol. Biol. 1974, 82, 371–391. [Google Scholar] [CrossRef]
- Koopmeiners, J.; Diederich, C.; Solarczek, J.; Voß, H.; Mayer, J.; Blankenfeldt, W.; Schallmey, A. HheG, a Halohydrin Dehalogenase with Activity on Cyclic Epoxides. ACS Catal. 2017, 7, 6877–6886. [Google Scholar] [CrossRef]
- Elgersma, A.V.; Ataka, M.; Katsura, T. Kinetic studies on the growth of three crystal forms of lysozyme based on the measurement of protein and Cl- concentration changes. J. Cryst. Growth 1992, 122, 31–40. [Google Scholar] [CrossRef]
- Quiocho, F.A.; Richards, F.M. Intermolecular cross linking of a protein in the crystalline state: Carboxypeptidase-A. Proc. Natl. Acad. Sci. USA 1964, 52, 833–839. [Google Scholar] [CrossRef]

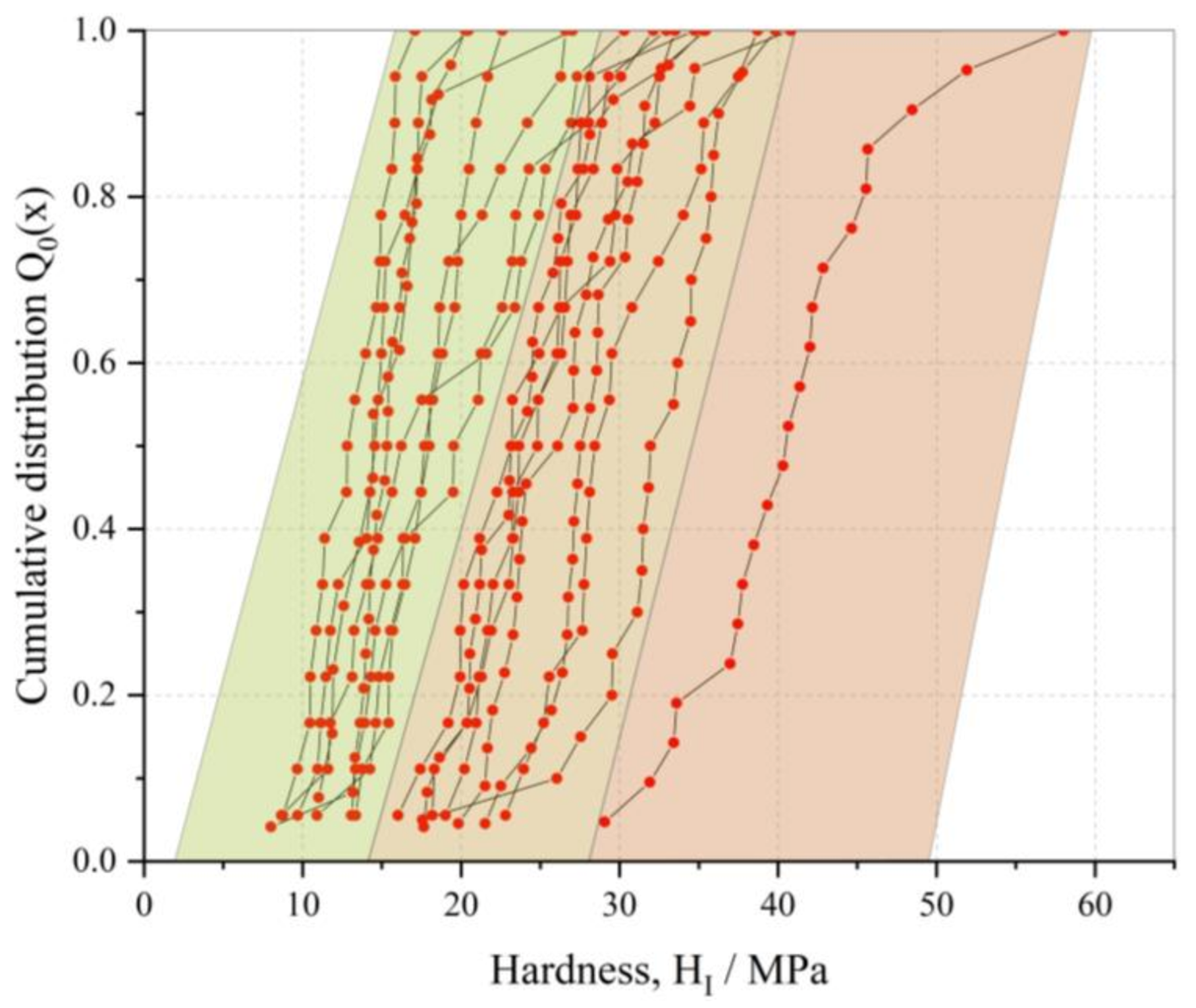

| FJAT-PGA CLECs (6NVX) a | BtPGA CLECs (6NVY) a | BmPGA CLECs (6NVW) a | |
|---|---|---|---|
| Median hardness (MPa) | 26 | 11 | 23 |
| Median Young´s modulus (MPa) | 1452 | 614 | 1170 |
| Melting temperature (°C) b | 74.3 | 64.5 | 56.0 |
| Solvent content (%) c | 54.6 | 64.8 | 43.5 |
| Number of symmetry mates (12 Å) c | 10 | 8 | 12 |
| Number of lysine residues c | 78 | 49 | 74 |
| FJAT-PGA CLECs (6NVX) a | BtPGA CLECs (6NVY) a | BmPGA CLECs (6NVW) a | HheG CLECs (5O30) a | Lysozyme CLECs (3LYZ) a | |
|---|---|---|---|---|---|
| Median hardness (MPa) | 26 | 11 | 23 | Prismatic face: 11 b, Basal face: 14.5 b | 12 b |
| Median Young´s modulus (MPa) | 1452 | 614 | 1170 | Prismatic face: 500 b, Basal face: not shownb | 1073 b |
| Solvent content (%) | 54.6 | 64.8 | 43.5 | 65.0 | 40.5 |
| Number of lysine residues per molecular weight (kDa−1) | 0.90 | 0.57 | 0.86 | 0.17 | 0.42 |
| Space group | P212121 | H3 | P21 | P3121 | P43212 |
| Crystal system | orthorhombic | hexagonal | monoclinic | trigonal | tetragonal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, M.; Mayer, J.; Kampen, I.; Schilde, C.; Biedendieck, R. Structure-Properties Correlation of Cross-Linked Penicillin G Acylase Crystals. Crystals 2021, 11, 451. https://doi.org/10.3390/cryst11040451
Kubiak M, Mayer J, Kampen I, Schilde C, Biedendieck R. Structure-Properties Correlation of Cross-Linked Penicillin G Acylase Crystals. Crystals. 2021; 11(4):451. https://doi.org/10.3390/cryst11040451
Chicago/Turabian StyleKubiak, Marta, Janine Mayer, Ingo Kampen, Carsten Schilde, and Rebekka Biedendieck. 2021. "Structure-Properties Correlation of Cross-Linked Penicillin G Acylase Crystals" Crystals 11, no. 4: 451. https://doi.org/10.3390/cryst11040451
APA StyleKubiak, M., Mayer, J., Kampen, I., Schilde, C., & Biedendieck, R. (2021). Structure-Properties Correlation of Cross-Linked Penicillin G Acylase Crystals. Crystals, 11(4), 451. https://doi.org/10.3390/cryst11040451






