Understanding the Reaction Crystallization Process of Glycidyl Trimethyl Ammonium Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Crystallization Procedure
2.4. Content Determination
3. Results and Discussion
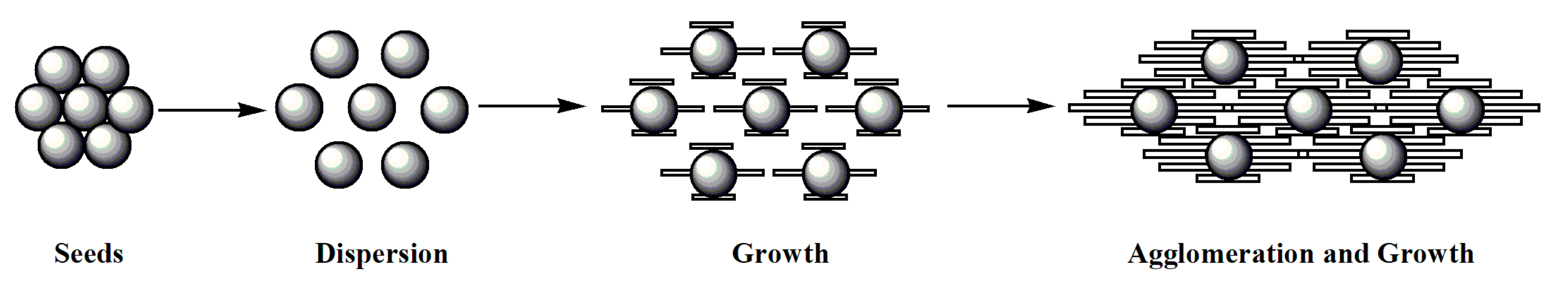
3.1. Process Analysis of Crystallization
- i.
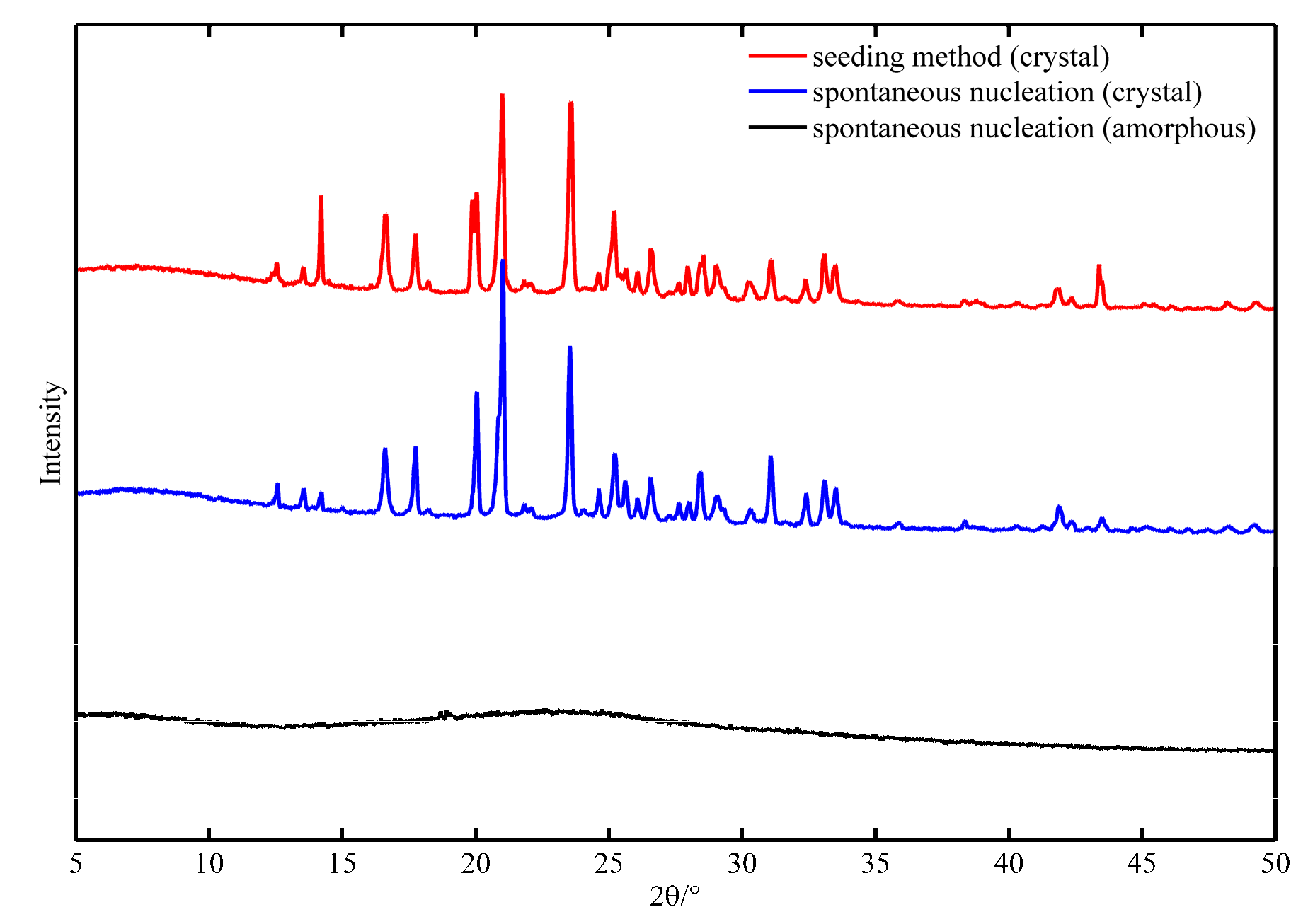
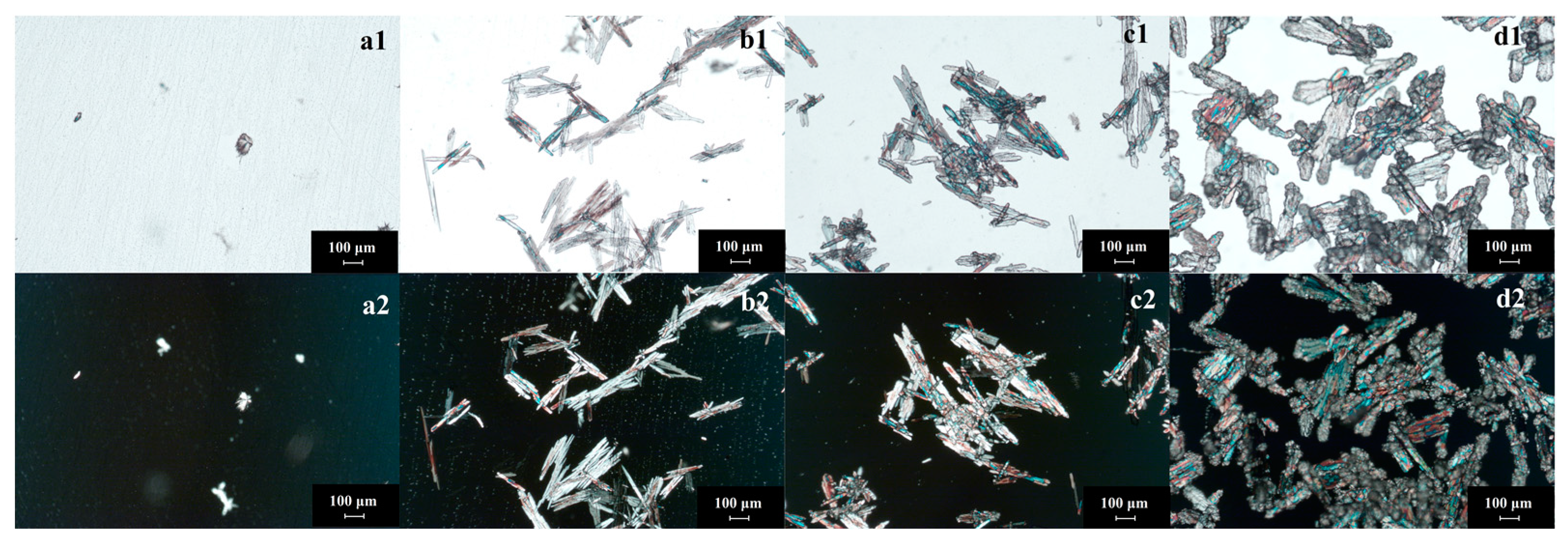
- Amorphous products prepared by the spontaneous nucleation method: Flow rate of the trimethylamine gas is the key factor affecting amorphous shape or crystallinity. When the flow rate is larger than 100 mL/min in this investigation, the product would be amorphous. As shown in Figure 5, there is no polarization phenomenon of amorphous products (catch with XRD without diffraction peak). In this process, the solids are formed gradually and they grow slightly (Figure 5(a1–d1)). The main behavior be-tween solids is agglomeration. The product has no fixed morphology and no polarization phenomenon (Figure 5(a2–d2)). On the other hand, the purity of amorphous products is low (less than 85% in fresh epichlorohydrin and less than 80% in the experiment of epichlorohydrin recycling). Amorphous solids tend to contain higher Gibbs free energy, which results in poor stability. Furthermore, amorphous solids are easy to preserve solvents (or reactant), which leading impurity content increased. GTA is a substance that absorbs moisture easily, especially in an amorphous state. Thus, amorphous GTA should be prevented during the reaction crystallization process.
- ii.
- Crystalline products prepared by spontaneous nucleation method: The spontaneously nucleated GTA is a rod-like crystal with high crystallinity (as shown in Figure 6(a2–d2)). The spontaneous nucleation process is fast, with the constant addition of trimethylamine gas (Figure 6(a1,b1)). The rod-like crystals of GTA gradually multi-ply and aggregate (Figure 6(c1,d1)). The purity of the crystals produced by this method could be larger than 90%, but the particle size is small. Solids agglomerate seriously during the storage procedure. Products prepared by the spontaneous nucleation method contains some uncertainty. The product may be crystalline or amorphous. When the reactant is recovered (epichlorohydrin), it is more difficult to obtain crystal products with high purity.
- iii.
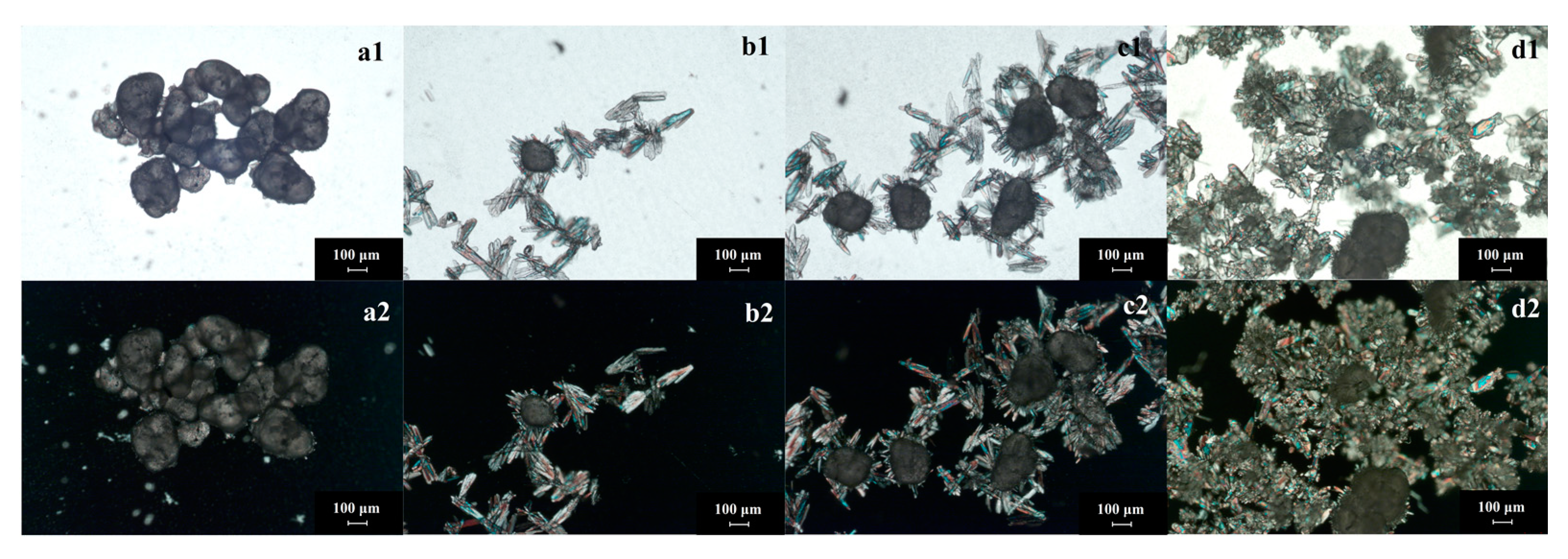
- Crystalline products prepared by the seed method: The seed method is widely used in the crystallization process. However, the application of seed in reaction crystallization—especially in gas- and liquid-phase reaction crystallization—is used relatively rarely. The investigation found that the seed method could produce high purity GTA solids no matter if the reactant of epichlorohydrin is recycled or not. The process involves first adding a certain amount of trimethylamine gas into epichlorohydrin (as mentioned in the experimental section). It must be emphasized that the seeds should be in crystal form (measured by XRD before using). After adding seed crystals, the seeds are aggregated pellets first (Figure 7(a1,b1)). At this time, the phenomenon of seed polarization is not obvious because the thickness of the solid is too large. Then, the seeds will spread out (Figure 7(a2,b2)). With the adding of trimethylamine gas, GTA grows and aggregates at the same time (Figure 7(c1,d1)). The crystal seeds regulate the number of crystal particles and the growth rate of crystal to a certain extent. The seed product has excellent crystallinity (Figure 7(c2,d2)) and pupurity (larger than 95%). This method will be examined emphatically in the following section.
3.2. Understanding of the Crystallization Process
3.3. Planning of Experiments
3.4. Factors Affecting Purity
3.5. Factors Affecting Particle Size
3.6. Factors Affecting Product Color and Yield
3.7. Process Identification and Scale-Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClure, J.D. Glycidyltrimethylammonium chloride and related compounds. J. Org. Chem. 1970, 35, 2059–2061. [Google Scholar] [CrossRef]
- Bendoraitiene, J.; Kavaliauskaite, R.; Klimaviciute, R.; Zemaitaitis, A. Peculiarities of starch sationization with glycidyltrimethylammonium chloride. Starch Strke 2006, 58, 623–631. [Google Scholar] [CrossRef]
- Cote, A.; Erdemir, D.; Girard, K.P.; Green, D.A.; Lovette, M.A.; Sirota, E.; Nere, N.K. Perspectives on the current state, challenges, and opportunities in pharmaceutical crystallization process development. Cryst. Growth Des. 2020, 20, 7568–7581. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z. Gas–liquid reactive crystallization kinetics of hydromagnesite in the MgCl2–CO2–NH3–H2O system: Its potential in CO2 sequestration. Ind. Eng. Chem. Res. 2012, 51, 16299–16310. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, S.G.; Jung, W.M.; Kim, M.C.; Kim, W.-S.; Choi, C.K.; Feigelson, R.S. Effect of Taylor vortices on calcium carbonate crystallization by gas–liquid reaction. J. Cryst. Growth 2003, 254, 196–205. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, X.Z. Secondary nucleation kinetics of AIBN crystallisation in methanol: Online imaging-based measurement and modelling. Crystals 2020, 10, 506. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, Y.; Wang, X.Z. Study on co-crystallization of LCZ696 using in situ ATR-FTIR and imaging. Crystals 2020, 10, 922. [Google Scholar] [CrossRef]
- Li, K.; Wu, S.; Xu, S.; Du, S.; Zhao, K.; Lin, L.; Yang, P.; Yu, B.; Hou, B.; Gong, J. Oiling out and polymorphism control of pyraclostrobin in cooling crystallization. Ind. Eng. Chem. Res. 2016, 55, 11631–11637. [Google Scholar] [CrossRef]
- Yu, S.; Xing, W.; Xue, F.; Cheng, Y.; Liu, Y.; Chen, H.; Hao, C.; Sun, Y. Measurement and correlation of solubility and thermodynamic properties of fluoxetine hydrochloride in 15 pure solvents and a methanol + water binary solvent system. J. Chem. Eng. Data 2020, 65, 4656–4668. [Google Scholar] [CrossRef]
- Yu, S.; Xing, W.; Xue, F.; Cheng, Y.; Li, B. Solubility and thermodynamic properties of nimodipine in pure and binary solvents at a series of temperatures. J. Chem. Thermodyn. 2021, 152, 106259. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Xing, W.; Xue, F.; Cheng, Y. Solubility, thermodynamic parameters, and dissolution properties of gliclazide in seventeen pure solvents at temperatures from 278.15 to 318.15 K. J. Mol. Liq. 2020, 312, 113425. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, J.; Cheng, Y.; Du, S.; Wang, Y.; Xue, F.; Xing, W. Solid-liquid phase equilibrium of clozapine in aqueous binary solvent mixtures. J. Mol. Liq. 2021, 329, 115371. [Google Scholar] [CrossRef]
- Tacsi, K.; Pataki, H.; Domokos, A.; Nagy, B.; Csontos, I.; Markovits, I.; Farkas, F.; Nagy, Z.K.; Marosi, G. Direct processing of a flow reaction mixture using continuous mixed suspension mixed product removal crystallizer. Cryst. Growth Des. 2020, 20, 4433–4442. [Google Scholar] [CrossRef]
- Takasuga, M.; Ooshima, H. Control of crystal aspect ratio and size by changing solvent composition in oiling out crystallization of an active pharmaceutical ingredient. Cryst. Growth Des. 2015, 15, 5834–5838. [Google Scholar] [CrossRef]
- Li, X.; Yin, Q.; Zhang, M.; Hou, B.; Bao, Y.; Gong, J.; Hao, H.; Wang, Y.; Wang, J.; Wang, Z. Process design for antisolvent crystallization of erythromycin ethylsuccinate in oiling-out system. Ind. Eng. Chem. Res. 2016, 55, 7484–7492. [Google Scholar] [CrossRef]
- Marone, P.A.; Thiyagarajan, P.; Wagner, A.M.; Tiede, D.M. Effect of detergent alkyl chain length on crystallization of a detergent-solubilized membrane protein: Correlation of protein–detergent particle size and particle–particle interaction with crystallization of the photosynthetic reaction center from Rhodobacter sphaeroides. J. Cryst. Growth 1999, 207, 214–225. [Google Scholar]
- Chen, C.W.; Lee, T. Round granules of dimethyl fumarate by three-in-one intensified process of reaction, crystallization, and spherical agglomeration in a common stirred rank. Org. Process Res. Dev. 2017, 21, 1326–1339. [Google Scholar] [CrossRef]
- Testa, C.J.; Shvedova, K.; Hu, C.; Wu, W.; Born, S.C.; Takizawa, B.; Mascia, S. Heterogeneous crystallization as a process intensification technology in an integrated continuous manufacturing process for pharmaceuticals. Org. Process Res. Dev. 2021, 25, 225–238. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Wang, X. Improved understanding of cefixime trihydrate reactive crystallization and process scale-up with the aid of PAT. Org. Process Res. Dev. 2019, 23, 177–188. [Google Scholar] [CrossRef]

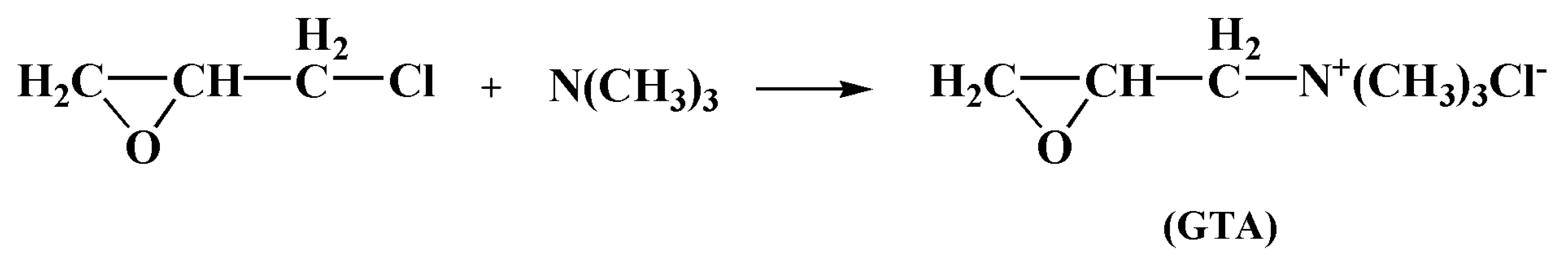


| Name | Supplier | Purity (wt %) |
|---|---|---|
| epichlorohydrin | Sinopec Qilu petrochemical Company, Zibo City, Shandong Province, China | 99% |
| trimethylamine | Sinopec Qilu petrochemical Company, Zibo City, Shandong Province, China | 99% |
| methyl tert-butyl ether | Sinopharm Chemical Reagent Co., Ltd., Shanghai City, China | 99% |
| sodium hydroxide (NaOH) | Sinopharm Chemical Reagent Co., Ltd., Shanghai City, China | 96% |
| phenolphthalein | Sinopharm Chemical Reagent Co., Ltd., Shanghai City, China | indicator |
| water | arium® advance EDI, Sartorius, Göttingen, Germany | ultrapure |
| Experiment No. | Reaction Temperature (°C) | Stirring Speed (r/min) | Flow Rate (mL/min) | Seed Amount (wt %) | Length of Breeding Time (h) |
|---|---|---|---|---|---|
| exps 1–5 | 10, 15, 20, 25, 30 | 80 | 40 | 1.0 | 2 |
| exps 6–10 | 20 | 60, 80, 100, 120, 140 | 40 | 1.0 | 2 |
| exps 11–16 | 20 | 100 | 30, 40, 50, 60, 70 | 1.0 | 2 |
| exps 17–22 | 20 | 100 | 50 | 0.2, 0.5, 1.0, 2.0, 3.0 | 2 |
| exps 23–27 | 20 | 100 | 50 | 1.0 | 1, 2, 3, 4, 5 |
| exp 28 | 20 | 100 | 50 | 1.0 | 3 |
| exp 29 | 20 | 60 | 150 L/min | 1.0 | 4 |
| Seed Amount (wt %) | 0 | 0.2 | 0.5 | 1.0 | 2.0 | 3.0 |
|---|---|---|---|---|---|---|
| Purity of GTA (%) | 89.4 | 95.7 | 96.4 | 97.3 | 97.0 | 97.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Chen, H.; Gao, X.; Feng, W.; Xing, W.; Du, S.; Wang, Y.; Xue, F.; Cheng, Y. Understanding the Reaction Crystallization Process of Glycidyl Trimethyl Ammonium Chloride. Crystals 2021, 11, 449. https://doi.org/10.3390/cryst11040449
Yu S, Chen H, Gao X, Feng W, Xing W, Du S, Wang Y, Xue F, Cheng Y. Understanding the Reaction Crystallization Process of Glycidyl Trimethyl Ammonium Chloride. Crystals. 2021; 11(4):449. https://doi.org/10.3390/cryst11040449
Chicago/Turabian StyleYu, Shuai, Hui Chen, Xujie Gao, Weichun Feng, Wenguo Xing, Shichao Du, Yan Wang, Fumin Xue, and Yan Cheng. 2021. "Understanding the Reaction Crystallization Process of Glycidyl Trimethyl Ammonium Chloride" Crystals 11, no. 4: 449. https://doi.org/10.3390/cryst11040449
APA StyleYu, S., Chen, H., Gao, X., Feng, W., Xing, W., Du, S., Wang, Y., Xue, F., & Cheng, Y. (2021). Understanding the Reaction Crystallization Process of Glycidyl Trimethyl Ammonium Chloride. Crystals, 11(4), 449. https://doi.org/10.3390/cryst11040449








