Characterising Supramolecular Architectures in Crystals Featuring I⋯Br Halogen Bonding: Persistence of X⋯X’ Secondary-Bonding in Their Congeners
Abstract
1. Introduction
2. Methods
3. Supramolecular Aggregation Featuring I⋯Br Secondary-Bonding Interactions
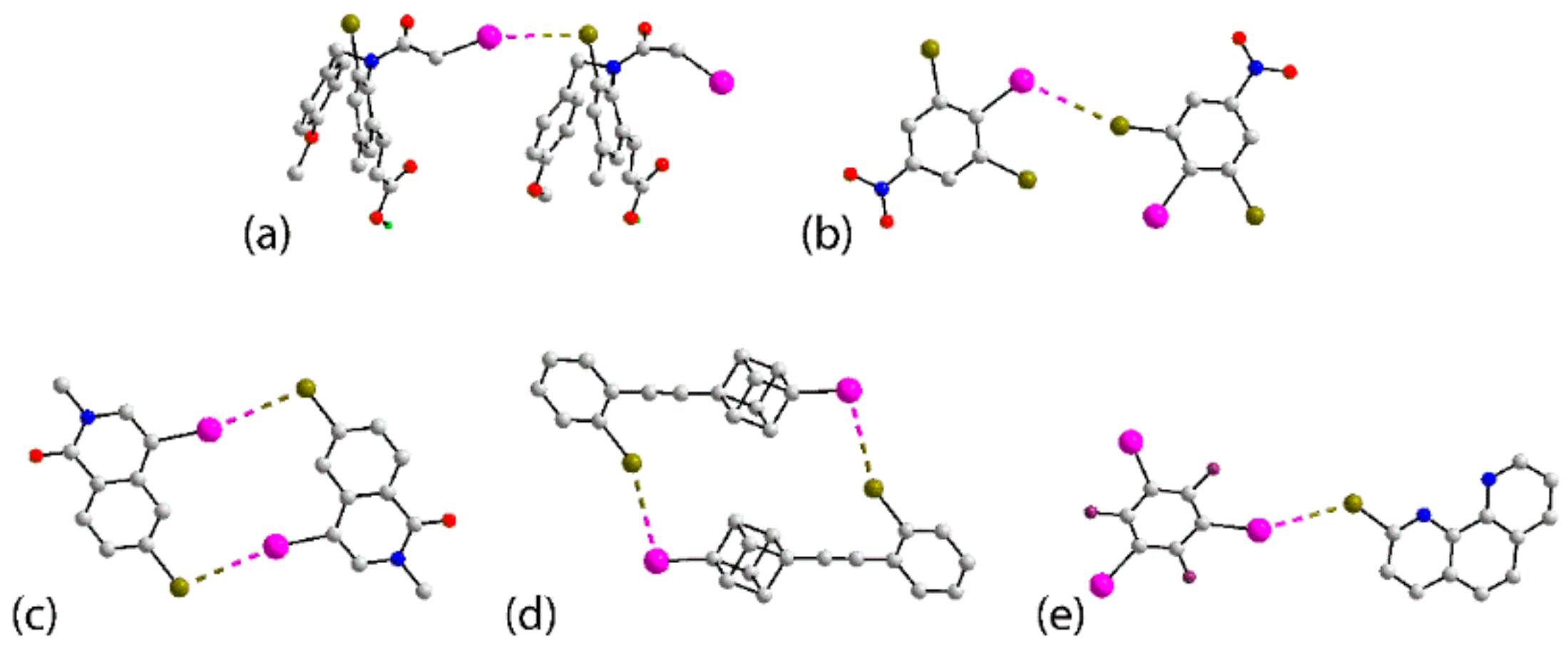
3.1. Zero-Dimensional Aggregates Featuring I⋯Br Interactions
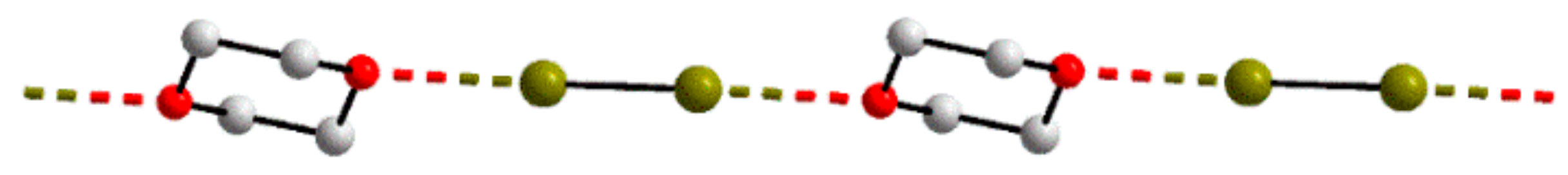
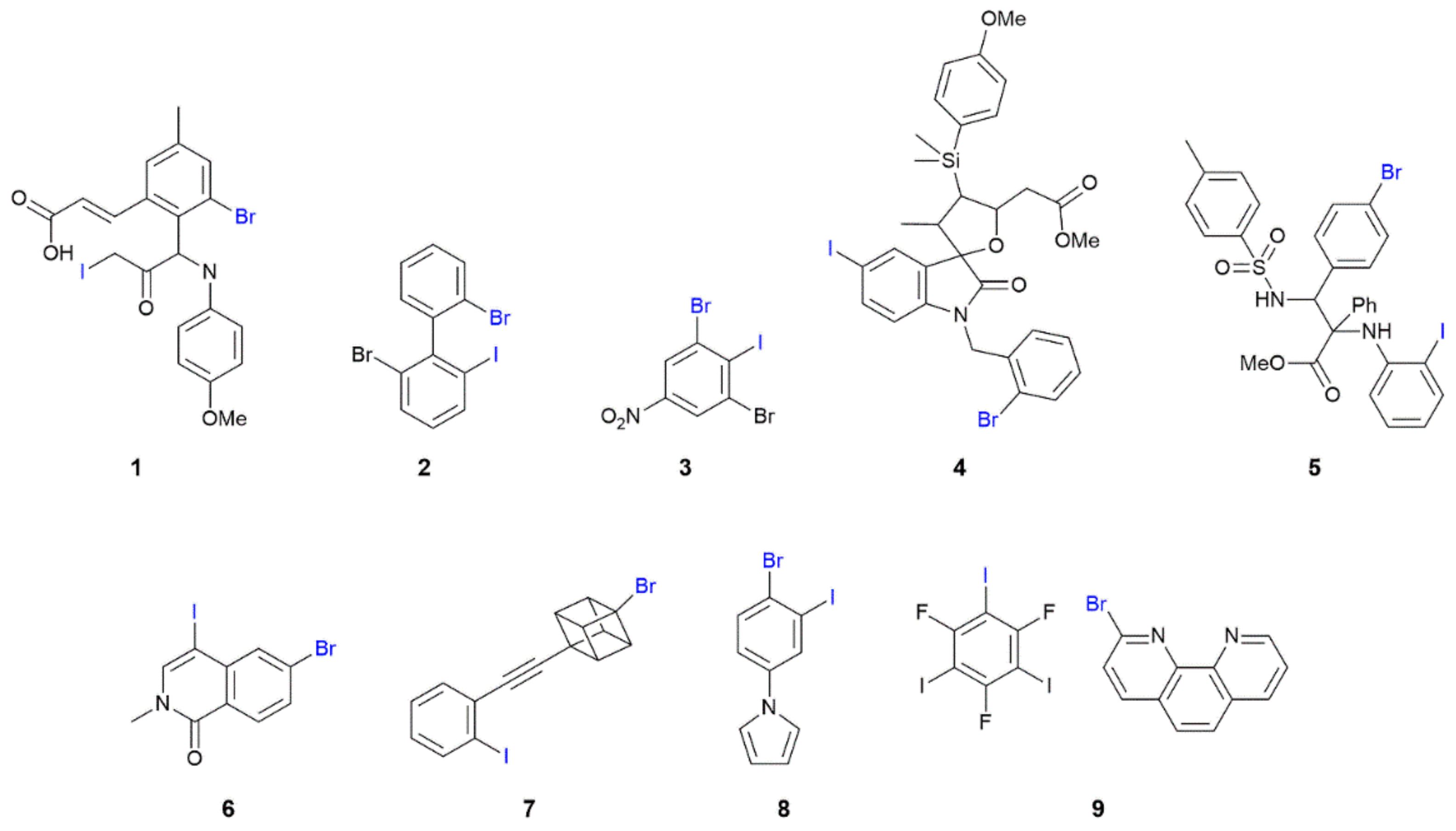
3.2. One-Dimensional Aggregates Featuring I⋯Br Interactions
3.3. Two- and Three-Dimensional Architectures Featuring I⋯Br Interactions
4. Relation to Congeners
5. Overview
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassel, O.; Hvoslef, J. The Structure of Bromine 1,4-Dioxanate. Acta Chem. Scand. 1954, 8, 873. [Google Scholar] [CrossRef]
- Spek, A.L. CheckCIF Validation ALERTS: What They Mean and How to Respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef]
- Alcock, N.W. Secondary Bonding to Nonmetallic Elements. Adv. Inorg. Chem. Radiochem. 1972, 15, 1–58. [Google Scholar] [CrossRef]
- Mulliken, R.S. Structures of Complexes Formed by Halogen Molecules with Aromatic and with Oxygenated Solvents. J. Am. Chem. Soc. 1950, 72, 600–608. [Google Scholar] [CrossRef]
- Bent, H.A. Structural Chemistry of Donor-Acceptor Interactions. Chem. Rev. 1968, 68, 587–648. [Google Scholar] [CrossRef]
- Hassel, O. Structural Aspects of Interatomic Charge-Transfer Bonding. Science 1970, 170, 497–502. [Google Scholar] [CrossRef]
- Colin, M.M.; Gaultier de Claubry, H. Sur le Combinaisons de I’iode Avec les Substances Végétales et Animales. Ann. Chim. 1814, 90, 87–100. [Google Scholar]
- Guthrie, F. On the Iodide of Iodammonium. J. Chem. Soc. 1863, 16, 239–244. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The Sigma-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Kolář, M.H.; Hobza, P. Computer Modeling of Halogen Bonds and Other σ-hole Interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen Bonding Based Recognition Processes: A world Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen bonds Yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hypervalency, Secondary Bonding and Hydrogen Bonding: Siblings under the Skin. Chem. Soc. Rev. 2017, 46, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Riel, A.M.S.; Rowe, R.K.; Ho, E.N.; Carlsson, A.-C.C.; Rappé, A.K.; Berryman, O.B.; Ho, P.S. Hydrogen Bond Enhanced Halogen Bonds: A Synergistic Interaction in Chemistry and Biochemistry. Acc. Chem. Res. 2019, 52, 2870–2880. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Christopherson, J.-C.; Topić, F.; Barrett, C.J.; Friščic, T. Halogen-Bonded Cocrystals as Optical Materials: Next-Generation Control over Light−Matter Interactions. Cryst. Growth Des. 2018, 18, 1245–1259. [Google Scholar] [CrossRef]
- Pancholi, J.; Beer, P.D. Halogen Bonding Motifs for Anion Recognition. Coord. Chem. Rev. 2020, 416, 213281. [Google Scholar] [CrossRef]
- Saccone, M.; Catalano, L. Halogen Bonding beyond Crystals in Materials Science. J. Phys. Chem. B 2019, 123, 9281–9290. [Google Scholar] [CrossRef] [PubMed]
- Beale, T.M.; Chudzinski, M.G.; Sarwar, M.G.; Taylor, M.S. Halogen Bonding in Solution: Thermodynamics and Applications. Chem. Soc. Rev. 2013, 42, 1667–1690. [Google Scholar] [CrossRef]
- Tepper, R.; Schubert, U.S. Halogen Bonding in Solution: Anion Recognition, Templated Self-Assembly, and Organocatalysis. Angew. Chem. Int. Ed. 2018, 57, 6004–6016. [Google Scholar] [CrossRef] [PubMed]
- Von der Heiden, D.; Vanderkooy, A.; Erdélyi, M. Halogen Bonding in Solution: NMR Spectroscopic Approaches. Coord. Chem. Rev. 2020, 407, 213147. [Google Scholar] [CrossRef]
- Parisini, E.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen Bonding in Halocarbon–Protein Complexes: A Structural Survey. Chem. Soc. Rev. 2011, 40, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, M.R.; Vander Zanden, C.M.; Carter, M.; Ho, P.S. Halogen Bonding (X-Bonding): A Biological Perspective. Prot. Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Brammer, L.; Espallargas, G.M.; Libri, S. Combining Metals with Halogen Bonds. CrystEngComm 2008, 10, 1712–1727. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Egido, I.; Hortelano, C.; López-López, M.; Gómez-Sal, P. Comparison of Halogen Bonding Networks with Ru(II) Complexes and Analysis of the Influence of the XB Interactions on Their Reactivity. Faraday Discuss. 2017, 203, 257–283. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polyhalide-Bonded Metal Complexes: Structural Diversity in an Eclectic Class of Compounds. Coord. Chem. Rev. 2018, 367, 1–17. [Google Scholar] [CrossRef]
- Fourmigué, M. Halogen Bonding: Recent Advances. Curr. Opin. Solid State Mater. Sci. 2009, 13, 36–45. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Liu, Y.; Tiekink, E.R.T. Supramolecular Associations in Binary Antimony(III) Dithiocarbamates: Influence of Ligand Steric Bulk, Influence on Coordination Geometry, and Competition with Hydrogen Bonding. CrystEngComm 2005, 7, 20–27. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Aggregation Patterns in the Crystal Structures of Organometallic Group XV 1,1-Dithiolates: The Influence of the Lewis Acidity of the Central Atom, Metal- and Ligand-Bound Steric Bulk, and Coordination Potential of the 1,1-Dithiolate Ligands upon Supramolecular Architecture. CrystEngComm 2006, 8, 104–118. [Google Scholar] [CrossRef]
- Lai, C.S.; Tiekink, E.R.T. Prevalence of Intermolecular Bi⋯S Interactions in Bismuth Dithiocarbamate Compounds: Bi(S2CNR2)3. Z. Kristallogr. 2007, 222, 532–538. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Stereochemical Activity of Lone Pairs of Electrons and Supramolecular Aggregation Patterns Based on Secondary Interactions Involving Tellurium in Its 1,1-Dithiolate Structures. Coord. Chem. Rev. 2010, 254, 46–76. [Google Scholar] [CrossRef]
- Lee, S.M.; Heard, P.J.; Tiekink, E.R.T. Molecular and Supramolecular Chemistry of Mono- and Di-Selenium Analogues of Metal Dithiocarbamates. Coord. Chem. Rev. 2018, 375, 410–423. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. A Survey of Supramolecular Aggregation Based on Main Group Element⋯Selenium Secondary Bonding Interactions – A Survey of the Crystallographic Literature. Crystals 2020, 10, 503. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Zero-, One-, Two- and Three-Dimensional Supramolecular Architectures Sustained by Se⋯O Chalcogen Bonding: A Crystallographic Survey. Coord. Chem. Rev. 2021, 427, 213586. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular Architectures Sustained by Delocalised C–I⋯π(Arene) Interactions in Molecular Crystals and the Propensity of Their Formation. CrystEngComm 2021, 23, 904–928. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New Software for Searching the Cambridge Structural Database and Visualizing Crystal Structures. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Brandenburg, K.; Berndt, M. DIAMOND, Version 3.2k; GbR: Bonn, Germany, 2006. [Google Scholar]
- Guthrie, D.B.; Geib, S.J.; Curran, D.P. Synthesis of Highly Enantioenriched 3,4-Dihydroquinolin-2-Ones by 6-Exo-Trig Radical Cyclizations of Axially Chiral α-Halo-Ortho-Alkenyl Anilides. J. Am. Chem. Soc. 2009, 131, 15492–15500. [Google Scholar] [CrossRef]
- Leroux, F.R.; Berthelot, A.; Bonnafoux, L.; Panossian, A.; Colobert, F. Transition-Metal-Free Atropo-Selective Synthesis of Biaryl Compounds Based on Arynes. Chem.-Eur. J. 2012, 18, 14232–14236. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.A.; Aguirre Hernández, G.; Bernès, S. Anomalous Halogen Bonds in the Crystal Structures of 1,2,3-TriBromo-5-NitroBenzene and 1,3-DiBromo-2-Iodo-5-NitroBenzene. Acta Crystallogr., Sect. E: Cryst. Commun. 2015, 71, 960–965. [Google Scholar] [CrossRef]
- Franz, A.K.; Dreyfuss, P.D.; Schreiber, S.L. Synthesis and Cellular Profiling of Diverse Organosilicon Small Molecules. J. Am. Chem. Soc. 2007, 129, 1020–1021. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, D.; Lv, F.; Guo, X.; Hu, W.; Yang, L.; Liu, S. Three-Component Reactions Based on Trapping Ammonium Ylides with N-Sulfonyl Aldimines via Cooperative Catalysis of Squaramides and Rh2(OAc)4. Tetrahedron 2014, 70, 1471–1477. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, Y.; Wang, Y. Synthesis of 4-Iodoisoquinolin-1(2H)-Ones by a Dirhodium(II)-Catalyzed 1,4-Bisfunctionalization of Isoquinolinium Iodide Salts. Org. Lett. 2019, 21, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.; Bernhard, S.S.R.; Plunkett, S.; Senge, M.O. Not Your Usual Bioisosteres: Solid State Study of 3D Interactions in Cubanes. Chem. Eur. J. 2019, 25, 6941–6954. [Google Scholar] [CrossRef]
- Messaoud, M.Y.A.; Bentabed-Ababsa, G.; Hedidi, M.; Derdour, A.; Chevallier, F.; Halauko, Y.S.; Ivashkevich, O.A.; Matulis, V.E.; Picot, L.; Thiéry, V.; et al. Deproto-Metallation of N-Arylated Pyrroles and Indoles Using a Mixed Lithium–Zinc Base and Regioselectivity-Computed CH Acidity Relationship. Beilstein J. Org. Chem. 2015, 11, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.G.; Wang, W. Noncovalent Interactions Between 1,3,5-Trifluoro-2,4,6-Triiodobenzene and a Series of 1,10-Phenanthroline Derivatives: A Combined Theoretical and Experimental Study. Crystals 2019, 9, 140. [Google Scholar] [CrossRef]
- Hong, L.; Sun, W.; Liu, C.; Wang, L.; Wong, K.; Wang, R. Enantioselective Friedel–Crafts Alkylation of 4,7-Dihydroindoles with Enones Catalyzed by Primary–Secondary Diamines. Chem. Eur. J. 2009, 15, 11105–11108. [Google Scholar] [CrossRef] [PubMed]
- Jardim, G.A.M.; da Silva Júnior, E.N.; Bower, J.F. Overcoming Naphthoquinone Deactivation: Rhodium-Catalyzed C-5 Selective C–H Iodination as A Gateway to Functionalized Derivatives. Chem. Sci. 2016, 7, 3780–3784. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.L.; Tang, S.Z.; Chen, M.E.; Zhang, X.M.; Lv, J.W.; Chen, X.W.; Qi, F.M.; Chen, S.W.; Zhang, F.M. Transition-Metal-Free site-Selective γ-C(sp2)–H Monoiodination of Arenes Directed by an Aliphatic Keto Group. Org. Lett. 2020, 22, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, X.; Shen, P.; Jiang, S. 1-(3-Bromopropoxy)-4-Iodobenzene. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o4760. [Google Scholar] [CrossRef]
- Rheingold, A.L. Private Communication to the Cambridge Structural Database. Refcode CORZER 2019. [Google Scholar] [CrossRef]
- Ning, J.H.; Xu, X.W. 3-Bromo-N’-(2-Hydroxy-3,5-Diiodobenzylidene)benzohydrazide Monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o905–o906. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Nong, X.R.; Yin, H. 2,4-Dibromo-6-(4-IodoPhenylIminoMethyl)Phenol. Acta Crystallogr. Sect. E: Struct. Rep. Online 2007, 63, o4640. [Google Scholar] [CrossRef]
- Tang, G.M.; Chi, R.H.; Wan, W.Z.; Wang, Y.T.; Cui, Y.Z.; Ng, S.W. Synthesis, Crystal Structures, and Nonlinear Optic and Thermal Properties of Two Diiodocarbazole Derivatives. J. Chem. Res. 2017, 41, 79–81. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.H.; Luo, W.; Liu, S.; Zhu, H.J. 5-Bromo-2-Iodo-1,3-Dimethylbenzene. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o219. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, N.M.; Schollmeyer, D.; Nubbemeyer, U. Total Synthesis of (–)-C/D-Cis-DeHydro-3-O-Methyl-Estradiols. Eur. J. Org. Chem. 2016, 2, 357–366. [Google Scholar] [CrossRef]
- Gaefke, G.; Enkelmann, V.; Höger, S. A Practical Synthesis of 1,4-Diiodo-2,5-Bis(chloromethyl)benzene and 1,4-Diiodo-2,5-Bis(bromomethyl)benzene. Synthesis 2006, 17, 2971–2973. [Google Scholar] [CrossRef]
- Gholap, S.P.; Jangid, D.; Fernandes, R.A. Metal-Free Brønsted Acid-Catalyzed Rearrangement of δ-Hydroxyalkynones to 2,3-Dihydro-4H-Pyran-4-Ones: Total Synthesis of Obolactone and a Catechol Pyran Isolated from Plectranthus Sylvestris. J. Org. Chem. 2019, 84, 3537–3551. [Google Scholar] [CrossRef] [PubMed]
- Maji, B.; Bhattacharya, A.; Hazra, S. Halogen-Induced Friedel-Crafts Alkenylation Reactions with Haloalkynes: Direct Access to Gem-1, 1-Dihaloalkenes. Chem. Sel. 2017, 2, 10375–10378. [Google Scholar] [CrossRef]
- Hu, T.; Shen, M.; Chen, Q.; Li, C. Pushing Radical Cyclization from Regioselective to Regiospecific: Cyclization of Amidyl Radicals Controlled by Vinylic Halogen Substitution. Org. Lett. 2006, 8, 2647–2650. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; He, Y.; Fan, X. Selective Synthesis of Pyrrolidin-2-Ones and 3-Iodopyrroles via The Ring Contraction and Deformylative Functionalization of Piperidine Derivatives. Org. Biomol. Chem. 2019, 17, 156–164. [Google Scholar] [CrossRef]
- Pandeeti, O.; Bijigiri, S.K.; Panda, P.K. One-Pot Synthesis of Benzotripyrrole Derivatives from 1H-Pyrroles. New J. Chem. 2019, 43, 18437–18841. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, S.; Yang, Y.; Yang, Y.; Hammond, G.B.; Xu, B. Regio- and Stereoselective Synthesis of 1,2-Dihaloalkenes Using In-Situ-Generated ICl, IBr, BrCl, I2, and Br2. Cell Press Chem. 2020, 6, 1018–1031. [Google Scholar] [CrossRef]
- Betz, R.; Klüfers, P. 1-Bromomethyl-2-Iodobenzene. Acta Crystallogr. Sect. E: Struct. Rep. Online 2007, 63, o4753. [Google Scholar] [CrossRef]
- Mamane, V.; Peluso, P.; Aubert, E.; Cossu, S.; Pale, P. Chiral Hexahalogenated 4,4′-Bipyridines. J. Org. Chem. 2016, 81, 4576–4587. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, D.; Alzola, J.M.; Lobkovsky, E.B.; Dichtel, W.R. Regioselective Synthesis of Polyheterohalogenated Naphthalenes via the Benzannulation of Haloalkynes. Chem. Eur. J. 2015, 21, 18122–18127. [Google Scholar] [CrossRef] [PubMed]
- Barát, V.; Csókás, D.; Bates, R.W. Synthesis of (−)-Cytisine Using a 6-Endo Aza-Michael Addition. J. Org. Chem. 2018, 83, 9088–9095. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Luo, F.; Zhang, Q.; Liang, Y.; Li, D.; Zhan, Y.; Kong, L.; Wang, Z.-X.; Peng, B. Asymmetric Iodonio-[3,3]-Sigmatropic Rearrangement to Access Chiral α-Aryl Carbonyl Compounds. J. Am. Chem. Soc. 2020, 142, 6884–6890. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, S.; Zhang, J.; Oswald, V.F.; Amassian, A.; Marder, S.R.; Blakey, S.B. KOtBu-Initiated Aryl C–H Iodination: A Powerful Tool for the Synthesis of High Electron Affinity Compounds. J. Am. Chem. Soc. 2016, 138, 3946–3949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Li, C.; Liu, P.; Xu, W.; Tang, L.; Wang, J.; Zhao, G. Facile Synthesis of Enantiomerically Pure 1-(5-Bromo-2-Chlorophenyl)-1-(4-Ethoxyphenyl)ethane. Chem. Res. Chin. Univ. 2014, 30, 250–256. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Liu, Z.; Lv, Y. Eco-Friendly C–I and C–O Bond Formation of Simple Alkenes: Direct Access to β-Iodo Oxyamines. Chem. Sel. 2018, 3, 5766–5768. [Google Scholar] [CrossRef]
- Gelbrich, T.; Threlfall, T.L.; Hursthouse, M.B. XPac Dissimilarity Parameters as Quantitative Descriptors of Isostructurality: The Case of Fourteen 4,5′-Substituted Benzenesulfonamido-2-Pyridines Obtained by Substituent Interchange Involving CF3/I/Br/Cl/F/Me/H. CrystEngComm 2012, 14, 5454–5464. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, G.R. Halogen Bonds in Some Dihalogenated Phenols: Applications to Crystal Engineering. IUCrJ 2014, 1, 49–60. [Google Scholar] [CrossRef]
- Caracelli, I.; Haiduc, I.; Zukerman-Schpector, J.; Tiekink, E.R.T. M⋯π(arene) Interactions for M = Gallium, Indium and Thallium: Influence upon Supramolecular Self-Assembly and Prevalence in Some Proteins. Coord. Chem. Rev. 2014, 283, 50–63. [Google Scholar] [CrossRef]
- Balmohammadi, Y.; Khavasi, H.R.; Naghavi, S.S. Existence of Untypical Halogen-Involving Interactions in Crystal Packings: A Statistical and First-Principles Study. CrystEngComm 2020, 22, 2756–2765. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The Use and Misuse of van der Waals Radii. Struct. Chem. 2021, 32, 623–629. [Google Scholar] [CrossRef]
- Sakurai, T.; Sundaralingam, M.; Jeffrey, G.A. A Nuclear Quadrupole Resonance and X-Ray Study of Crystal Structure of 2,5-Dichloroaniline. Acta Crystallogr. 1963, 16, 354–363. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The Nature of Halogen⋯Halogen Interactions - Are Short Halogen Contacts due to Specific Attractive Forces or due to Close Packing of Nonspherical Atoms. J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Rheingold, A.L. Private Communication to the Cambridge Structural Database. Refcode COMJUM 2019. [Google Scholar] [CrossRef]
- Liu, R.; Wu, W.Y.; Li, Y.H.; Deng, S.P.; Zhu, H.J. 2-Bromo-5-Iodo-1, 3-Dimethylbenzene. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o280. [Google Scholar] [CrossRef]
- Bhar, A.; Aune, J.P.; Benali-Cherif, N.; Benmenni, L.; Giorgi, M. Three Polychloromononitrobenzenes: C6H3Cl2NO2, C6H2Cl3NO2 and C6HCl4NO2. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1995, 51, 256–260. [Google Scholar] [CrossRef]
- Näther, C.; Jess, I.; Kuś, P.; Jones, P.G. Dimorphism of 1,4-Dibromo-2,5-bis(bromomethyl)benzene: Crystallographic and Physicochemical Investigations. CrystEngComm 2016, 18, 3142–3149. [Google Scholar] [CrossRef]
- Kersten, K.; Kaur, R.; Matzger, A. Survey and Analysis of Crystal Polymorphism in Organic Structures. IUCrJ 2018, 5, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, P.; Lusi, M.; Cruz-Cabeza, A.J.; Nauha, E.; Bernstein, J. Same or Different – That Is the Question: Identification of Crystal Forms from Crystal Structure Data. CrystEngComm 2020, 22, 7170–7185. [Google Scholar] [CrossRef]
- Guo, M.L.; Zhang, L. 2,4-Dibromo-6-(4-bromophenyliminomethyl)phenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o4558. [Google Scholar] [CrossRef]
- Ji, H.; Ma, H.P.; Yang, Y.A.; Zhu, H.L. 2-[(4-BromPhenylimino)methyl]-4,6-Diiodophenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o788. [Google Scholar] [CrossRef]
- Zhang, X.L.; Guo, Y.N. Crystal Structure of 2-Bromo-4-Chloro-6-[(4-Bromophenylimino)methyl]phenol, C13H8Br2ClNO. Z. Kristallogr. New Cryst. Struct. 2011, 226, 521–522. [Google Scholar] [CrossRef]
- Zhang, X.L. Crystal Structure of 2-Bromo-4-Chloro-6-[(4-chlorophenylimino)methyl]phenol, C13H8BrCl2NO. Z. Kristallogr. New Cryst. Struct. 2011, 226, 567–568. [Google Scholar] [CrossRef]
- Puthilibai, G.; Vasudhevan, S.; Rajagopal, G. 2-Bromo-4-Chloro-6-(4-fluorophenyliminomethyl)phenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o1333. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Zhu, H.; Zhu, H.L. Crystal Structure of 2[(4-Fluorophenylimino)methyl]-4,6-Diiodophenol, C13H8FI2NO. Z. Kristallogr. New Cryst. Struct. 2012, 227, 447–448. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. A Structural Survey of Meta⋯π Heteroaromatic Supramolecular Synthons for Metal =Tellurium, Tin, and Gold. CrystEngComm 2009, 11, 2701–2711. [Google Scholar] [CrossRef]
- Allen, F.H.; Motherwell, W.D.S.; Raithby, P.R.; Shields, G.P.; Taylor, R. Systematic Analysis of the Probabilities of Formation of Bimolecular Hydrogen-Bonded Ring Motifs in Organic Crystal Structures. New J. Chem. 1999, 23, 25–34. [Google Scholar] [CrossRef]
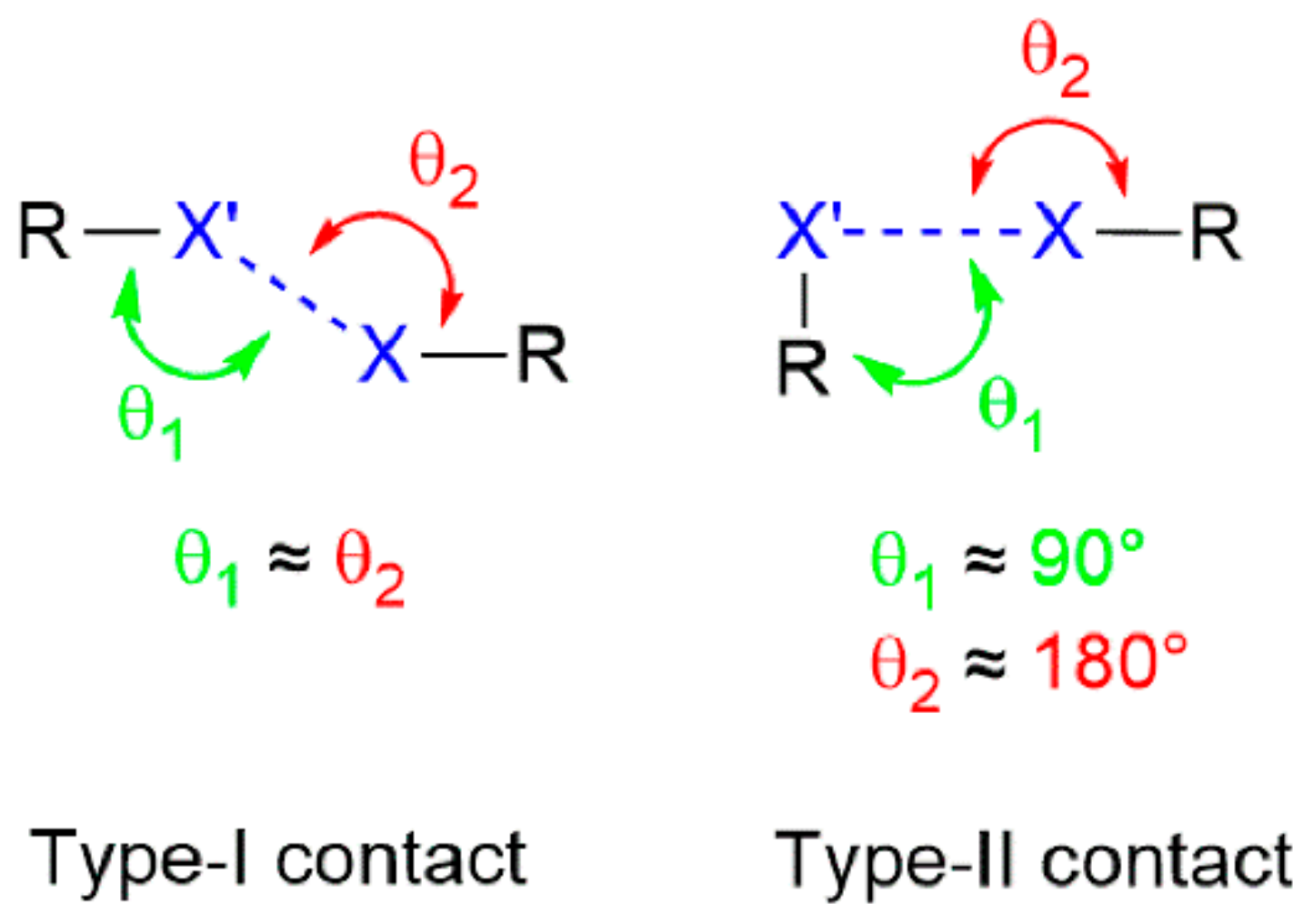
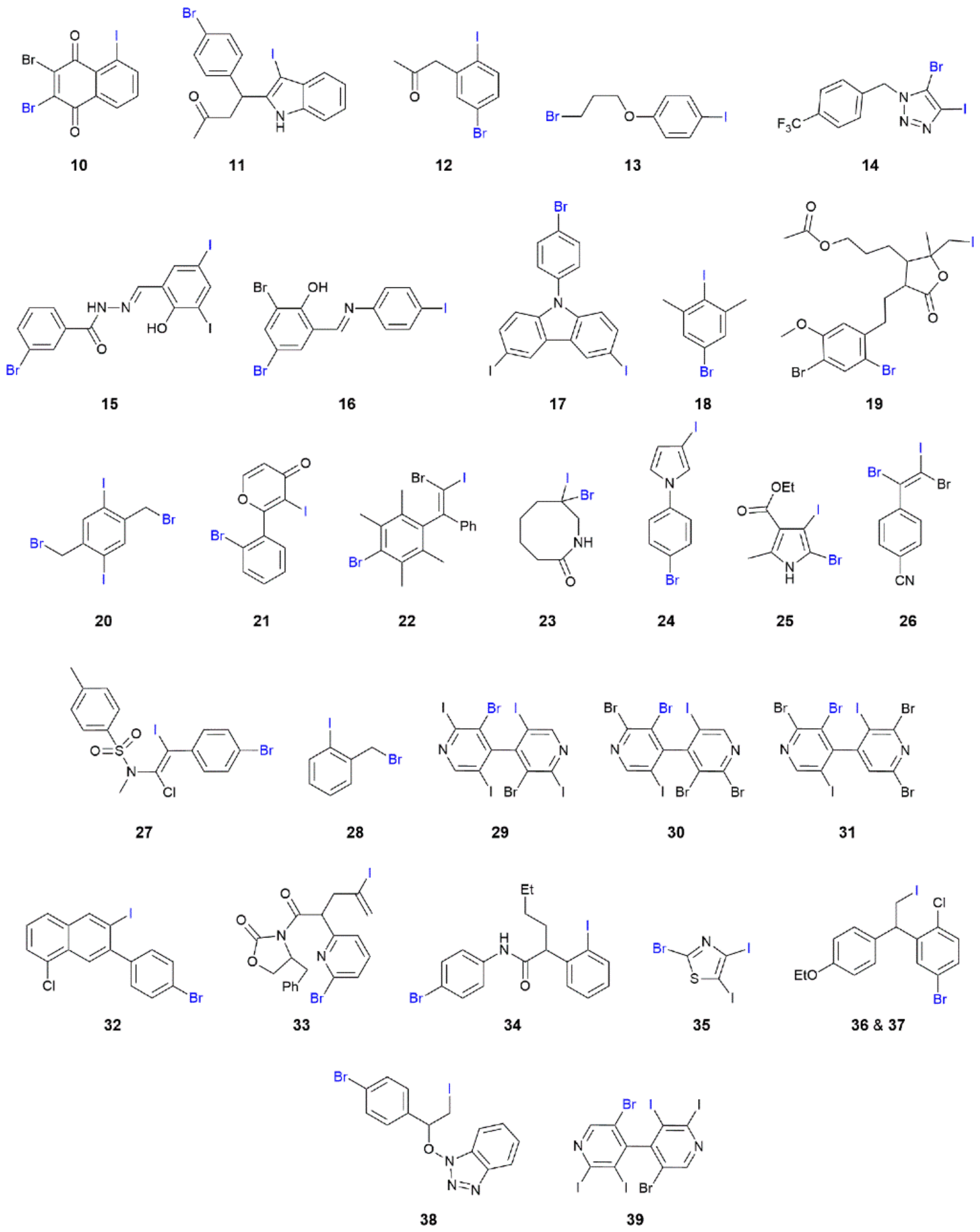
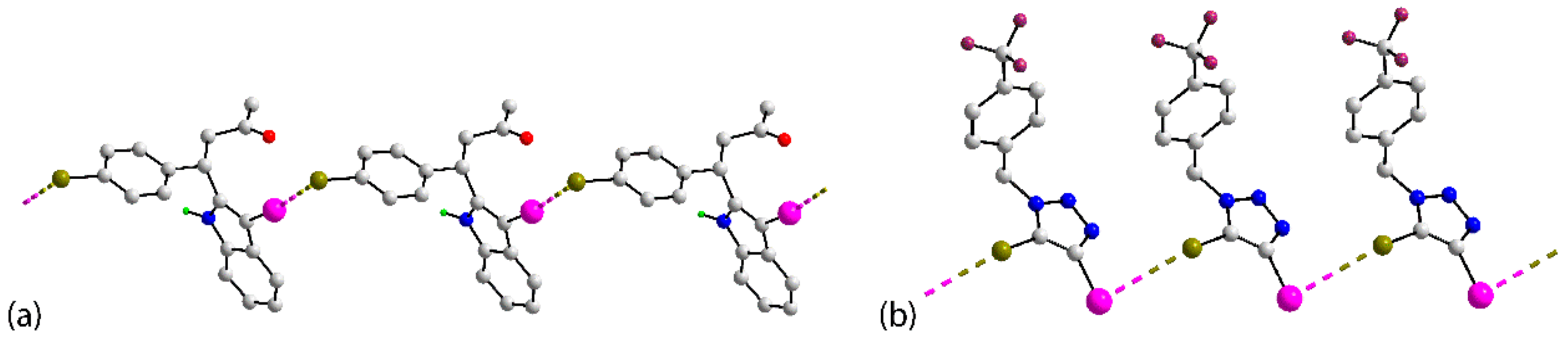
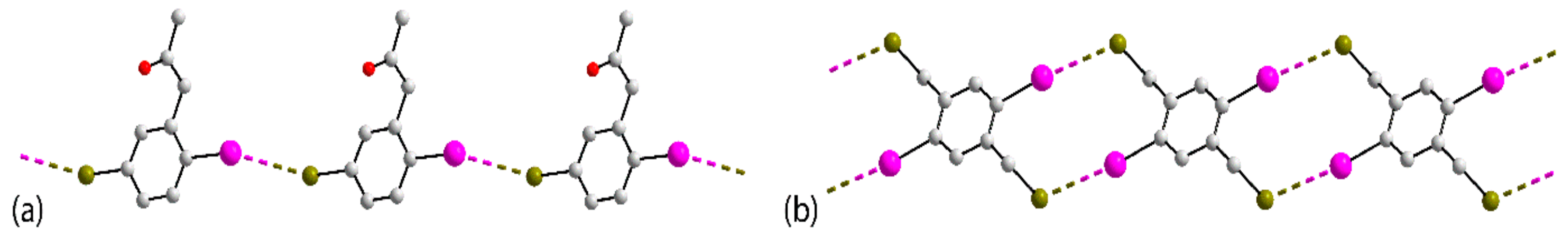
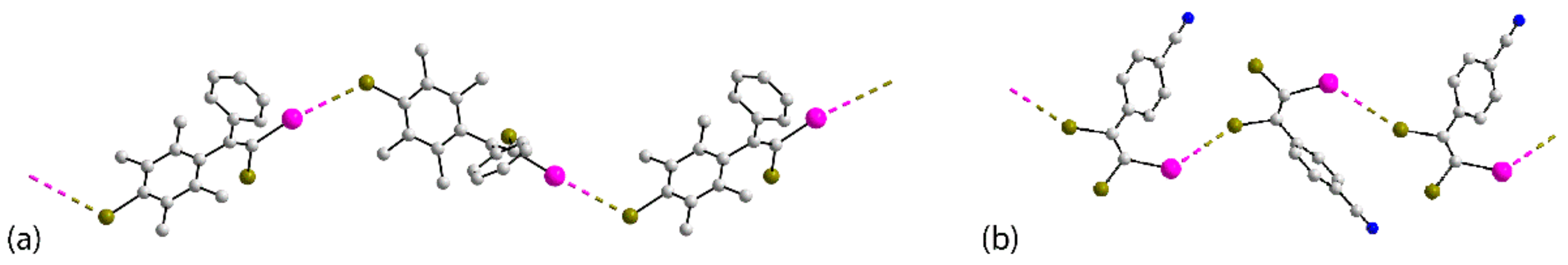
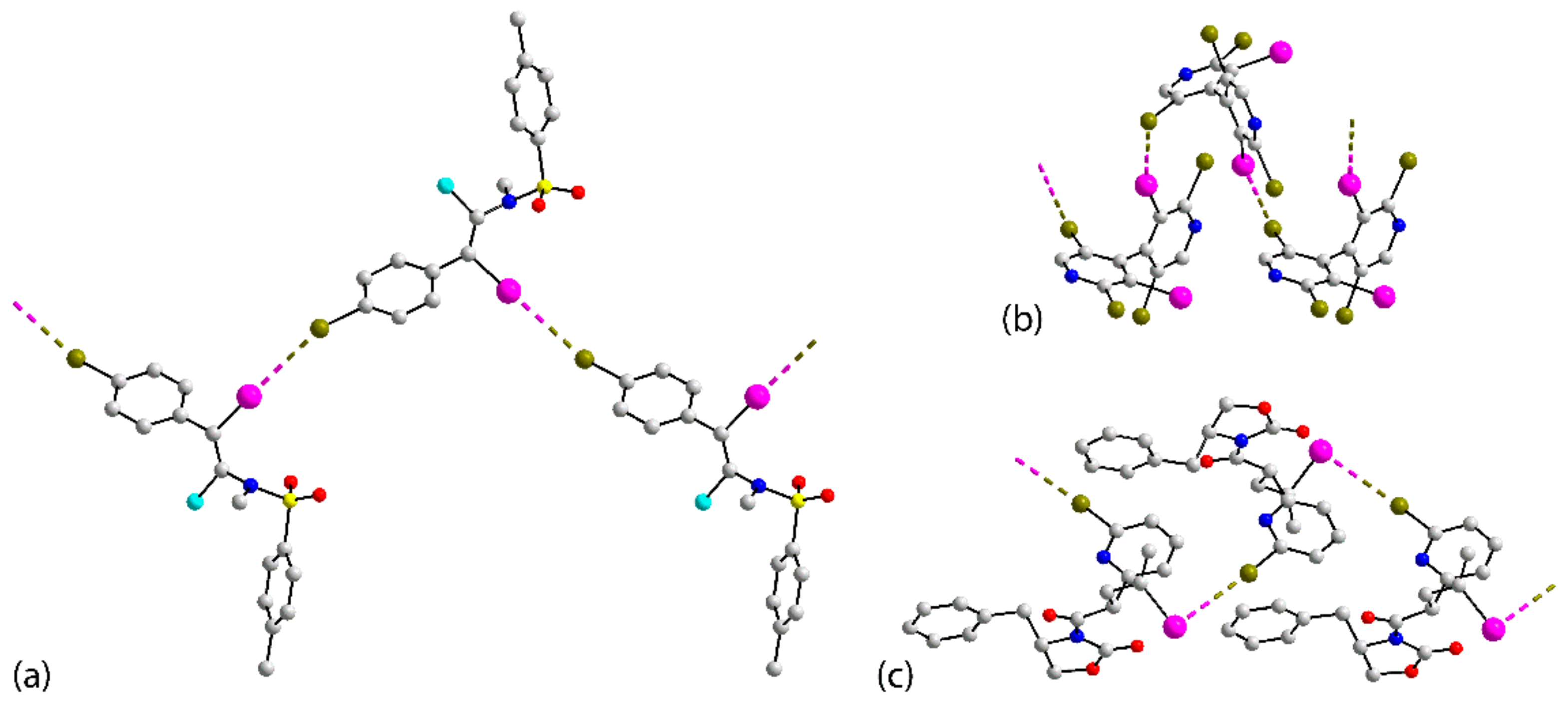
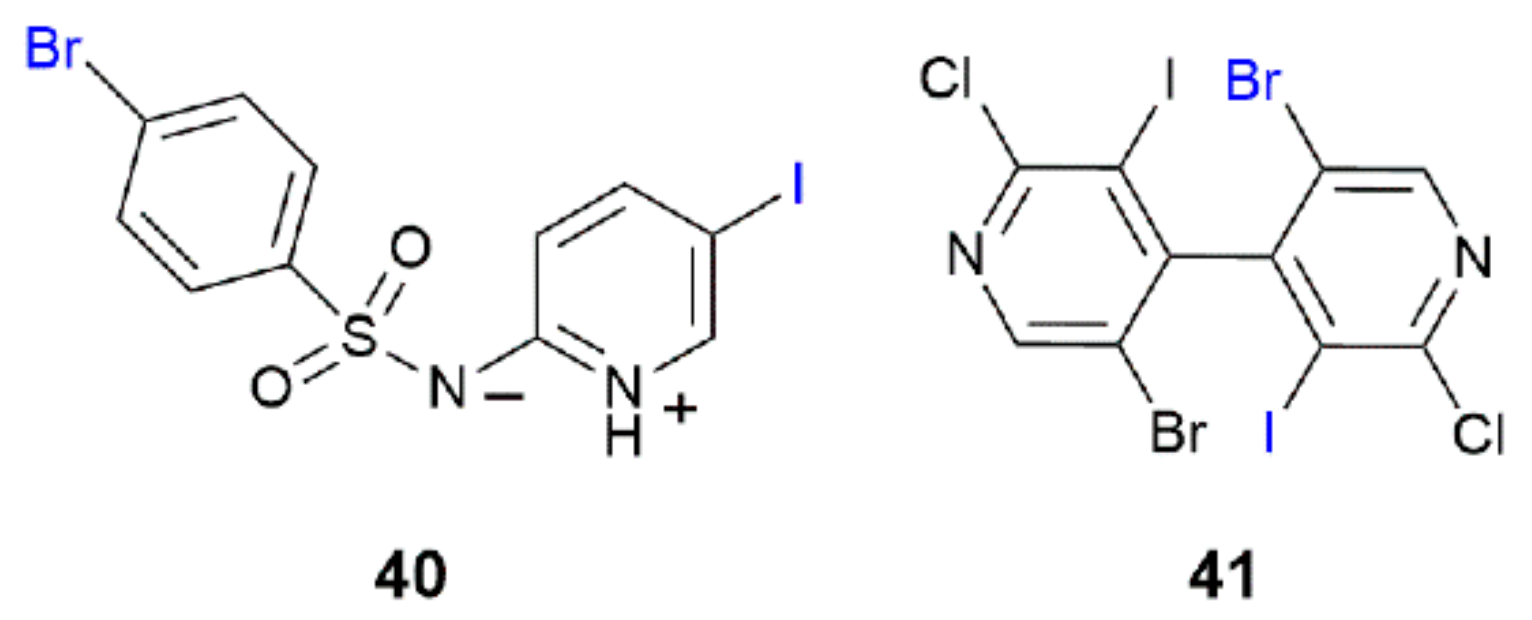
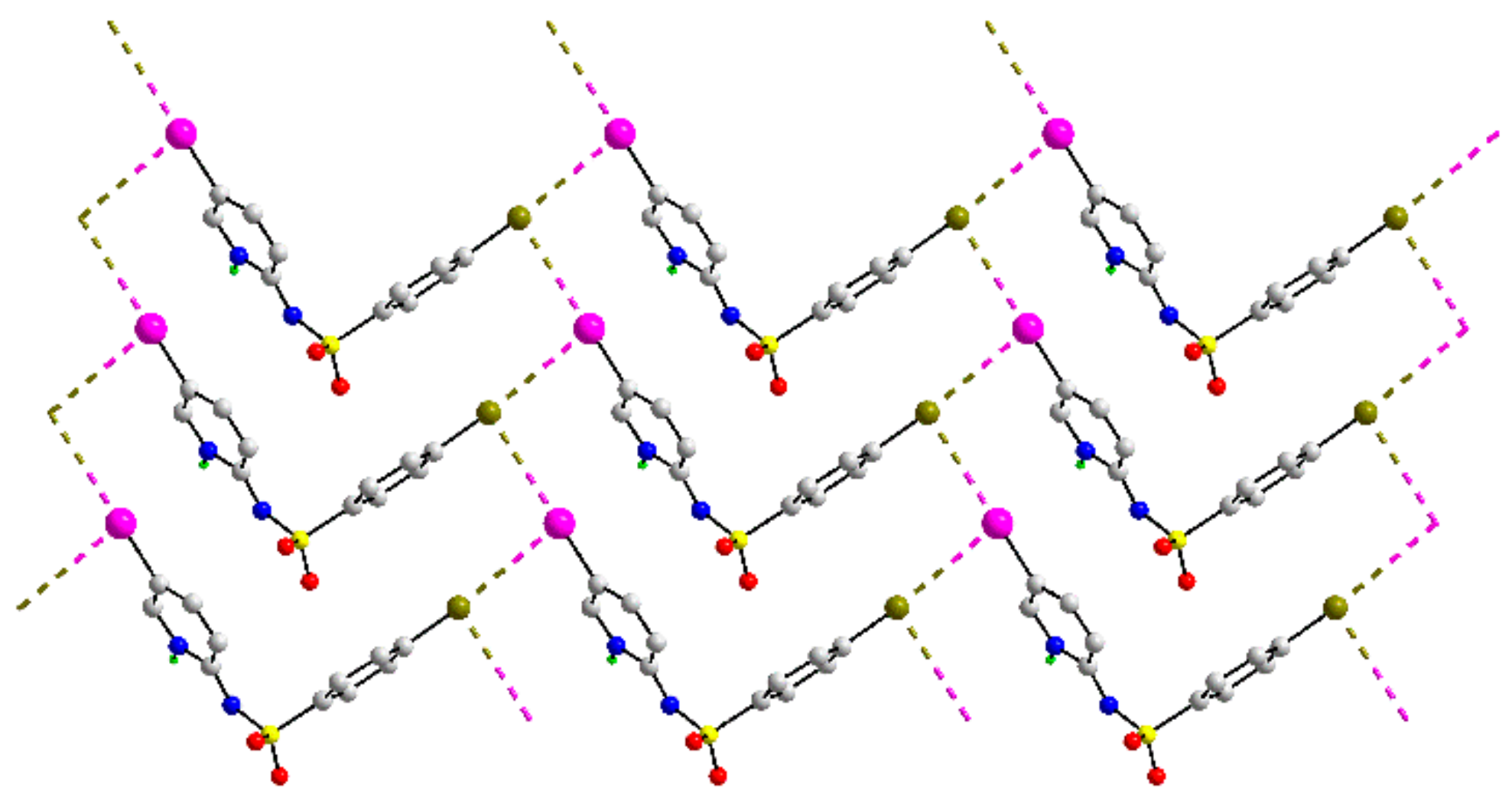
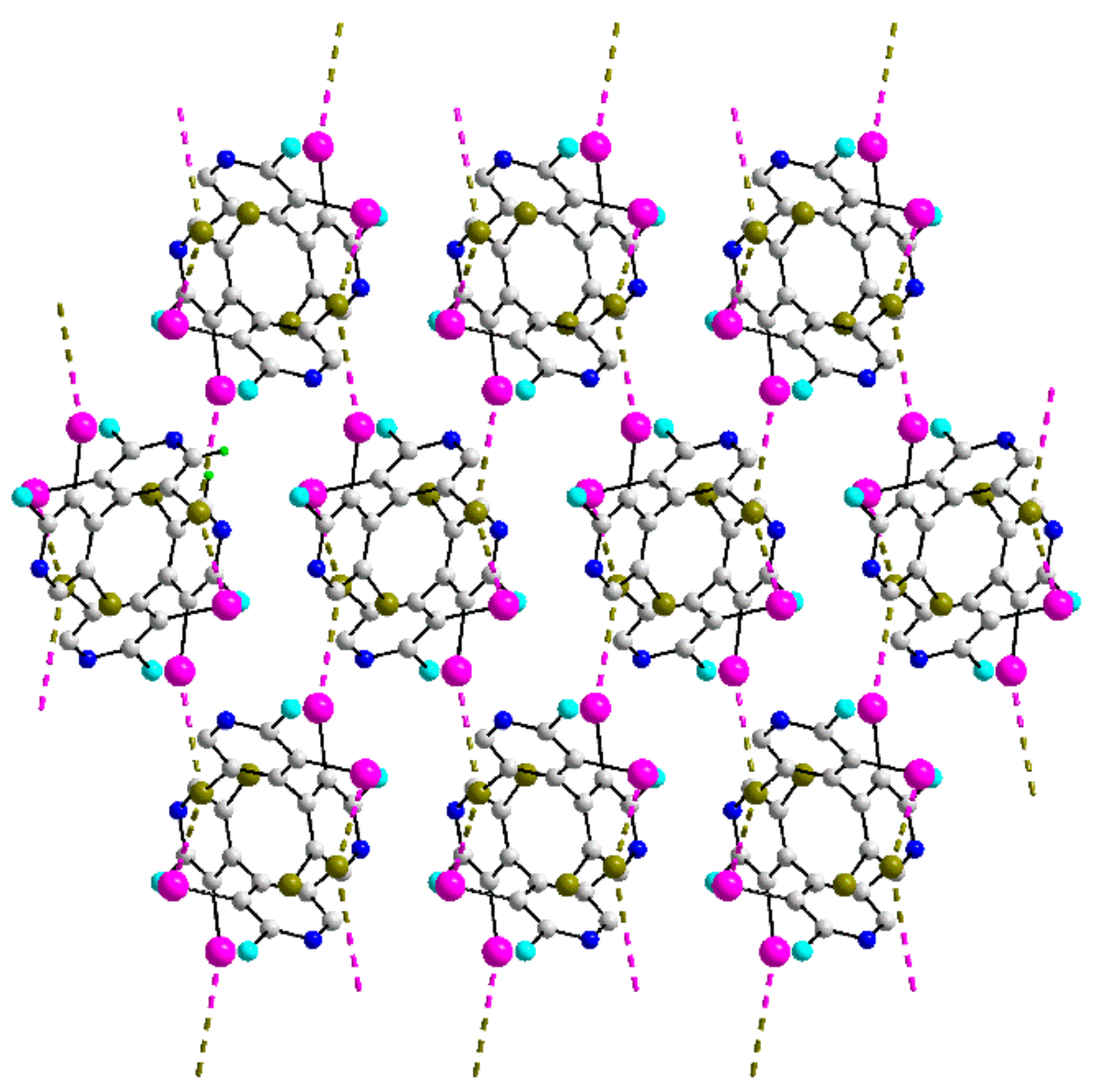

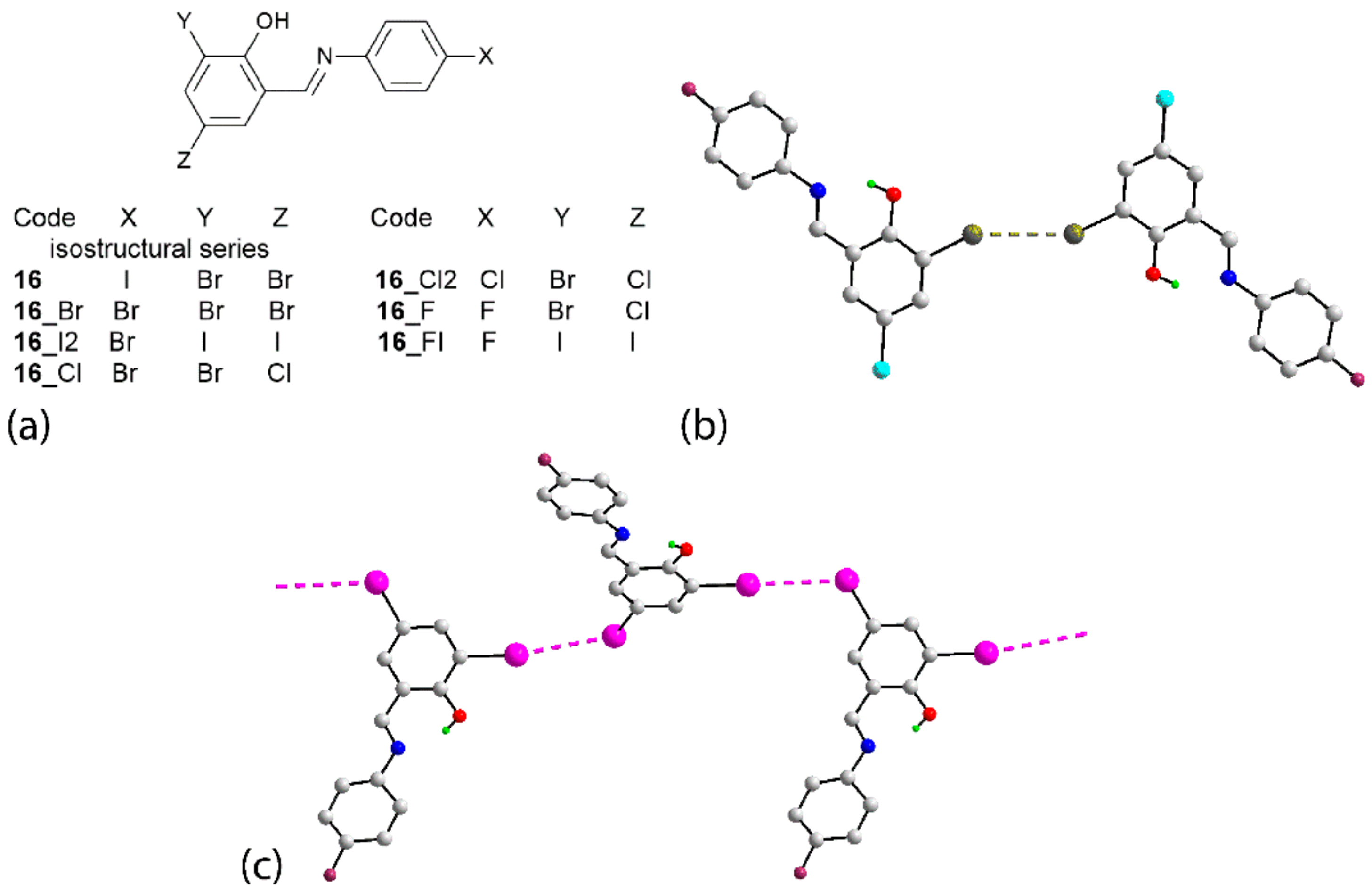
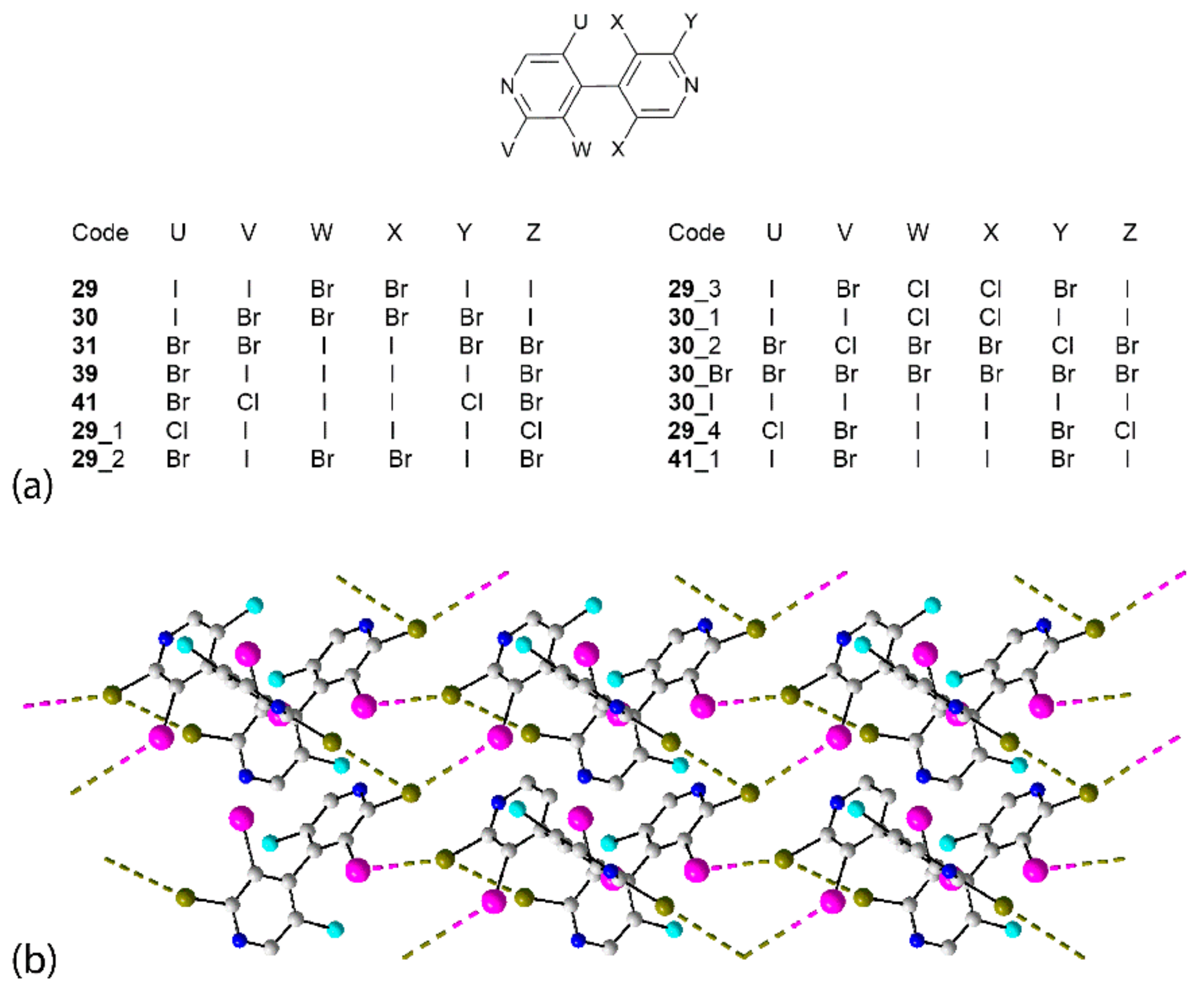
| Crystal | d(I⋯Br) | C–I⋯Br (θ1) | C–Br⋯I (θ2) | Motif | CSD REFCODE | Ref. |
|---|---|---|---|---|---|---|
| 1 | 3.6830(11) | 161.3(2) | 102.6(2) | Two-molecule–one contact | OJACUY | [41] |
| 2 | 3.7397(9) | 172.38(19) | 85.37(16) | Two-molecule–one contact | RECROI | [42] |
| 3 | 3.813(3) | 117.2(5) | 161.2(5) | Two-molecule–one contact | DUPNIN | [43] |
| 4 | 3.5640(3) | 73.4(3) | 159.70(4) | Centrosymmetric dimer | NEYSUG | [44] |
| 5 | 3.6449(9) | 160.07(13) | 124.72(15) | Centrosymmetric dimer | LOBHOB | [45] |
| 6 | 3.7068(13) | 168.8(2) | 102.8(3) | Centrosymmetric dimer | SIZTED | [46] |
| 7 | 3.7556(4) | 93.01(5) | 155.75(6) | Centrosymmetric dimer | QOCSUZ | [47] |
| 8 | 3.8122(5) | 117.83(10) | 166.26(12) | Centrosymmetric dimer | KAGQUH | [48] |
| 9 | 3.6927(7) | 156.68(11) | 130.84(14) | Two-molecule–one contact | VIXWIL | [49] |
| 10 | 3.5402(4) | 169.74(9) | 121.58(9) | 1-D: linear | MUKKUY | [50] |
| 11 | 3.540(2) | 173.67(16) | 91.44(19) | 1-D: linear | MAMFIS | [51] |
| 12 | 3.6421(9) | 161.23(11) | 160.90(13) | 1-D: linear | SUWKUT | [52] |
| 13 | 3.6623(10) | 155.87(8) | 160.15(9) | 1-D: linear | CIQMEV | [53] |
| 14 | 3.7163(11) | 84.5(3) | 148.8(3) | 1-D: linear | CORZER | [54] |
| 15 | 3.7169(12) | 149.09(17) | 140.37(19) | 1-D: linear | VIQQIC | [55] |
| 16 | 3.7226(16) | 166.3(3) | 112.8(3) | 1-D: linear | HIRHUM | [56] |
| 17 | 3.7228(12) | 158.5(2) | 135.2(2) | 1-D: linear | QAPQAC | [57] |
| 18a | 3.6869(15) | 162.8(2) | 161.1(3) | 1-D: linear | YIRTOJ | [58] |
| 3.7413(15) | 159.8(3) | 156.0(3) | 1-D: linear | |||
| 19 | 3.7558(13) | 84.5(3) | 171.2(4) | 1-D: linear | CABCAM | [59] |
| 20 | 3.6271(4) | 166.66(7) | 97.45(10) | 1-D: linear | MESMED | [60] |
| 21 | 3.6011(6) | 175.22(11) | 103.29(13) | 1-D: zigzag | YIZFUL | [61] |
| 22 | 3.640(1) | 168.25(17) | 118.41(17) | 1-D: zigzag | JIPTEK | [62] |
| 23 | 3.6666(12) | 175.7(2) | 115.3(2) | 1-D: zigzag | SELYOY | [63] |
| 24 | 3.691(3) | 151.5(4) | 126.0(5) | 1-D: zigzag | JINJUO | [64] |
| 25b | 3.7380(17) | 127.2(3) | 151.3(4) | 1-D: zigzag | MOZTED | [65] |
| 3.7905(14) | 126.8(3) | 158.3(4) | ||||
| 26 | 3.8025(5) | 127.28(11) | 152.94(12) | 1-D: zigzag | BULZIU | [66] |
| 27 | 3.6889(8) | 169.87(9) | 138.90(11) | 1-D: helical | BUNBAQ | [66] |
| 28 | 3.6943(4) | 170.95(7) | 90.81(11) | 1-D: helical | CIQLEU | [67] |
| 29 | 3.7136(15) | 78.41(9) | 170.13(11) | 1-D: helical | IMURAL | [68] |
| 30 | 3.7281(18) | 71.91(14) | 161.19(15) | 1-D: helical | INADIM | [68] |
| 31 | 3.7330(7) | 162.62(7) | 81.66(7) | 1-D: helical | IMUQOY | [68] |
| 32 | 3.7592(4) | 173.40(6) | 104.68(8) | 1-D: helical | FABWIR | [69] |
| 33 | 3.7631(7) | 87.09(15) | 168.75(18) | 1-D: helical | ZIMLIT | [70] |
| 34 | 3.7724(9) | 167.18(16) | 76.25(18) | 1-D: helical | WUJYUY | [71] |
| 35 | 3.7966(13) | 107.0(3) | 160.7(3) | 1-D: helical | IKUZAR | [72] |
| 36 | 3.7973(9) | 103.12(13) | 159.62(14) | 1-D: helical | GUMMEI | [73] |
| 37 | 3.8053(7) | 103.40(10) | 159.58(11) | 1-D: helical | GUMLUZ | [73] |
| 38 | 3.8001(10) | 165.44(11) | 121.92(14) | 1-D: helical | VEXDAG | [74] |
| 39 | 3.8140(9) | 169.03(7) | 77.36(8) | 1-D: helical | IMURUF | [68] |
| 40c | 3.7436(5) | 158.64(11) | 87.49(12) | 2-D; flat topology | VEGSOR | [75] |
| 3.7465(6) | 80.73(11) | 172.51(12) | ||||
| 41 | 3.6944(7) | 161.40(5) | 83.20(5) | 3-D | INADOS | [68] |
| 3.7405(5) | 95.99(5) | 155.52(5) |
| Crystal | X | X’ | d(X⋯X’) | C–X⋯X’ (θ1) | C–X’⋯X (θ2) | %[d(X⋯X)/ΣvdW] a | CSD REFCODE | Ref. |
|---|---|---|---|---|---|---|---|---|
| 20 | I | Br | 3.6271(4) | 166.66(7) | 97.45(10) | 94.7 | MESMED | [60] |
| 20_Cl | I | Cl | 3.5373(9) | 166.82(10) | 99.27(14) | 94.8 | MESMIH | [60] |
| 21 | I | Br | 3.6011(6) | 175.22(11) | 103.29(13) | 94.0 | YIZFUL | [61] |
| 21_Br | Br | Br | 3.5512(5) | 175.86(9) | 100.68(9) | 96.0 | YIZFOF | [61] |
| 21_Cl | Br | Cl | 3.4969(6) | 174.70(6) | 100.98(7) | 97.1 | YIZFIZ | [61] |
| 23_I | I | I | 3.7541(7) | 173.90(16) | 112.67(16) | 94.8 | SELYUE | [63] |
| 23 | I | Br | 3.6666(12) | 175.7(2) | 115.3(2) | 95.7 | SELYOY | [63] |
| 40_X = I | I | I | 3.9277(8) | 160.50(19) | 88.39(19) | 99.2 | VEGSIL | [75] |
| 3.8761(9) | 83.8(2) | 175.2(2) | 97.9 | |||||
| 40 | I | Br | 3.7436(5) | 158.64(11) | 87.49(12) | 97.7 | VEGSOR | [75] |
| 3.7465(6) | 80.73(11) | 172.51(12) | 97.8 | |||||
| 40_X = Cl | I | Cl | 3.6890(9) | 156.80(8) | 87.45(12) | 98.9 | VEGSUX | [75] |
| 3.7310(11) | 78.73(8) | 169.42(13) | 100.0 | |||||
| 40_X = F | I | F | 3.742(2) | 152.21(9) | 80.44(16) | 109.5 | VEGTAE | [75] |
| 3.512(3) | 69.70(9) | 159.44(19) | 108.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiekink, E.R.T. Characterising Supramolecular Architectures in Crystals Featuring I⋯Br Halogen Bonding: Persistence of X⋯X’ Secondary-Bonding in Their Congeners. Crystals 2021, 11, 433. https://doi.org/10.3390/cryst11040433
Tiekink ERT. Characterising Supramolecular Architectures in Crystals Featuring I⋯Br Halogen Bonding: Persistence of X⋯X’ Secondary-Bonding in Their Congeners. Crystals. 2021; 11(4):433. https://doi.org/10.3390/cryst11040433
Chicago/Turabian StyleTiekink, Edward R. T. 2021. "Characterising Supramolecular Architectures in Crystals Featuring I⋯Br Halogen Bonding: Persistence of X⋯X’ Secondary-Bonding in Their Congeners" Crystals 11, no. 4: 433. https://doi.org/10.3390/cryst11040433
APA StyleTiekink, E. R. T. (2021). Characterising Supramolecular Architectures in Crystals Featuring I⋯Br Halogen Bonding: Persistence of X⋯X’ Secondary-Bonding in Their Congeners. Crystals, 11(4), 433. https://doi.org/10.3390/cryst11040433






